Abstract
Sciatic nerves from allogeneic Sprague-Dawley rats were pretreated with chondroitinase ABC and were used to bridge damaged sciatic nerves in Wistar rats. Chondroitin sulfate proteoglycans were removed from the chemically extracted acellular nerves. At 3 months after grafting, the footplate pinch test result was positive in the Wistar rats. Autotomy scores decreased, and increased muscular contraction tension appeared when triceps surae muscles were stimulated. In addition, the recovery rate of wet triceps surae muscle weight increased, and the distal segment of the chondroitinase ABC-treated graft exhibited Schwann cells next to the nerve fibers. These results suggested that chondroitinase ABC pretreatment enhanced repair of long nerve defects via acellular nerve grafting.
Keywords: chondroitinase ABC, neural regeneration, peripheral nerve, sciatic nerve, Schwann cell
INTRODUCTION
The most common method of repairing injured nerves is to directly suture the severed nerve ends together. However, this method is not feasible if the gap is too large to allow for tension-free nerve repair. In these cases, autografting, synthetic nerve guide conduits, and venous and allograft materials are used to repair nerve gaps[1,2]. Acellular nerve grafting, with its lower immune response, as well as the internal structure and extracellular matrix components of the nerve, is an alternative to autografting for repair of peripheral nerve defects[3].
Different techniques have been used to create acellular nerves, such as thermal treatment[4], radiation, and chemical treatment[5,6]. Acellular nerve grafts obtained by chemical extraction are intact cylindrical collagen tubes with a basal lamina component and have been adapted for reoccupation by Schwann cells after allografting[7]. During extraction, some protein components, such as chondroitin sulfate proteoglycans and laminin, remain in the extracellular matrix[3,6]. Chondroitin sulfate proteoglycans were previously identified to inhibit the neurite-promoting activity of laminin[8,9]. A previous study[10] reported that nerve regeneration was significantly enhanced in freeze-thawed allografts following treatment with chondroitinase ABC (ChABC). However, there is a distinct limit to the effective length of acellular grafts. In the present study, chemically extracted nerve grafts were pre-treated with ChABC to optimize the nerve-grafted material. The experiment tested whether the optimized chemically acellular nerve could repair a long nerve gap in a rat model of sciatic nerve injury.
RESULTS
Quantitative analysis of experimental animals
A total of 20 Wistar rats were included in the study and were randomly assigned to a ChABC treatment group and a control group, with 10 rats in each group. The ChABC treatment group underwent sciatic nerve impairment through the use of a 25-mm long acellular nerve allograft treated with ChABC. The control group was treated with an acellular nerve allograft without ChABC treatment (Figure 1). Functional and histological feature were evaluated at 12 weeks post-surgery. Functional evaluation included autotomy scores, pinch stimuli to the hind paw, tension of triceps surae muscle, and weight of triceps surae muscles. Grafted nerves were processed by immunofluorescence staining to visualize regenerated nerve structures. All rats were included in the final analysis without loss.
Figure 1.
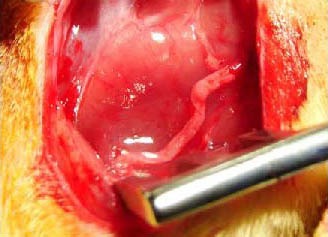
25-mm long acellular nerve graft used to bridge the gap in the sciatic nerve.
General observations of rats following sciatic nerve injury bridging
Muscular atrophy, trophic ulcerations, or autonomous paws were observed in rats following sciatic nerve injury. The legs exhibited muscular atrophy between 3 and 4 weeks post-surgery. Trophic ulcerations or autonomous paws were observed on the operated legs of both groups.
Autotomy scores following sciatic nerve injury bridging
Autotomy scores are used to reflect the degree of neuropathic pain. The autotomy score of the ChABC treatment group was 0.166, and the control group was 3.125. The Wilcoxon rank-sum test was used to compare between the two groups. The autotomy score in the ChABC treatment group was significantly lower than in the control group (P < 0.05).
Pinch stimulation of the hindpaw
A toothed forceps was used to pinch the planter aspect of the hind paw. A forceful and reflexive withdrawal of the both legs represented pain sensory recovery. Eight rats in the ChABC treatment group exhibited forceful and reflexive withdrawal of both legs when the footplates were pinched with a toothed forceps. Only one rat in the control group had a positive response, and there was significant difference between the two groups (P < 0.05).
Tension of the triceps surae muscle
The greatest muscular contraction tension was recorded when the triceps surae muscles were stimulated. Triceps surae muscular tension on the operated side was expressed as a percentage of the non-lesioned side. The ratio reflected motor function recovery. Tension in the operated triceps surae muscle was measured in 8 rats with ChABC-treated grafts and results were compared to the control group (no rat in the control group could be measured) (the ratio: 20.61 ± 2.59%, P < 0.05).
Weight of triceps surae muscles
The ratio of triceps surae muscle weight was the index for muscle atrophy. Triceps surae muscles from the operated and non-lesioned sides were weighed using an electronic scale (0.000 1 precision). The percentage weight of triceps surae muscles with ChABC-treated grafts was 21.09 ± 2.25%, but control grafts were 13.90 ± 1.86%. The ratio of conserved muscle to mass was greater with ChABC-treated grafts than with control grafts (P < 0.05).
Histological observations (Figure 2)
Figure 2.
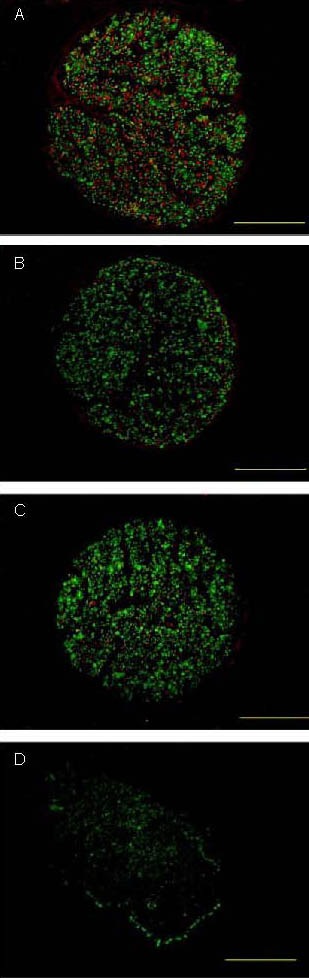
Histological observations of rats following sciatic nerve injury bridging.
Neurofilament-200 (green)/S-100 (red) double immunofluorescent cross-section of the proximal nerve segment from the chondroitinase ABC (ChABC) treatment group (A), distal segment of the ChABC treatment group (B), proximal nerve segment of the control group (C), and distal segment of the control group (D). Scale bars: 200 μm.
Sections were collected from each specimen at two different sites: 3 mm from the proximal nerve stump and 3 mm into the distal nerve stump. Lesioned sciatic nerves were immunohistochemically examined 12 weeks after surgery. Transverse sections were double-labeled for neurofilament-200 (green) and S-100 (red). The distal graft of ChABC-treated grafts showed that Schwann cells were next to regenerated nerve axons. In contrast, in the distal nerve segments of untreated grafts, Schwann cells were not aligned along regenerated nerve axons (Figure 2).
DISCUSSION
Acellular nerve grafting stimulates a slight immune response, but contains internal structures and extracellular matrix components of the nerve, and is currently the best available scaffold for repairing peripheral nerve defects[11,12,13]. However, there may be a limit to the extent of nerve regeneration that can occur in acellular grafts. Results from most rat studies have shown that axonal regeneration through acellularized grafts is limited to 1–2 cm[14,15,16,17]. The reasons for this limitation remain controversial. However, it is generally accepted that maximal axonal regeneration into acellular nerve grafts is achieved by 6–8 weeks after implantation; the distal graft degrades soon thereafter with inadequate axonal and cellular infiltration. In the present study, nerve regeneration largely failed prior to reaching the distal suturing point of the 25-mm acellular nerve graft in the control group. In contrast, ChABC treatment supported significantly better nerve regeneration and resulted in better functional outcomes.
Results from present study confirmed that ChABC treatment enhanced nerve regeneration function. ChABC treatment decreased nerve pain due to nerve damage and also increased sensation recovery. Autotomy scores were associated with nerve pain caused by nerve damage. Autotomy scores in the ChABC treatment group were significantly lower than in the control group. The footplate pinch test was designed to evaluate pain sensation recovery. Most ChABC-treated rats regained pain sensations, and ChABC treatment also helped to preserve muscle atrophy, allowing the motor nerve fibers to regenerate through the graft and into the muscular target. These experimental results suggested that the speed and amount of nerve fiber regeneration increased, because ChABC degraded chondroitin sulfate proteoglycans.
ChABC treatment in acellular nerve grafts mimics a key degeneration process by enhancing growth-promoting properties of the basal lamina scaffold. Modification of acellular nerve grafts by ChABC markedly increases the rate of ingress and extent of axonal regeneration, such as in vivo nerve pre-degeneration[3,18]. Results now show that ChABC treatment also improved functional recovery of the long acellular nerve grafts. Therefore, selective degradation of chondroitin sulfate proteoglycans provides distinct advantages compared to in vivo nerve pre-degeneration. For example, pre-degeneration involves extensive remodeling and may compromise basal lamina integrity, which is then exacerbated in acellular nerve grafts. In addition, inhibitory chondroitin sulfate proteoglycans are highly expressed after nerve injury, which might interfere with the regenerative potential of pre-degenerated grafts.
In a previous study[6], substantial nerve regeneration and reinnervation was achieved in the rat with 4-cm freeze-killed nerve grafts pretreated with ChABC. However, that study did not evaluate functional recovery of the nerves with ChABC-treated acellular grafts. In the present study, ChABC treatment of acellular allografts improved functional recovery in a long nerve gap. The optimized chemically extracted nerve allograft proved to be more suitable as a scaffold for repair of peripheral nerve defects.
MATERIALS AND METHODS
Design
Tissue engineering, randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Institute of Orthopedics, General Hospital of Chinese PLA, China from September 2008 to October 2010.
Materials
A total of ten healthy, 80- to 90-day-old, Sprague Dawley rats, weighing 250–300 g and irrespective of gender, were utilized for the preparation of acellular nerve allografts (20 nerve grafts from both sides). A total of 20 healthy, 80- to 90-day-old, Wistar rats, weighing 250–300 g and irrespective of gender, were used as graft recipients. All animals were provided by the Animal Center, General Hospital of Chinese PLA (license No. SCXK (Jun) 2007-004). The animals were housed at 25°C and a relative humidity of 70%. Experimental protocols were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[19].
Methods
Preparation of acellular nerve allografts
Sprague Dawley rats were sacrificed by intraperitoneal injection of sodium pentobarbital (0.5 mL, 60 mg/mL). Excised sciatic nerve segments (30 mm-long) were cleaned from the external debris and were treated with chemical detergents to obtain acellular allografts[6]. Acellular nerves were incubated in phosphate-buffered saline (PBS) containing 2 U/mL ChABC (affinity purified; Sigma, St. Louis, MO, USA) or in PBS alone for 16 hours at 37°C. The segments were rinsed twice with cold, sterile Ringer's solution, and then irradiated for 12 hours utilizing Co60 (Academy of Military Medical Sciences, Beijing, China) for sterilization and stored in the same solution at 4°C.
Acellular nerve allograft
Surgery was performed using aseptic techniques, and microsurgical dissection and repair was performed under a surgical microscope. The peritoneal cavity of Wistar rats was injected with sodium pentobarbital (0.5 mL, 60 mg/mL) for anesthetization. The left sciatic nerve was exposed by mobilizing the posterior margin of the gluteus maximus and lifting the muscle. The nerve was sharply transected at 8.0 mm distal to the sciatic notch with microsurgery scissors. The 25-mm long acellular nerve grafts were attached using 11-0 nylon interrupted microepineurial sutures at the proximal and distal stumps, which resulted in a 25-mm gap. Muscle and skin were closed using 4-0 vicryl and 4-0 nylon sutures, respectively. After recovery from anesthesia, the animals were returned to standard housing.
Autotomy scores following sciatic nerve injury bridging
Autotomy in paws was scored as previously described[20]. The scores of this scale ranged from 0 to 5 and were relative to a normal undamaged hindpaw: (1) nail (s) nibbled, damaged, or removed; (2) one digit bitten upon or loss of up to one-third of the digit; (3) one or two digits bitten or loss of one-third to one half of the digit; (4) one or two digits bitten by more than 50%; (5) more digits chewed or > 50% loss.
Pinch stimulation of the hind paw
The rats were intraperitoneally anesthetized with sodium pentobarbital (0.5 mL, 60 mg/mL). However, as the rat recovered from anesthesia and sodium pentobarbital levels decreased, it was possible to for observation of theobserve the eye-blink response to touch. A toothed forceps was used to pinch the planter aspect of the hind paw. When both footplates were pinched with the toothed forceps, a forceful and reflexive withdrawal of both legs was considered a positive response. If the non-injured leg exhibited a forceful, reflexive withdrawal and the operative leg had no leg movement, the footplate pinch test result was considered negative. Footplate pinch response was assessed by an observer blinded to the treatment conditions and validated by a second observer.
Tension measurement of the triceps surae muscle
Immediately after the nerve pinch test, rats were deeply anesthetized. The triceps surae muscles from both operated and non-lesioned sides were cleanly dissected from both sides and detached from the bone at the terminal point. The terminal point was connected to the muscle tension transducer (BL-420F biological function experimental system, Chengdu TaiMeng, China) using a 3-0 suture thread. The stimulation protocol to induce muscle contraction was a single stimulation with a delay time of 0.1 ms and a wave width of 0.1 ms. The greatest muscular contraction tension was recorded when intension stimulation increased from 0.5 to 1.5 V. Triceps surae muscular tension on the operated side was expressed as a percentage of the non-lesioned side.
Weight measurement of the triceps surae muscles
Rats were euthanized by a lethal dose of intraperitoneally injected sodium pentobarbital. The triceps surae muscles from the operated and non-lesioned sides were cleanly dissected, detached from the bone at their origin and terminal points, and weighed using an electronic scale (0.000 1 precision). The ratio of conserved muscle to mass was recorded for each animal by dividing the experimental muscle mass by the non-lesioned muscle mass.
Histological observations
Sections were collected from each specimen at two different sites: 3 mm from the proximal nerve stump and 3 mm into the distal nerve stump. Sections were processed for a particular antigen pair using immunohistochemical methods with the following primary antibodies: mouse anti-neurofilament-200 (1: 500; Sigma) and rabbit anti-S-100 (1: 500; Abcam, Cambridge, MA, USA). Secondary antibodies were diluted (1: 220) in 0.5% Triton-X100 in PBS and included goat anti-mouse IgG1 Alexa 488 (Molecular Probes, Eugene, Oregon, USA) for neurofilament-200 and goat anti-rabbit IgG Alexa 594 (Molecular Probes) for S-100. The immunostained axons and Schwann cells were visualized with a fluorescence microscope and captured with a digital camera (Olympus, Tokyo, Japan).
Statistical analysis
Data were analyzed utilizing SPSS 13.0 (SPSS, Chicago, IL, USA), and expressed as mean ± SD. Data in accordance with normal distribution were analyzed using the two-sample t-test. The chi-square test was used to evaluate hind paw pinch stimulation data from both groups. P < 0.05 was considered statistically significant.
Acknowledgments:
We would like to thank Xuemei Cui (Institute of Orthopaedics, General Hospital of Chinese PLA) for reagents. We sincerely thank Jingxiang Huang (Institute of Orthopaedics, General Hospital of Chinese PLA) for caring the experimental animals.
Footnotes
Conflicts of interest: None declared.
Funding: This study was funded by the Liaoning Scientific Research Foundation of Doctor, No. 20101134; and the Beijing Natural Science Foundation (The oriented micro-structure, double-aligned nerve-derived extracellular matrix scaffolds promote peripheral nerve long defects regeneration).
Ethical approval: This study was approved by the Animal Ethics Committee, General Hospital of Chinese PLA. All surgical procedures were performed according to the Institutional Animal Care and Use Committee of our institution.
(Edited by Jiang XF, Zhang KG/Qiu Y/Wang L)
REFERENCES
- [1].Shin RH, Friedrich PF, Crum BA, et al. Treatment of a segmental nerve defect in the rat with use of bioabsorbable synthetic nerve conduits: a comparison of commercially available conduits. J Bone Joint Surg Am. 2009;91(9):2194–2204. doi: 10.2106/JBJS.H.01301. [DOI] [PubMed] [Google Scholar]
- [2].Kim BS, Yoo JJ, Atala A. Peripheral nerve regeneration using acellular nerve grafts. J Biomed Mater Res A. 2004;68(2):201–209. doi: 10.1002/jbm.a.10045. [DOI] [PubMed] [Google Scholar]
- [3].Krekoski CA, Neubauer D, Zuo J, et al. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21(16):6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ide C, Tohyama K, Tajima K, et al. Long acellular nerve transplants for allogeneic grafting and the effects of basic fibroblast growth factor on the growth of regenerating axons in dogs: A preliminary report. Exp Neurol. 1998;154(1):99–112. doi: 10.1006/exnr.1998.6921. [DOI] [PubMed] [Google Scholar]
- [5].Hiles RW. Freeze dried irradiated nerve homograft: a preliminary report. Hand. 1972;4(1):79–84. doi: 10.1016/0072-968x(72)90019-8. [DOI] [PubMed] [Google Scholar]
- [6].Sondell M, Sundler F, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795(1-2):44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- [7].Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9-10):1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- [8].Muir D, Engvall E, Varon S, et al. Schwannoma cellderived inhibitor of the neurite-promoting activity of laminin. J Cell Biol. 1989;109(5):2353–2362. doi: 10.1083/jcb.109.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite- inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998;34(1):41–54. [PubMed] [Google Scholar]
- [10].Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207(1):163–170. doi: 10.1016/j.expneurol.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10(11-12):1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- [12].Zhong H, Chen B, Lu S, et al. Nerve regeneration and functional recovery after a sciatic nerve gap is repaired by an acellular nerve allograft made through chemical extraction in canines. J Reconstr Microsurg. 2007;23(8):479–487. doi: 10.1055/s-2007-992340. [DOI] [PubMed] [Google Scholar]
- [13].Yu H, Peng J, Guo Q, et al. Improvement of peripheral nerve regeneration in acellular nerve grafts with local release of nerve growth factor. Microsurgery. 2009;29(4):330–336. doi: 10.1002/micr.20635. [DOI] [PubMed] [Google Scholar]
- [14].Sun XH, Che YQ, Tong XJ, et al. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol. 2008;29(3):347–353. doi: 10.1007/s10571-008-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Z, Peng J, Wang G, et al. Effects of local release of hepatocyte growth factor on peripheral nerve regeneration in acellular nerve grafts. Exp Neurol. 2008;214(1):47–54. doi: 10.1016/j.expneurol.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [16].Yu H, Peng J, Sun H, et al. Effect of controlled release nerve growth factor on repairing peripheral nerve defect by acellular nerve graft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22(11):1373–1377. [PubMed] [Google Scholar]
- [17].Muir D. The potentiation of peripheral nerve sheaths in regeneration and repair. Exp Neurol. 2010;223(1):102–111. doi: 10.1016/j.expneurol.2009.05.038. [DOI] [PubMed] [Google Scholar]
- [18].Jubran M, Widenfalk J. Repair of peripheral nerve transections with fibrin sealant containing neurotrophic factors. Exp Neurol. 2003;181(2):204–212. doi: 10.1016/s0014-4886(03)00041-4. [DOI] [PubMed] [Google Scholar]
- [19].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]


