Abstract
Changes in social and emotional behaviour have been consistently observed in patients with traumatic brain injury. These changes are associated with emotion recognition deficits which represent one of the major barriers to a successful familiar and social reintegration. In the present study, 32 patients with traumatic brain injury, involving the frontal lobe, and 41 age- and education-matched healthy controls were analyzed. A Go/No-Go task was designed, where each participant had to recognize faces representing three social emotions (arrogance, guilt and jealousy). Results suggested that ability to recognize two social emotions (arrogance and jealousy) was significantly reduced in patients with traumatic brain injury, indicating frontal lesion can reduce emotion recognition ability. In addition, the analysis of the results for hemispheric lesion location (right, left or bilateral) suggested the bilateral lesion sub-group showed a lower accuracy on all social emotions.
Keywords: traumatic brain injury, facial emotion recognition, social emotions
INTRODUCTION
Pathological changes in social interaction patterns of patients with traumatic frontal brain injury have been commonly described[1,2,3]. These changes can be partly explained by impaired basic and social emotion processing[4,5,6]. Previous study has indicated that basic and social emotion processing plays an important role in behaviour regulation, human communication and interaction and, overall, in human survival[7]. Nevertheless, a few studies have dedicated to understanding how social emotions are processed. Social emotions derive from a combination between genetics and social learning[8]. In other words, social emotions generate from basic emotions and thus share innate and universal characteristics and their adequate processing represent the ability to understand mental states in oneself and others[9,10]. The capacity to infer mental states and predict behaviours in others (beliefs, emotions, values) is highlighted by the “Theory of Mind (TOM)”[11]. This concept arose from the necessity to justify the atypical behaviour and emphatic inability observed in subjects with brain lesions or neurological developmental disorders.
A study of clinical groups reported that this human ability (TOM) can be sub-divided into two major components: the ability to understand cognitive mental state of others and the ability to infer an emotional state, and these components can be expressed individually[12], i.e. subjects are able to infer others’ mental state without inferring their emotions[13].
Subjects with difficulty in recognising emotional states in others are considered to present insufficient social cognition. The concept of social cognition can be described as a set of cognitive processes that allow more adequate behaviours when relating with other people[14,15,16]. According to previous studies[16,17], the cognitive processes involved in social cognition can be grouped into two major categories: one consists of unconscious, automatic processes activated by the stimulus, and another of deliberate and conscious cognitive processes controlled by individual goals and intentions.
The multiplicity of social cognitive processes depends on the quality of the stimulus. This seems to have influence in the access to cerebral basis and neuronal pathways supporting the cognitive processes. Studies of neurological disorders, both developmental (from the spectrum of autism) and traumatic brain lesions have significantly contributed to understanding neurological correlates for social cognition, specifically which brain areas are associated with social and emotional behaviour regulation and prediction. Pre-frontal cortex has been shown to participate in social emotion processing[6,16]. It acts like a containment program, assuming its automatic and implicit contribution for adequate emotional and social conducts[18]. In spite of the determinant role of pre-frontal cortex in emotion processing, the connectionist perspective defends that this brain structure is not the sole responsible. Connectionists refer that the regulation of social affective states is submitted to a neural circuit that involves pre-frontal cortex, basal nuclei and amygdala[19,20,21]. By comparing patterns of changes in facial social emotion recognition in patients with pre-frontal and/or amygdala brain damages[14,22], it is postulated that although pre-fontal cortex appears to be responsible for interpreting social signals, production of adequate answers for each social context is regulated by the whole neural circuit. In order to investigate the independent role of each of these neural structures in social emotion processing, some researchers[6] compared the performance on social emotional states recognition (e.g. “looks good-natured?” or “looks hostile?”) and cognitive states (e.g. “looks thoughtful?”) of two brain damaged groups: amygdala damaged and pre-frontal damaged patients. The amygdala damaged patients presented difficulties in recognising both emotional and cognitive states. On the other hand, pre-frontal cortex damaged patients showed impairment only in recognising social emotions, mainly those with negative valence (e.g. hostile). One previous study suggested that the amygdala was fundamental for decoding facial stimulus with social meaning such as guilt, admiration and seduction[10]. However, recent evidence[16] admitted that the amygdala may not play a specific role in social cognition as it seems to be equally responsible for decoding non-emotional cognitive stimuli. These results are consistent with previous study[6]. Conversely, the frontal cortex was proposed as an exclusive structure for social information processing[9]. Indeed, studies of traumatic frontal brain patients[22] and others with developmental neurological disorders (e.g. Asperger) have consistently referred to difficulties in recognizing social emotions (such as envy and gloating) in frontal brain impaired subjects. Some studies also reported that social emotions are combinations of two or more basic emotions[9]. Thus, if these patients present difficulties in recognising basic emotions, especially the negative ones such as anger and sadness, it is expected that they would also show difficulties in recognising envy. Considering the debate regarding the brain structures supporting social cognition, several studies[23,24,25,26,27,28] tried to further understand if there are differences in social emotion processing according to lesion lateralization. Their findings suggest a hemispheric specialization according to the type of emotion in process: the right hemisphere might be associated with processing of basic emotions whereas the left hemisphere with processing of social emotions. However, recent evidence has suggested that adequate processing of social emotions requires inter-hemispheric participation[7]. These results built a paradigm with facial expressions of social emotions (arrogance and seduction) for healthy participants. Each stimulus was first presented individually for each visual field (i.e., right visual field vs. left visual field) and then simultaneously for both visual fields, indicating that when the stimuli were presented in a bilateral way the answers were faster and more accurate. Most of previous studies focus mainly on the evaluation of developmental disorders or amygdale damage, but performance of patients with traumatic frontal brain injury on social emotion recognition remains understudied. The present study assessed a sample of this type of subjects to investigate hemispheric participation (unilateral or bilateral) in social emotions processing and specific contribution of different frontal brain regions in this process. In addition, we implemented visual (context integrated or not), auditory and prosodic paradigms in order to assess social emotion processing. Although the use of statically non-integrated facial expressions for assessment of social emotions processing is controversial, we support results that the ability to recognise emotional states through the face is a crucial element which provides individuals with the capacity to understand their social environment[29]. Moreover, it is much easier to recognise dynamic facial expressions because of the complexity involving the human face[29]. A high agreement has achieved among the participants on the recognition of statically faces representative of social mental states (admiration, arrogance, seduction, guilt, interest, shame, doubt and curiosity) presented without context[30]. Therefore, the present study built an experimental task with statically facial expressions representing three social emotions (arrogance, jealousy and guilt) according to previously testing[10] to understand if frontal lobe lesions altered the facial social emotion recognition.
RESULTS
Quantitative analysis and baseline data of subjects
Forty-three traumatic brain injury (TBI) patients were initially selected, and 11 were excluded for two reasons: six abandoned the experiment due to fatigue and physical indisposition and five did not show images of significant frontal brain damage. Thirty-two traumatic frontal lobe injury patients (TBI group) and 41 healthy age- and education-matched controls (control group) were included in the final analysis. The baseline data of subjects are shown in Table 1.
Table 1.
Sociodemografic, clinical and cognitive characterization of frontal lobe injured patients and healthy controls
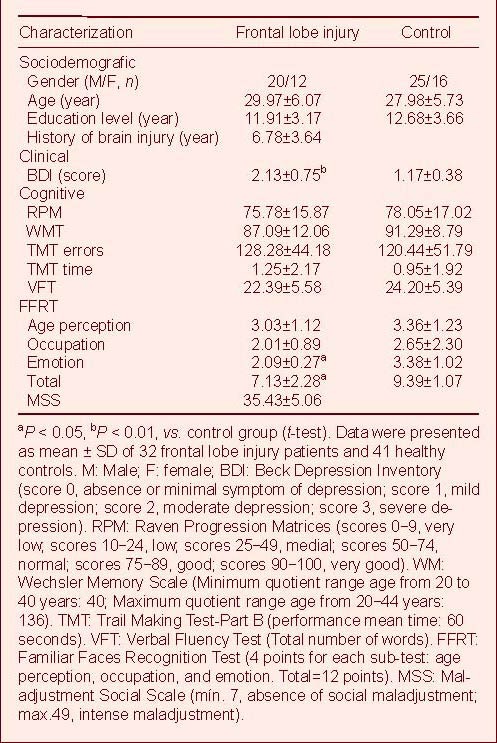
Accuracy of emotion recognition
Accuracy of the Go/No-Go paradigm was assessed by calculating the means of the correct answers for both groups (control and TBI). Next, the percentages of correct answers were also calculated using a d-prime [d’= Z (H) – Z (F)] performance discrimination model. This model allowed calculation and deletion of raw performance false alarms. The performance means were transformed in d-prime values. These values were analyzed using analysis of variance repeated measures which included the following factors: (1) group (control vs. TBI) as a between-subjects factor, (2) emotion (jealousy, guilt and arrogance) as a within-subjects factor and the performance on the social emotion recognition task (d-prime values) as a dependent variable.
According to analysis (Figure 1), the emotion factor exhibited no significant effect [F (2, 146) = 0.10; P = 0.907]. However, the group factor exhibited a significant effect [F (1, 146) = 35.93; P ≤ 0.000] – the TBI group presented lower results – and a group × emotion interaction was also observed for this task [F (2, 146) = 6.74; P ≤ 0.01] (Figure 1). Although it was possible to observe a worse performance for TBI group in all three emotions, there were more accentuated differences on arrogance and jealousy recognition.
Figure 1.
Accuracy (d-prime values) of emotion recognition in traumatic frontal lobe injury patients and healthy controls. Compared with the controls, the traumatic lobe injury (TBI) group presented lower emotion recognition (analysis of variance repeated measures). Data were presented as mean±SD of 32 frontal lobe injury patients and 41 healthy controls.
In order to better understand this interaction, a post-hoc Scheffé comparison test was performed. Results showed differences between groups for the following emotions under assessment: arrogance [M (control) = 3.03 ± 0.75; M (TBI) = 1.83 ± 0.76; P ≤0.001]; and jealousy
[M (control) = 2.94 ± 0.84 and M (TBI) = 1.84 ± 0.97; P ≤ 0.005] – with lower results for the control group. Guilt was the only emotion in which both groups presented similar results.
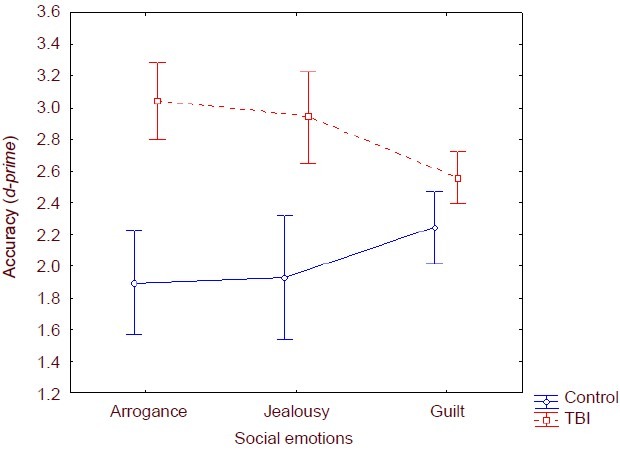
In order to understand if there were differences in emotion recognition according to the injury localization, the TBI group was divided into three sub-groups: right frontal, left frontal and bilateral. Accuracy comparison for the three subgroups was analyzed with Kruskal-Wallis test. The bilateral sub-group presented lower accuracy values for all social emotions. The right frontal sub-group registered similar accuracy pattern except for guilt recognition. However, the differences were not significant for jealousy and guilt recognition. On arrogance recognition the differences were only marginally significant [H = 5.364; P = 0.068]. The left frontal sub-group presented lower accuracy (Figure 2).
Figure 2.
Accuracy (d-prime values) of emotion recognition in right frontal/ left frontal/bilateral injury patients (Kruskal-Wallis test). Accuracy indicated correct answers. The bilateral sub-group presented lower accuracy values for all social emotions. The right frontal sub-group registered similar accuracy pattern except for guilt recognition. However, the differences were not significant for jealousy and guilt recognition. On arrogance recognition the differences were only marginally significant [H = 5.364; P =0.068]. The left frontal sub-group presented lower accuracy. Data were shown as mean ± SD of 32 frontal lobe injury patients and 41 healthy controls.
We also sought to analyse the performance on emotion recognition according to the damaged frontal lobe and therefore we divided the TBI group into three new sub-groups: orbitofrontal, medial and dorsolateral and analyzed using a Kruskal-Wallis test. No significant differences between the sub-groups were found for arrogance (H = 1.194; P = 0.551), jealousy (H = 1.101; P = 0.951) or guilt (H = 1.202; P = 0.548) (data not shown).
Reaction time for emution recognition
In order to calculate the means for the reaction time, only the reaction time of correct answers was included. Reaction time was also analysed with analysis of variance repeated measures (Figure 3) using group (control vs. TBI) as a between-subject factor and emotion (jealousy, guilt and arrogance) as a within-subject factor. The analysis showed a significant group effect [F (1,146) = 18.09; P ≤ 0.001] – higher reaction time registered for the TBI group. Furthermore, there was also a significant effect of the emotion factor [F (2, 146) = 14.19; P ≤ 0.001]. It was also observed a group x emotion interaction [F (2, 146) = 3.11; P ≤ 0.05] (Figure 3). Higher reaction time was observed for all the social emotions assessed for the TBI group.
Figure 3.
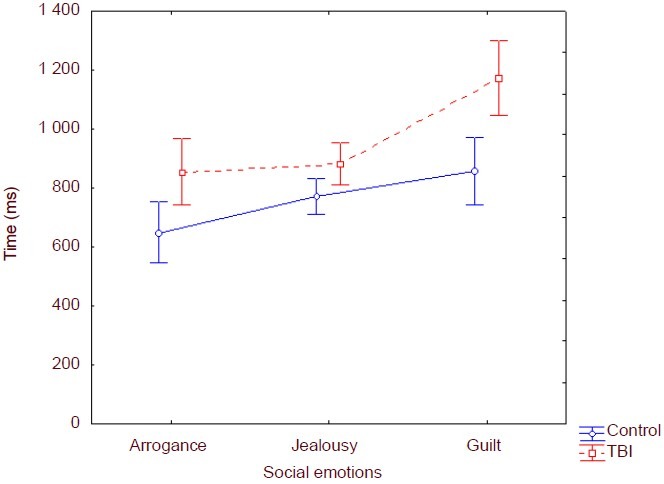
Reaction time (ms) in traumatic brain injury and control groups. Reaction time indicated response time to each stimulus. Data were shown as mean ± SD of 32 frontal lobe injury patients and 41 healthy controls.
Although the TBI group presented lower reaction time on every social emotion, the post-hoc Scheffé comparison (Table 2) shows that the difference was significant between groups only for guilt recognition (P =0.008).
Table 2.
Comparison of reaction time (ms) between frontal lobe injury patients (TBI) and healthy controls

Correlation between social emotion adjustment and visual emotion recognition
Some studies have shown that TBI can lead to patients’ social and emotional unsuitability[31]. Correlation between the social unsuitability scale and the visual emotion recognition task was analyzed using Spearman correlation analysis. Results suggested a negative association between the two tasks (social emotion recognition and the social unsuitability scale; r = −0.484; P = 0.031), showing that the worse the ability to adjust social emotion, the worse performance on emotion recognition task was obtained.
Correlation between depressive symptoms and visual emotion recognition
Finally, the correlation between the scores of depressive symptoms observed in the TBI group and the mean for the emotion recognition task was analyzed using Pearson correlation analysis. Since the results did not show significant correlation (r = -0.069; P = 0.767), it seems that the scores for depressive symptoms presented by the TBI group did not influence their performance on the emotion recognition task.
DISCUSSION
The neuronal basis underlying behavior involved in social cognition is one of the topics of great interest in social cognitive neurosciences[32]. Considering this, several brain structures have been suggested to be closely related with processing of social information. The pre-frontal cortex is, more consensually, the brain structure associated with recognition, regulation and expression of emotions[16]. In order to analyze the involvement of the frontal lobe in the processing of social emotions we assessed a frontal TBI group of participants and compared them to a group of healthy participants using a social emotion recognition task.
Results from the present study suggest that pre-frontal cortex lesion appears to condition adequate visual recognition of social emotions, specifically arrogance and jealousy, consistent with previous results[33,34] that frontal TBI patients present difficulties when processing facial emotional stimuli, especially for negative emotions. Moreover, evaluations of brain damaged patients suggested that pre-frontal cortex might be the main mediator and regulator of social emotions processing (such as envy)[22]. The present study tested jealousy, a mental state intimately associated to envy, and observed results significantly lower for the TBI group. Another study[22] added that this difficulty decoding social emotions is the reason why these patients misattribute mental states and cannot predict emotional behaviors of others (TOM).
The analysis of the results for hemispheric lesion location (right, left or bilateral) seems to support the hypothesis of inter-hemispheric participation for social emotions processing. The bilateral lesion sub-group showed a lower accuracy on all social emotions in the study. However, the difference was only marginally significant for arrogance. The recognition of facial expressions of social emotions, such as arrogance, seems to imply different cognitive functions mediated by different neuronal structures distributed by both hemispheres in a homologous way[7]. Another question in the present study was how the performance in social emotions recognition task was affected by lesions in different regions of the frontal lobe (medial, dorsolateral and orbitofrontal). This question emerged from suggestions of several researchers that medial pre-frontal cortex is a dominant region for social cognition and TOM[7,35]. In spite of the lower performance of medial lesion sub-group for all the emotions in study, the results did not present statistical relevance.
Nevertheless, it has been suggested that while pre-frontal cortex appears to interact with neuroanatomical components responsible for social and affective states regulation[19,20,21] the different regions within frontal lobe do not act individually in emotion processing and thus damage to a specific pre-frontal region will functionally impair the others and provoke further difficulties in processing social emotions. The changes in emotion recognition observed in frontal damaged patients were not always associated with cognitive or executive functions, consistent with results of cognitive assessment in the present study. Although, we did find differences between groups (TBI vs. control) for social emotion recognition task, there were no differences in executive functioning. Severe TBI patients had difficulties in reading facial expressions of emotions, especially from the eyes[12]. On the other hand, the same patients presented normative values for executive functions. This dissociation may be explained according to TOM which has two distinct components (emotion vs. cognition), as we previously referred. According to TOM, results suggest that TBI patients maintain their cognitive component intact while the emotional component is pathologically altered[22].
Based on previous conclusion that unsuitable emotional behaviors exist in patients with frontal damage, especially with traumatic lesions, we tested the association between the social unsuitability scale and the social emotion recognition task. As expected, the more socially and emotionally unsuited the patients were, the worse the social emotion recognition through facial expressions was. Eslinger et al[31] referred the multiple difficulties showed by brain damaged patients in familiar professional and social reinsertion and they also suggested that these difficulties seem to be associated with deficits on emotional behavior recognition and prediction.
The difficulty of post-trauma reinsertion often implies the development of depressive symptoms in TBI patients. The TBI group in the present study revealed lower depressive symptoms scores compared with the control group. Since depressive symptoms may condition the results interpretation, we tested correlation between these symptoms and the social emotion recognition task. Results revealed no association between these two variables, supporting the idea that the depressive condition appears not be associated with the lower performance of TBI patients on the social emotion recognition task.
In conclusion, the mechanism of social emotion recognition is not yet completely understood, so it is important to clarify which neuronal structures are dominant in this process, especially to understand the role of the amygdala. The evaluation of facial recognition is primordial because it is the beginning of a effective intervention in these patients.
SUBJECTS AND METHODS
Design
A non-randomized, concurrent controlled study.
Time and setting
The participants were assessed in a quiet laboratory room of the Psychology Department at the University of Algarve, between November 2008 and July 2009.
Subjects
All participants were Portuguese native speakers. Informed consent was obtained from all subjects after the nature of the study had been explained.
TBI group
TBI patients was recruited from a database of Faro Hospital and from a private neurosurgical clinic in Algarve (n = 28) and from the Neurosurgical Department of Hospital of S. José in Lisbon (n = 4). Inclusion criteria were (1) clinical diagnosis of frontal TBI, (2) with a minimal of 12 months posterior to trauma, (3) post-traumatic amnesia: minimum 24 hours. Exclusion criteria were as follows: (1) previous clinical history of psychiatric disorder, (2) non-frontal TBI, and (3) age over 40 years-old. In order to get a more conclusive characterization of the traumatic brain injuries, the 32 TBI participants were submitted to a structural magnetic resonance imaging, with a Philips 1.5 Intera Scanner. Imaging parameters consisted of (1)sagital spin echo T1 (22 cuts of 5 mm), (2) axial turbo spin echo dual echo PD and T2 (22 cuts of 5 mm), (3) axial fluid attenuated inversion recovery (Flair- 22 cuts of 5 mm), (4) axial fast field echo (gradient echo) T2 (22 cuts of 6 mm), (5) axial diffusion weight (gradient) echo diffusion (20 cuts of 3 mm), (6) coronal turbo spin echo T2 (25 cuts of 3 mm), and (7) axial 3D fast field echo T1 (200 cuts of 0.6 mm).
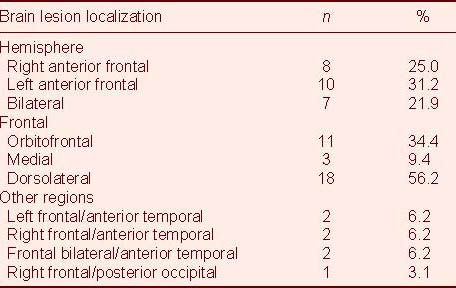
Subsequently, two independent physicians (a neurosurgeon and a neuroradiologist) rated neuroimaging data with the following grid criteria: frontal (anterior or posterior), frontal (orbitofrontal, medial, and dorsolateral), parietal (anterior or posterior), temporal (anterior or posterior) and occipital (anterior or posterior). Each lesion was characterized using the rules of abnormal focal signals (hypointense, hyperintense or mixed; Table 3).
Table 3.
Distribution of frontal lobe injury patients according to brain lesion localization
Control group
Forty-one healthy participants were students recruited in the University of Algarve for this experiment. Exclusion criteria included: (1) neurological disease, (2) self-reported depression and (3) history of TBI.
Methods
Procedure
An individual session was arranged for each participant, with a medium duration of 60 minutes. At the beginning, participants filled in a demographic questionnaire, where they also had to provide neurological and medical information. Afterwards, the battery of cognitive tests described above was introduced. In the end, all participants performed the visual emotion recognition task as earlier described.
Cognitive assessment
Participants were submitted to cognitive assessment through several tests: (1) Raven's Progressive Matrices, (2) Wechsler Memory Scale, (3) Verbal Fluency Test, (4) Familiar Faces Recognition Test, and (5) Trail Making Test–Part B. These tests were applied in the same order, allowing us to characterize participants’ cognitive functions and also to make sure that variables other than those proposed by us could not explain the differences that we would eventually find between the two groups.
Cognitive results of both groups are shown in Table 1. This comparison allowed us to confirm that groups did not differ in any of the cognitive variables except for Familiar Face Recognition Test, where control group scored higher. Furthermore, this difference was only observed when the TBI participants had to identify the emotional state of the famous face. There was a significant difference between groups for depressive symptoms. TBI group presented higher scores for Beck Depression Inventory scale compared with control group (however this value indicates low depressive symptoms in Table 1). As for the Social and Emotional Maladjustment Scale, the TBI group displayed high values of social maladjustment [M (TBI) = 35.43, DP = 5.06].
Go/No-Go task
In order to assess participants’ ability to recognize social emotions through facial expressions, a Go/No-Go Task was designed, which consisted of stimuli presentation scored according to accuracy and reaction time. Presentation software (0.7) (http://nbs.neurobs.com/presentation) was used to present stimuli and register accuracy rates and reaction times.
The stimuli material used for this task was selected according to previous study[36]. Three actors represented three social emotions and one neutral with an Alpha of Krippendorff index's < 70. Black and white photographs with 44.46 cm (large)/ 50 cm (length) were used for the stimuli.
The Go/No-Go task was comprised of three blocks of stimuli presentation, with one block for each social emotion (arrogance, jealousy and guilt) and blocks were all applied separately (Figure 4). Each block consisted of 24 stimuli. Twelve (50%) of these stimuli represented the emotion in study – Go (e.g. arrogance) and twelve (50%) were distractors – NoGo (e.g. photos representative of guilt, jealousy and neutral). The blocks were separated by an interval of 2 minutes (Figure 4). In total, each participant responded to 72 stimuli. Score “1” was considered for each correct answer (minimum score = 0; maximum score = 12).
Figure 4.
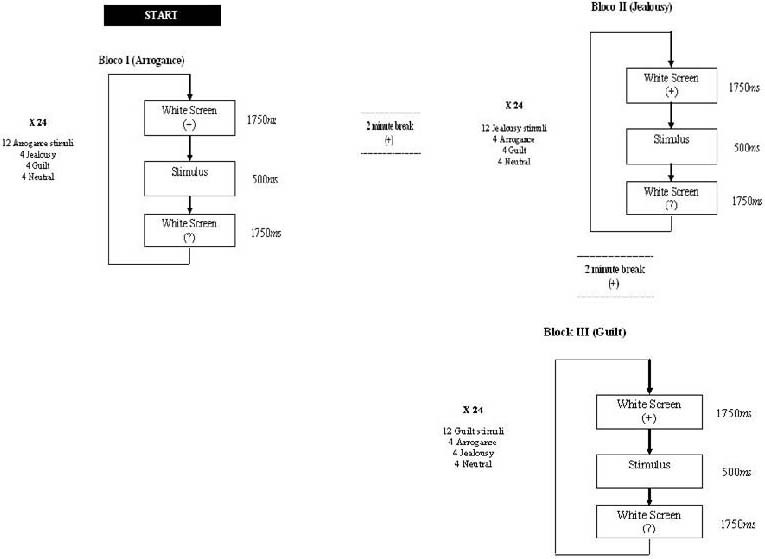
Presentation scheme for each block of emotions.
Before the task, participants were instructed which emotion they had to identify from the presented sequence. Therefore, they had to observe carefully each emotional photograph presented and, once they perceived the required one, they had to select it on the touch screen. For example, for the arrogance block (first), participants were instructed to observe each presented photograph and, once they recognized the photograph which represented arrogance, they had to mark it as quickly as possible on the touch screen. Thus, each participant perceived twenty-four stimuli per block, but only half of them were correct: twelve photographs represented arrogance and the other twelve represented jealousy, guilt and neutral facial (four photographs per each).
As shown in Figure 4, a blank screen with a fixation cross (+) was viewed for 1 750 ms, followed by one emotional stimulus presented for 500 ms. A question mark (?) appeared for 1 750 ms, which was the maximum time allowed to choose if the emotional stimulus was the correct one. Participants were instructed to press the key space, which was coloured, whenever they identified the correspondent emotion of each block (Figure 5). Once time expired, the next trial started automatically. Instructions were presented to participants with a test trial, and were refreshed for each different block.
Figure 5.
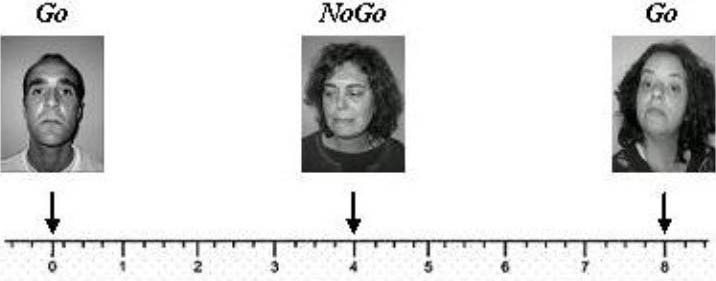
Presentation scheme of stimuli in a Go/NoGo task: first block (arrogance). In each block the stimuli were separated between them in 4 seconds.
A pre-test was performed on a healthy sample of five participants. Accuracy rates and reaction time were registered. Volunteers had lower accuracy rates for arrogance (arrogance: 70%; jealousy: 64.4%; and guilt: 60%) as well as lower reaction time (arrogance: 1 117.8 ms; jealousy: 1 525.2 ms; and guilt: 1 165.1 ms).
Statistical analysis
SPSS software (version 16.0) (Statistical Package for the social sciences) and Statistica (version 7) were used to analyze data. The comparison data (TBI vs. control) obtained from characterization battery was analyzed using a parametric t-test. Results obtained in the emotion recognition task between groups were compared using analysis of variance repeated measures and post-hoc Scheffι comparison test. Results in the emotion recognition task according TBI sub-groups were analyzed used a non-parametric test (Kruskal Wallis test) following analysis of variance repeated measures. To analyze the correlation between social emotion adjustment and visual emotion recognition, Spearman correlation analysis was used. Correlation between depressive symptoms and visual emotion recognition was analyzed by Pearson correlation analysis.
Acknowledgements:
Work reported in this paper was conducted with the assistance of Fernando Sancho Center of magnetic resonance. We thank Dr. Daniel Maymone neurologist in a clinical in Algarve for this contributions to the research reported here and for facilitating access to research participants as well as the people with TBI to participate in this study.
Footnotes
Conflicts of interest: None declared.
Ethical approval: All experiments and protocols of this study were approved by Ethics Committee of the Faro Hospital and from the Neurosurgical Department of Hospital of S. José in Lisbon.
(Edited by Qin YL, Zhang GM/Su LL/Wang L)
REFERENCES
- [1].Ardila A. On the evolutionary origins of executive functions. Brain Cogn. 2008;68(1):92–99. doi: 10.1016/j.bandc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [2].Kendall E, Terry DJ. Psychosocial adjustment following closed head injury: A model for understanding individual differences and predicting outcome. Neuropsychol Rehabil. 1996;6:101–132. [Google Scholar]
- [3].McDonald S. Are you crying or laughing? Emotion recognition deficits after severe traumatic brain injury. Brain Impairment. 2005;6:56–67. [Google Scholar]
- [4].Milders M, Ietswaart M, Crawford J, et al. Impairments in theory of mind shortly after traumatic brain injury and at 1-year follow-up. Neuropsychology. 2006;20:400–408. doi: 10.1037/0894-4105.20.4.400. [DOI] [PubMed] [Google Scholar]
- [5].Milders M, Ietswaart M, Crawford J, et al. Social behaviour following traumatic brain injury and its association with emotion recognition, understanding of intention, and cognitive flexibility. J Int Neuropsychol Soc. 2008;14:318–326. doi: 10.1017/S1355617708080351. [DOI] [PubMed] [Google Scholar]
- [6].Shaw P, Braham J, Lawrence EJ, et al. Differential effects of lesions of the amygdala and prefrontal cortex on recognizing facial expressions of complex emotions. J Cogn Neurosci. 2005;17:1410–1419. doi: 10.1162/0898929054985491. [DOI] [PubMed] [Google Scholar]
- [7].Tamietto M, Adenzato M, Geminiani G, et al. Fast recognition of social emotions takes the whole brain: Interhemispheric cooperation in the absence of cerebral asymmetry. Neuropsychologia. 2007;4:836–843. doi: 10.1016/j.neuropsychologia.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [8].Damásio AR. 12th ed. Lisbon: Europe America Publication; 1995. Descartes Error, Reason and the Human Brain. [Google Scholar]
- [9].Shamay-Tsoory SG. Recognition of ‘fortune of others’ emotions in Asperger syndrome and high functioning autism. J Autism Dev Disord. 2008;38:1451–1461. doi: 10.1007/s10803-007-0515-9. [DOI] [PubMed] [Google Scholar]
- [10].Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- [11].Premack DG, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–526. [Google Scholar]
- [12].Muller F, Simion A, Reviriego E, et al. Exploring Theory of Mind after traumatic brain injury. Cortex. 2009;45:1–12. doi: 10.1016/j.cortex.2009.08.014. [DOI] [PubMed] [Google Scholar]
- [13].Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:369–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- [14].Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- [15].Adolphs R, Spezio M. Role of the amygdale in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- [16].Adolphs R. The Social Brain: Neural Basis of Social Knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fiske ST, Taylor SE. 3rd ed. New York: McGraw-Hill; 2008. Social Cognition: From Brains to Culture. [Google Scholar]
- [18].Norman W, Shallice T. Attention to action. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self Regulation: Advances in Research and Theory. New York: Plenum; 1986. [Google Scholar]
- [19].Emery NJ, Capitanio JP, Mason WA, et al. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys. Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- [20].Heimer L, Olmos J, Alheid G, et al. “Perestroika” in the basal forebrain: Opening the border between neurology and psychiatry. Prog Brain Res. 1991;87:109–165. doi: 10.1016/s0079-6123(08)63050-2. [DOI] [PubMed] [Google Scholar]
- [21].Zaborszky L, Gaykema RP, Swanson DJ, et al. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- [22].Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia. 2007;45:3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- [23].Buck R. New York, NY: Guilford Press; 1984. The Communication of Emotion. [Google Scholar]
- [24].Buck R, Losow JI, Murphy MM, et al. Social facilitation and inhibition of emotional expression and communication. J Pers Soc Psychol. 1992;63:962–968. doi: 10.1037//0022-3514.63.6.962. [DOI] [PubMed] [Google Scholar]
- [25].Gainotti G. Disorders of emotional behaviour. J Neurol. 2001;248:743–749. doi: 10.1007/s004150170088. [DOI] [PubMed] [Google Scholar]
- [26].Leventhal H. The integration of emotion and cognition: A view from the perceptual-motor theory of emotion. In: Clarke MS, Fiske ST, editors. Affect and Cognition. Hillsdale, NJ: Erlbaum; 1982. [Google Scholar]
- [27].Ross ED, Homan RW, Buck R. Differential hemispheric lateralization of primary and social emotions. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:1–19. [Google Scholar]
- [28].Tucker DM. Lateral brain function, emotion, and conceptualization. Psychol Bull. 1981;89:19–46. [PubMed] [Google Scholar]
- [29].Back E, Jordan TR, Thomas S. The recognition of mental states from dynamic and static facial expressions. Vis Cogn. 2008;17:1271–1286. [Google Scholar]
- [30].Baron-Cohen S, Wheelwright S, Jolliffe T. Is there a “language of the eyes”? Evidence from normal adults, and adults with autism or Asperger syndrome. Vis Cogn. 1997;4:311–331. [Google Scholar]
- [31].Eslinger P, Parkinson K, Shamay SG. Empathy and social-emotional factors in recovery from stroke. Curr Opin Neurol. 2002;15:91–97. doi: 10.1097/00019052-200202000-00014. [DOI] [PubMed] [Google Scholar]
- [32].Ochsner KN. Social cognitive neuroscience: historical development, core principles, and future promise. In: Kruglanski A, Higgins ET, editors. Social Psychology: A Handbook of Basic Principles. 2nd ed. New York: Guilford; 2007. [Google Scholar]
- [33].Bornhofen C, McDonald S. Emotion perception deficits following traumatic brain injury: A review of the evidence and rationale for intervention. J Int Neuropsych Soc. 2008;14:511–525. doi: 10.1017/S1355617708080703. [DOI] [PubMed] [Google Scholar]
- [34].Ietswaart M, Milders M, Crawford J, et al. Longitudinal aspects of emotion recognition in patients with traumatic brain injury. Neupsychologia. 2008;46:148–159. doi: 10.1016/j.neuropsychologia.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [35].Baron-Cohen S, Wheelwright S, Hill J, et al. The “Reading the mind in the eyes” test revised version: a study with normal adults with Asperger syndrome or high-functioning autism. J Child Psychiatry. 2001;42:241–252. [PubMed] [Google Scholar]
- [36].Martins A, Reis A. Stimuli validation to the paradigms construction for the study of emotion recognition. Ability and scientific capacity proofs presented in University of Algarve. 2007 [Google Scholar]


