Abstract
The majority of all known diseases are accompanied by disorders of the cardiovascular system. Studies into the complexity of the interacting pathways activated during cardiovascular pathologies are, however, limited by the lack of robust and physiologically relevant methods. In order to model pathological vascular events we have developed an in vitro assay for studying the interaction between endothelium and whole blood. The assay consists of primary human endothelial cells, which are placed in contact with human whole blood. The method utilizes native blood with no or very little anticoagulant, enabling study of delicate interactions between molecular and cellular components present in a blood vessel.
We investigated functionality of the assay by comparing activation of coagulation by different blood volumes incubated with or without human umbilical vein endothelial cells (HUVEC). Whereas a larger blood volume contributed to an increase in the formation of thrombin antithrombin (TAT) complexes, presence of HUVEC resulted in reduced activation of coagulation. Furthermore, we applied image analysis of leukocyte attachment to HUVEC stimulated with tumor necrosis factor (TNFα) and found the presence of CD16+ cells to be significantly higher on TNFα stimulated cells as compared to unstimulated cells after blood contact. In conclusion, the assay may be applied to study vascular pathologies, where interactions between the endothelium and the blood compartment are perturbed.
Keywords: Immunology, Issue 93, In vitro human model system, whole blood, endothelial cells, vascular activation, inflammation, blood coagulation
Introduction
Methods for analyzing blood-vascular interactions in cardiovascular disease generally involve animal research experiments. Results created in experimental research animal models may, however, have little or no implication for human disease.1,2 As such, there is a need for good and reliable in vitro models to investigate cellular interactions between the blood compartment and vascular endothelial cells in a human-like system. We have therefore established a blood endothelial cell chamber model. This is based on a previously described model used to investigate interactions between biomaterials and human whole blood.3 Contrary to other in vitro setups where usually limited number of purified components, i.e., thrombocytes, leukocytes or endothelial cells are available, the present model incorporates all the components present in a blood vessel.
The setup of the blood endothelial cell chamber model is designed to enable use of freshly drawn human blood with little or no added anticoagulant. Blood stored during longer time periods acquire so called storage lesions where breakdown of erythrocytes may interfere with delicate interactions between blood and endothelial cells.4
To avoid clot formation during handling of whole blood ex vivo requires either high doses of anticoagulant or that all material in contact with blood have to be non-activating. As non-activating surfaces are rare in the laboratory environment, materials may alternatively be equipped with a protective layer of immobilized heparin. A protective layer of immobilized heparin (hereafter referred to as Corline heparin surface (CHS)) that may be applied to most materials creating a surface where blood contact can occur without causing an activation of the coagulation cascade.5 Thus, by furnishing the chambers with CHS, the blood endothelial cell chamber model enables use of very low concentrations of anticoagulant in the blood. Avoiding the addition of high concentrations of anticoagulant in the blood endothelial chamber model makes it possible to study sensitive interactions within a blood vessel that might otherwise be masked.6
The blood endothelial cell chamber model consists of two chambers formed by attaching plastic cylinders (height: 8 mm; radius: 9 mm) to a plastic microscope slide. The edges facing upward on the cylinders are equipped with grooves that are fitted with rubber O-rings used for sealing the chambers against the cell culture slide. The chambers are only partially filled with blood as the air left in the chamber keeps blood in movement when the chambers are subsequently rotated in a vertical position (Figure 1).
In order to assess the functionality of the model, we performed experiments with either a completely CHS coated chamber or Human Umbilical Vein Endothelial Cells (HUVEC). Two separate blood volumes were assayed and the formation of thrombin antithrombin (TAT) complexes (an indirect marker of coagulation) was measured with enzyme linked immunosorbent assay (ELISA). We then assessed the effect of blood volumes and tumor necrosis factor (TNFα) stimulated endothelial cells on leukocyte recruitment by image analysis.
 Figure 1. Setup of the blood endothelial cell chamber model. (A) Top view schematic drawing of the blood chamber made in PMMA. (B) Primary human endothelial cells are cultured on 1-well chamber slides. Fresh human whole blood is added to two chambers on a microscope slide. The chambers and all material used for handling the blood are treated with a protective HS coating. After removing the walls of the cell culture slide, the cells are placed facing the blood and the system is clamped shut. (C) The blood endothelial cell chambers are then incubated under rotation in 37 °C. Afterwards, the reactions in the blood compartment may be analyzed by ELISA and the endothelial cells may be analyzed by microscopy.
Figure 1. Setup of the blood endothelial cell chamber model. (A) Top view schematic drawing of the blood chamber made in PMMA. (B) Primary human endothelial cells are cultured on 1-well chamber slides. Fresh human whole blood is added to two chambers on a microscope slide. The chambers and all material used for handling the blood are treated with a protective HS coating. After removing the walls of the cell culture slide, the cells are placed facing the blood and the system is clamped shut. (C) The blood endothelial cell chambers are then incubated under rotation in 37 °C. Afterwards, the reactions in the blood compartment may be analyzed by ELISA and the endothelial cells may be analyzed by microscopy.
Protocol
NOTE: Blood was drawn from healthy individuals by open system venipuncture in approval with the Ethical Review Board in Uppsala (Permit No. 2008/264).
1. Seeding Cells
Culture primary human umbilical vein endothelial cells (HUVEC) at 37 °C in 5% CO2 in T75 flasks coated with 1% gelatin in PBS pH 7.4.
Remove culture medium from a T75 flask containing HUVEC (or other type of primary human endothelial cells) and wash the cells with 2.1 mM EDTA in PBS pH 7.4. Detach cells by adding 1 ml 0.25% trypsin-EDTA and incubating for 2 - 3 min in 37 °C.
Collect cells by rinsing the flask with 9 ml 10% FBS in PBS pH 7.4 and transfer the cell suspension to a 15 ml tube. Spin down the cells at 735 x g for 5 min.
Remove the supernatant and resuspend the cell pellet carefully in endothelial cell growth medium microvascular (EC GMMV) and count the cells in a Bürker chamber. Seed 21,000 cells/cm2 in 1-well cell culture slides and incubate at 37 °C. The HUVEC will be confluent after 1 - 2 days of culture. Monitor the cell morphology and confluency daily under a light microscope.
2. Preparation of Whole Blood
Prepare all artificial surfaces that will be in contact with blood with a double layer of immobilized heparin (CHS) according to the manufacturer’s instructions. CHS coated materials protected from dust can be stored at room temperature up to 6 months without loss of function.
Use open system venipuncture to draw blood from a healthy donor shortly (<30 min) before starting the blood endothelial chamber experiment. Couple a hypodermic needle (18 G x 50 mm) to CHS coated silicon tubing (inner diameter: 2 mm) before carefully collecting blood in a CHS coated 50 ml tube. Supplement blood with unfractionated heparin to reach a final concentration of 0.75 IU/ml. Mix the blood gently by inverting the tube 2 - 3 times.
3. Blood Endothelial Chamber Model
Fabricate blood chambers (Figure 1A; top view) in acrylic polymethyl methacrylate (PMMA) consisting of two cylinders (height 8 mm and radius 9 mm) that are glued to a PMMA slide (25 x 75 mm). Fit the edges of the cylinders facing upwards with rubber O-rings to seal the blood chamber against the glass slides with cultured endothelial cells (Figure 1B). Rotate the blood chambers connected to the culture slide with endothelial cells thereafter in a vertical position (Figure 1C).
Use a CHS coated pipette tip to transfer 1.5 ml blood into each CHS coated chamber taking care not to activate the blood.
Transfer 1 ml blood into microcentrifuge tubes containing 30 μl 0.34 M K3EDTA to reach a final concentration of 10 mM K3EDTA for determination of initial plasma protein concentrations using an enzyme linked immunosorbent assay (ELISA). Carefully mix the blood and keep the tubes on ice.
Remove the plastic walls of the cell culture glass slides according to manufacturer’s instructions and carefully wash the cells in PBS pH 7.4. Make sure to subsequently remove as much liquid as possible from the slides. Do not let the cells dry out.
Place the slide on top of the blood chambers, with cells facing the blood. Secure the slide by placing a clamp around the blood endothelial cell chamber. Use a plastic slide for additional support to avoid breakage of the cell culture glass slide when placing the clamp.
Fix the blood endothelial cell chamber onto a rotating wheel (40 cm in diameter) place in a 37 °C water bath. Rotate each chamber on the wheel at 0.5 x g for 30 min.
After incubation, dismantle the blood endothelial cell chamber. Wash the cell culture slides in PBS pH 7.4 and fix the cells in ice cold 1% paraformaldehyde (PFA) for 10 min. Transfer 1 ml blood from each chamber to microcentrifuge tubes containing 30 μl 0.34 M K3EDTA to reach a final concentration of 10 mM K3EDTA and mix the tubes gently. Keep the tubes on ice.
Centrifuge the tubes at 4,560 x g for 10 min in 4 °C. Transfer the blood plasma to new microcentrifuge tubes and store in -70 °C until further analysis.
4. Analysis of Blood Endothelial Interactions
Perform immunofluorescent staining with mouse anti-human CD16 followed by secondary goat anti-mouse Alexa 488 and phalloidin-TexasRed. Perform each staining step for 1 hr at room temperature and wash the slides in PBS pH 7.4 between every step. Counterstain the nuclei with DAPI for 10 min at room temperature. Analyze blood components bound to the endothelial cells by fluorescence microscopy in combination with image analysis using suitable image analysis software such as CellProfiler.
Quantify the reactions in the blood compartment with thrombin-antithrombin (TAT) ELISA as per manufacturer’s instructions of the plasma samples.
Use appropriate statistical tools, e.g., an unpaired t test, to analyze the data.
Representative Results
The Necessity of CHS Coating
In order to measure reactions in whole blood specific to those elicited by the endothelial cells, all materials used for the blood endothelial cell chamber must be furnished with a CHS coating prior to use. Figure 2A (left) shows an uncoated chamber after 30 min of blood contact with a clearly visible clot present. Treating the chamber with CHS (Figure 2A, right) on the other hand protects the blood from uncontrolled activation, ensuring that the results are due to the endothelial cell-blood interactions.
The Effect of the Blood Volume in the Chamber
Activation of coagulation is more likely in stagnant blood as the probability of interaction between activated coagulation factors is increased. In the blood endothelial cell chamber model, blood is kept in motion due to the circulation of an air bubble inside the chamber. In order to determine the effect of changes in the blood volume, and thus also air bubble size, a CHS coated chamber was connected to a CHS coated glass slide that was tested with 1.5 ml or 1.75 ml whole blood for 30 min. The volume of blood without any movement by the air bubble is 2.5 times larger when 1.75 ml of blood is added to the chamber compared to when 1.5 ml of blood is used (Figure 2B). The measured TAT-values suggested increased TAT-formation with increased blood volume (Figure 2D). When HUVEC (Figure 2C) were incubated with either 1.5 or 1.75 ml of whole blood, no difference was noted between the two groups, suggesting that endothelial cells may modulate the activation of coagulation (Figure 2D).
The Effect of TNFα on Blood Cell Recruitment and Activation of Coagulation
In order to study the effect of TNFα on leukocyte recruitment, HUVEC were treated with 20 ng/ml TNFα 4 hr prior to blood contact. Blood contact - with either 1.5 ml or 1.75 ml blood - was continued for 30 min after which the culture slides were stained for CD16 (Figure 2F), imaged and the number of CD16+ cells was quantified. The recruitment of CD16+ cells increased significantly with TNFα treatment (Figure 2E) from 100 to 256 CD16+ cells/mm2 (p < 0.0001) with 1.5 ml blood and from 172 to 378 (P < 0.0001) with 1.75 ml blood. The formation of TAT complexes was measured in the corresponding plasma samples of the blood incubated with either TNFα treated or untreated cells (Figure 2G). TNFα stimulated cells induced an approximate doubling of TAT complexes as compared to untreated cells.
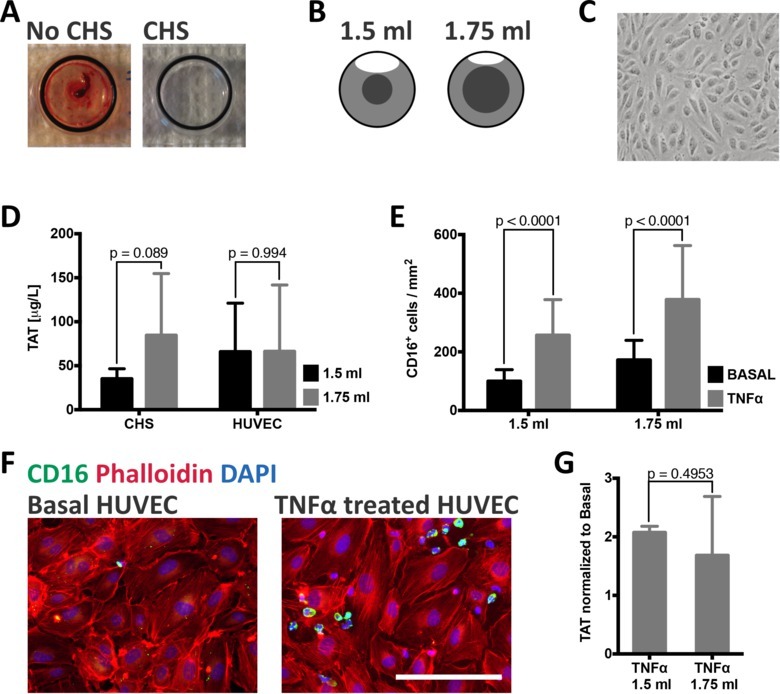 Figure 2: Functionality of the blood endothelial cell chamber model. (A) Chambers with or without CHS were incubated with whole blood. Without CHS, a clot was formed after 30 min. Measurement of thrombin-antithrombin (TAT) complexes, an indirect marker of coagulation, in blood incubated without CHS was > 6,000 μg/ml. (B) The volume of blood not kept moving by the rotating air bubble will vary with different blood volumes. With the addition of 1.5 ml of blood, this volume will be 0.3 ml, whereas it will increase to 0.74 ml with the addition of 1.75 ml. (C) HUVEC imaged with phase contrast microscopy show typical endothelial cell morphology of a confluent monolayer prior to blood contact. (D) TAT values for completely CHS coated chambers show a slight difference when different blood volumes are added. The addition of 1.5 ml blood resulted in 35 ± 11 μg/ml (n = 7) in comparison to 1.75 ml, which resulted in 85 ± 70 μg/ml TAT (n = 8). This difference is no longer present when HUVEC are incubated with the same blood volumes; 66 ± 55 μg/ml for 1.5 ml (n = 5) and 66 ± 29 μg/ml for 1.75 ml (n = 7). (E) Quantification of the number of CD16+ cells present on either unstimulated or TNFα stimulated HUVECs after blood contact. The number of CD16+ cells is significantly increased with TNFα stimulated cells incubated either with 1.5 ml (basal: 100 ± 40 cells/mm; TNFα: 256 ± 121 cells/mm) or 1.75 ml (basal: 172 ± 67 cells/mm; TNFα: 378 ± 185 cells/mm) of whole blood. (F) Representative images of unstimulated and TNFα stimulated HUVEC stained for phalloidin (red), CD16 (green) and DAPI (blue). Scale bar = 250 μm. (G) The formation of TAT complexes is roughly doubled by TNFα stimulated cells as compared to untreated cells incubated with blood (1.5 ml: 2.1 ± 0.1 times more TAT, n = 3; 1.75 ml: 1.7 ± 1.0 times more TAT, n = 4). All values are presented as mean ± standard deviation; p values were calculated by unpaired t tests. Please click here to view a larger version of this figure.
Figure 2: Functionality of the blood endothelial cell chamber model. (A) Chambers with or without CHS were incubated with whole blood. Without CHS, a clot was formed after 30 min. Measurement of thrombin-antithrombin (TAT) complexes, an indirect marker of coagulation, in blood incubated without CHS was > 6,000 μg/ml. (B) The volume of blood not kept moving by the rotating air bubble will vary with different blood volumes. With the addition of 1.5 ml of blood, this volume will be 0.3 ml, whereas it will increase to 0.74 ml with the addition of 1.75 ml. (C) HUVEC imaged with phase contrast microscopy show typical endothelial cell morphology of a confluent monolayer prior to blood contact. (D) TAT values for completely CHS coated chambers show a slight difference when different blood volumes are added. The addition of 1.5 ml blood resulted in 35 ± 11 μg/ml (n = 7) in comparison to 1.75 ml, which resulted in 85 ± 70 μg/ml TAT (n = 8). This difference is no longer present when HUVEC are incubated with the same blood volumes; 66 ± 55 μg/ml for 1.5 ml (n = 5) and 66 ± 29 μg/ml for 1.75 ml (n = 7). (E) Quantification of the number of CD16+ cells present on either unstimulated or TNFα stimulated HUVECs after blood contact. The number of CD16+ cells is significantly increased with TNFα stimulated cells incubated either with 1.5 ml (basal: 100 ± 40 cells/mm; TNFα: 256 ± 121 cells/mm) or 1.75 ml (basal: 172 ± 67 cells/mm; TNFα: 378 ± 185 cells/mm) of whole blood. (F) Representative images of unstimulated and TNFα stimulated HUVEC stained for phalloidin (red), CD16 (green) and DAPI (blue). Scale bar = 250 μm. (G) The formation of TAT complexes is roughly doubled by TNFα stimulated cells as compared to untreated cells incubated with blood (1.5 ml: 2.1 ± 0.1 times more TAT, n = 3; 1.75 ml: 1.7 ± 1.0 times more TAT, n = 4). All values are presented as mean ± standard deviation; p values were calculated by unpaired t tests. Please click here to view a larger version of this figure.
Discussion
Multiple interactions between the blood and vessel wall are normally maintained in a quiescent state due to non-adhesive and anti-thrombotic nature of the endothelium.7 During pathological conditions involving inflammation, the endothelium is activated with a resulting increase in adhesion receptor expression8 and a reduced ability to inhibit hemostasis.9 Activation of the cascade systems in the blood in turn amplifies the thrombogenicity of the endothelium causing further thrombosis and leukocyte recruitment.10 To gain a better understanding of this delicate interactions between endothelial cells and whole blood, we have developed a novel in vitro method where cultured endothelial cells are placed in contact with whole blood. To our knowledge, our setup is the first to show endothelial cells incubated with whole human blood with no or very little anticoagulant for longer time periods.
The sensitivity of this system is enabled by open system venipuncture in combination with a protective layer of immobilized heparin on all surfaces placed in contact with blood. The use of an open system during blood procurement reduces the activation of the cascade systems during venipuncture whilst permitting the use of a self-determined amount of anticoagulant. Commercially available evacuated blood tubes may, however, be used depending on the intended end points of the study. The final concentration of anticoagulant added to most commercially available closed system tubes may inhibit sensitive, and otherwise hard to study, interactions in the setup.6 A protective heparin surface further eliminates activation of blood through surface contact by any surfaces other than the endothelial cells.5 Indeed, incubation of whole blood supplemented with a small amount of unfractionated heparin in chamber without the protection of CHS resulted in clot formation.
The air bubble inside the chamber enables mixing of the blood during incubation with the endothelial cells. In order to assess the effect of the air bubble size, we used two different blood volumes. In this model, we measured similar TAT levels regardless of air bubble size in chambers incubated with HUVEC. TAT was, however, increased with a smaller air bubble size in the fully CHS treated chambers. This, in accordance with previously described endothelial cell properties11, indicates a regulatory effect upon the increased activation of coagulation provided by the endothelial cells that is lacking in the inert CHS chamber. The air bubble in the system creates a flow upon rotation of the chamber. The size of the bubble will affect the forces within this rotating system. This is shown by our results in Figure 2D were a smaller bubble creates higher TAT values representing a decreased force upon the blood i.e., the blood movement within the chamber is lower and thereby activation of the cascade system occurs to a higher extent. It should be mentioned that in this system we are creating a rotating flow that will be different in speed across the chamber with the highest speed along the walls and the lowest in the center. This is obviously not an optimal environment with regard to flow and shear stress for the endothelial cells, but still we have a system producing stable and repeatable results. More optimal conditions would include circulating laminar flow in the absence of turbulence. This is, however, not possible in the model represented here and to our knowledge such a system is not available to date. Although, there are several microfluidic systems available commercially that are not possible to combine with whole blood with no or low levels of anticoagulant added to the system thereby fail to enable proper sensitive evaluation of the interactions between all components present in blood and endothelial cells.
Furthermore, there was a 2-fold increase in recruitment of blood cells towards TNFα activated HUVEC in combination with a doubled formation of TAT independently of the blood volume. This verifies stability of the model system and also shows the possibility to use an activated endothelium in combination with whole blood. Future on, the blood endothelial cell chamber can be used to investigate blood cell recruitment towards activated endothelial cells by a variety of markers expressed on cells under various conditions and stages of activation. Furthermore, the model may be used in combination with pharmacological agents in order to evaluate effects on inflammatory conditions.
In summary, we show the great benefit of combining a chamber model with human blood and vascular cells to create a complete human in vitro system to perform relevant investigations of vascular disease.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by grants from the Swedish Research Council (90293501, A0290401, A0290402), the European Community's Seventh Framework Programme under grant agreement n°602699 (DIREKT), the NovoNordisk Foundation, the Gurli and Edward Brunnberg Foundation, Stem Therapy, Vleugel Foundation and Åke Wiberg Foundation.
References
- Mestas J, Hughes CCW. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172(2):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Ekdahl KN, Reynolds H, Larsson R, Nilsson B. A new in vitro model to study interaction between whole blood and biomaterials. Studies of platelet and coagulation activation acid the effect of aspirin. Biomaterials. 1999;20(7):603–611. doi: 10.1016/s0142-9612(98)00210-5. [DOI] [PubMed] [Google Scholar]
- Doctor A, Spinella P. Effect of Processing and Storage on Red Blood Cell Function In. Vivo. Semin Perinatol. 2012;36(4):248–259. doi: 10.1053/j.semperi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, et al. Optimal heparin surface concentration and antithrombin binding capacity as evaluated with human non-anticoagulated blood in vitro. J Biomed Mater Res A. 2003;67(2):458–466. doi: 10.1002/jbm.a.10104. [DOI] [PubMed] [Google Scholar]
- Ekdahl KN, Hong J, Hamad OA, Larsson R, Nilsson B. Evaluation of the blood compatibility of materials, cells, and tissues: basic concepts, test models, and practical guidelines. Adv Exp Med Biol. 2013;735:257–270. doi: 10.1007/978-1-4614-4118-2_18. [DOI] [PubMed] [Google Scholar]
- Aird WC. Spatial and temporal dynamics of the endothelium. J Thromb Haemost. 2005;3(7):1392–1406. doi: 10.1111/j.1538-7836.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- Kirchhofer D, Tschopp TB, Hadvary P, Baumgartner HR. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J Clin Invest. 1994. pp. 2073–2083. [DOI] [PMC free article] [PubMed]
- Esmon CT. Molecular circuits in thrombosis and inflammation. Thromb Haemost. 2013;109(3):416–420. doi: 10.1160/TH12-08-0634. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Aird WC. Vascular-Bed–Specific Hemostasis and Hypercoagulable States. N Engl J Med. 1999;340(20):1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]


