Abstract
The subcutaneous matrigel plug assay in mice is a method of choice for the in vivo evaluation of pro- and anti-angiogenic factors. In this method, desired factors are introduced into cold-liquid ECM-mimic gel which, after subcutaneous injection, solidifies to form an environment mimicking the cancer milieu. This matrix permits the penetration of host cells, such as endothelial cells, and therefore, the formation of vasculature.
Herein we propose a new modified matrigel plug assay, which can be exploited to illustrate the angiogenic potential of a pool of factors secreted by cancer cells, as opposed to a specific factor (e.g., bFGF and VEGF) or agent. The plug containing ECM-mimic gel is utilized to introduce the host (i.e., mouse) with a pool of factors secreted to the C.M. of fast-growing tumor-generating glioblastoma cells. We have previously described an extensive comparison of the angiogenic potential of U-87 MG human glioblastoma and its dormant-derived clone, in this system model, showing induced angiogenesis in the U-87 MG parental cells. The C.M. is prepared by filtering collected media from confluent tissue culture plates of either cell line following 48 hr incubation. Hence, it contains only factors secreted by the cells, without the cells themselves. Described here is the combination of two imaging modalities, microbubbles contrast-enhanced ultrasound imaging and intravital fibered-confocal endomicroscopy, for an accurate, real-time characterization of the extent, morphology and functionality of newly-formed blood vessels within the plugs.
Keywords: Cancer Biology, Issue 93, Matrigel plug, angiogenesis, microbubbles, ultrasound, fibered-confocal endomicroscopy, conditioned media.
Introduction
The matrigel plug angiogenesis assay was first described by Kibbey et al. in 1992, where it was used to evaluate angiogenesis stimulation by the peptide SIKVAV (Ser-Ile-Lys-Val-Ala-Val)1. In contrast to other in vivo angiogenesis assays, like the mouse corneal angiogenesis or chick chorioallantoic membrane assays, this assay is relatively easy to perform2. The injected ECM-mimic gel may contain cells, pro-angiogenic or an anti-angiogenic compounds and/or factors. When evaluating the activity of an anti-angiogenic compound, the plug normally contains a mixture of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), and the anti-angiogenic substance can be administered directly into the plug or systemically3,4.
The ECM-mimic gel is injected subcutaneously where it solidifies within minutes. Assessment of blood vessel recruitment in the resulting plug is typically performed by measuring hemoglobin levels within the plug, by fluorescence signal measurement following injection of fluorescein isothiocyanate (FITC)-labeled dextran, by immunostaining of histology sections for endothelial-specific markers or by fluorescence-activated cell sorting (FACS)2,5,6. However, this assessment only allows end-point analysis and lacks information about the functionality and morphology of blood vessels. In addition, measurements of plasma volume, either by hemoglobin levels or by using dextran-FITC as an indicator, may be misleading since blood content is affected by the size of blood vessels and the extent of stagnant pools of blood. This is especially crucial when the recruited vasculature is characterized by the enhanced permeability and retention (EPR) effect7.
We herein propose a new, more precise, method for visualization of the recruited blood vessels by combining two complementary imaging modalities. High-resolution microbubbles contrast-enhanced ultrasound (US) combined with intravital fibred-confocal endomicroscopy can provide information not only about blood vessel density, but also on their morphology and functionality. Moreover, this analysis can be performed at several time points thereby enabling monitoring of angiogenesis kinetics. US is a widely-used imaging modality which possesses high spatial resolution for blood vessels imaging following intravenous (i.v.) injection of microbubbles that remain exclusively in the vascular compartment8-11. The microbubbles are gas-filled microbubbles that produce a strong echogenic signal when excited with an US pulse and thus serve as a good contrast agent. 3D images of the plug are acquired using the US imaging system software following microbubbles destruction. The resulting images are composed from image frames of pre- and post-destruction of microbubbles and reflect the difference in video intensity in color. These overlayed images are automatically displayed by the software. Consequently, the percent of functional vessels within the plug can be quantified. High resolution fibered confocal endomicroscopy serves as a complementary imaging modality by reporting on blood vessels morphology. In this minimally invasive method, images are acquired following i.v. administration of FITC-conjugated dextran12. The fluorescent polymer dyes the bloodstream and thus serves as a 'contrast agent' for blood vessels morphology and blood flow. Moreover, since the injected polymer is at a size of 70 KDa, it can only cross leaky vessels as found in tumor vasculature. This will result in high background from the FITC-labeled dextran outside the blood vessels. Consequently, fibered confocal endomicroscopy can be used to visualize typical characteristics of the EPR effect in tumor neovasculature- enlarged, leaky, highly-tangled vessels, with blunt ends.
This system model was described in the comparison of the angiogenic potential of U-87 MG human glioblastoma and its dormant clone13. Vascularization within the plugs was evaluated three weeks post ECM-mimic gel inoculation. It was shown that the vascularization within plugs containing C.M. from U-87 MG fast-growing tumor-generating cells was significantly increased in terms of number, density and functionality, when compared with vascularization within plugs containing C.M. from the dormant tumor-generating cells. Hence, the authors were able to conclude that C.M. isolated from U-87 MG fast-growing tumor-generating cells contains higher levels of pro-angiogenic factors, compared with the C.M. from dormant tumor-generating cells. These factors stimulate the formation of functional blood vessels by positively affecting all steps of the angiogenic cascade (i.e., proliferation, sprouting, migration and finally, formation of tubular structures of endothelial cells).
Protocol
NOTE: All animal procedures were approved and performed in compliance with the standards of Tel Aviv University Sackler School of Medicine Institutional Animal Care and Use Committee (IACUC).
1. Conditioned Media Preparation
Seed 2 x 106 U-87 MG cells in a 10 cm culture plate in a volume of 8 ml Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% pencillin/streptomycin/nystatin solution.
Incubate cells at 37°C; 5% CO2 until achieving full confluency at 48 hr.
Using a 10 ml syringe, withdraw all medium from the plate and filter through a 0.45 µm filter into a 15 ml tube.
Without washing the cells plate, add 1 ml trypsin solution (0.25%) and incubate.
When all cells are detached, inactivate the trypsin using at least 5 ml DMEM supplemented with 10% FBS and preform a cell count.
Count the number of cells using a hemocytometer or other cell-counting device and calculate the number of cells according to the counting method used. NOTE: It will be used later in order to normalize C.M. concentration.
Transfer the C.M. to a glass round-bottom flask and concentrate under high vacuum rotary evaporator. In order to accelerate the process, increase the bath temperature to 37 °C, if desired. Continue until the C.M. achieves a paste-like texture.
Resuspend the C.M. from each culture plate in 400 µl saline. When using more than one plate, use equivalent volume. For example, for 2 culture plates, use 800 µl. Use each plate for 4 mice.
In order to compare different cell lines or treatments, adjust the volume according to the cell ratio received in step 1.6. For example, if one plate contains 1.5x more cells, add 200 µl of saline to the pre-existing 400 µl. This will be the adjusted C.M.
2. ECM-mimic Gel Plug Inoculation into Mice
- Before inoculating the ECM-mimic gel into mice, prepare the following materials:
- Growth-factors reduced phenol-free ECM-mimic gel (thaw overnight, a day before the experiment, at 4 °C on ice).
- Electric shaver.
- Ketamine and xylazine in order to anesthetize the mice.
- Ice-cold 1,000 µl sterile tips.
- Ice-cold 1 ml syringe and 27 G needles.
Prepare for each mouse a 1.5 ml Eppendorf tube containing 80 µl adjusted C.M. and keep on ice.
To each tube, add 600 µl ice-cold growth-factors reduced phenol-free ECM-mimic gel that was thawed overnight at 4 °C, on ice. The ECM-mimic gel is liquid in 4 °C and solidifies at body temperature; in order to prevent the ECM-mimic gel from solidifying, use ice-cold 1,000 µl tips. Mix tubes carefully without forming bubbles and keep on ice.
Anesthetize 6 week old BALB/c mice using ketamine (100 mg/kg) and xylazine (12 mg/kg). Confirm proper anesthesia according to the institutional animal care and use committee, i.e. prior to beginning the experiment. Animals have reached their proper plane of anesthesia when they no longer respond to a firm toe pinch.
Shave the abdominal area of the mice and disinfect using a surgical scrub/alcohol.
Use a cold 1 ml syringe and a 27 G needle to inject the contents of the Eppendorf tube subcutaneously. Perform the injection very slowly to prevent bubble formation. Once all volume is administered, do not eject the needle for several minutes, until solidification occurs.
3. Evaluation of ECM-mimic Gel Plug Vascularization Using Ultrasound
NOTE: This part of the protocol requires previous knowledge of operating an ultrasound imaging system, or the assistance of an authorized technician.
- Before starting, have the following materials and instruments ready:
- Electric shaver.
- Depilatory cream.
- Ketamine and xylazine in order to anesthetize the mice.
- Non-targeted microbubbles.
- Activation device.
- US imaging system including a 55 MHz 708 probe and a 3D acquisition micromanipulator.
- Measure vascularization within the plugs three weeks post inoculation: NOTE: In the U-87 MG-based animal model, 3 weeks was found to represent the ideal time point for imaging. Under different settings, calibrate vascularization kinetics starting from a few days following inoculation of plugs.
- Anesthetize mice using ketamine (100 mg/kg) and xylazine (12 mg/kg). Confirm proper anesthesia according to the institutional animal care and use committee, i.e. prior to beginning the experiment. Animals have reached their proper plane of anesthesia when they no longer respond to a firm toe pinch.
- Shave the abdominal area of the mice and treat with depilatory cream.
- Fix the mice supinely on the stage of the micro-manipulator.
- Using a 1 ml syringe containing saline and a 30 G needle, place the needle inside the tail vein, fix the needle firmly and loosen the syringe.
- Set up for the Contrast 3D-mode image acquisition session:
- Press “3D” to initialize the 3D motor stage and place the transducer in the middle of the desired scanning area.
- Press “3D” and input the scan distance and step size, then press “Scan”.
- Make sure that the entire plug was scanned in the 3D image data displayed. If not, adjust the transducer so that the entire plug is visible in the scan and repeat step 3.5.2.
- Press “Contrast” and push the time gain compensation (TGC) controls to the left to darken the image.
- Activate the microbubbles using a vial mixer that is designated for the activation of microbubbles for 45 sec.
- Mix in a 1 ml syringe 50 µl of microbubbles with 50 µl saline.
- Replace the saline-containing loosened syringe with a syringe containing activated microbubbles. Inject the diluted microbubbles into the tail vein of the mice, wait a few seconds for the bubbles to reach a steady state, and then acquire 3D contrast-enhanced cine loop of the plug, using the 3D acquisition motor:
- Press “3D” and select "Scan" to acquire 3D contrast-enhanced images and then press “Cine Store” to save the image data.
- Press “3D” and click “Destroy 3D” to destruct the microbubbles.
- Press “3D” again and select "Scan" to acquire a post-destruction cine loop, then press “Cine Store”.
- Generate the overlay image frames pre- and post-destruction of microbubbles:
- In the “Reference Settings” on the left side of the screen click “Create Reference”.
- Press “Study Management” to open the pre-destruction cine loop acquired.
- Click “Process Cine” to generate the green contrast overlay, then click “Load Into 3D”.
- Use the US imaging software in order to quantitate the resulting 3D image for blood volume within the plugs:
- Press “Scan/Freeze” and then press “Mode settings”.
- Click “Volume” and select “Parallel”.
- Create a 3D volume of the target tissue by segmenting a series of contours.
- Click “Finish” to complete the segmentation. NOTE: The volume of the contrast agent will be displayed on the lower left side of the screen.
- At the end of the procedure, place mice in a warm, clean, dry, quiet environment away from other animals. Provide warmth using a commercially-available surgical heating pad or an incandescent lamp (50-75 watt), placed 12-14 inches away from rodent. Monitor mice continuously until maintaining upright posture and walking normally.
4. Evaluation of ECM-mimic Gel Plug Vasculature Morphology Using Fibered Confocal Endomicroscopy
NOTE: this part of the protocol is performed immediately following US imaging and it requires previous knowledge of operating the fibered confocal endomicroscopy imaging system, or the assistance of an authorized technician.
- Before starting, have the following materials and instruments ready:
- Surgery tools like forceps, scissors and absorbable sewing threads.
- FITC-conjugated dextran (70 KDa).
- Calibration solutions (three tubes each containing hydrogen peroxide, water or fluorescent solution).
- The imaging system and a probe suitable for imaging of blood vessels according to manufacturer.
Anesthetize mice using ketamine (100 mg/kg) and xylazine (12 mg/kg).
Administer i.v., via the tail vein, 200 µl of 10 mg/ml FITC-labeled dextran.
Disinfect the abdominal area using a surgical scrub/alcohol and apply a small incision approximately 1 cm from the plug.
Place the endomicroscopy probe on top of the plug gently while keeping the plug intact.
- Acquisition:
- Once the probe is set on the plug, press “Start laser” using the foot pedal. The fluorescence should be shown on the screen.
- Acquire short movies (up to 30 sec) by pressing 'Record' on the screen or 'Select' using the foot pedal.
- Between movies, wash the probe by placing the tip in a tube containing water.
- When finished, remove the probe and clean the tip with a cotton tip.
- To analyze mean vessel diameter:
- Click on the “Detect microvessels” icon in the upper panel to open the vessel detection module window.
- Click “Preferences” and mark “Mean vessel diameter” in the “Statistic of interest” and “Display histogram bars” in the “Display mode”.
- Click “Detect” to display the selected measurements.
- To analyze the fluorescent signal intensity in areas adjacent to blood vessels:
- Click on “Create circle ROI” in the left panel and create a circle in the area of interest.
- Click “Compute histogram within ROI” to compute the values related to this region.
5. Post-procedural Treatment of Mice
At the end of each imaging procedure, place mice in a warm, clean, dry, quiet environment away from other animals. Provide warmth using a commercially-available surgical heating pad or an incandescent lamp (50-75 W), placed 12-14 in away from rodent. Monitor mice continuously until maintaining upright posture and walking normally.
- For further analysis of plugs' vascularization at additional time points, apply the following recovery conditions:
- Suture the small incision using absorbable sutures.
- Give mice analgesia such as buprenorphine (1 mg/kg) subcutaneously, and return to individual cages.
- Observe all animals to determine if further analgesic treatment is necessary and re-evaluate daily to monitor and assess for distress.
At the end-point of the experiment, euthanize mice by first applying isoflurane, followed by cervical dislocation.
If additional imaging will be performed in the weeks following the initial imaging steps, all procedures should be kept as sterile as possible. A surgical skin scrub should be performed, sterile water and sterile cotton tips should be used to clean the endomicroscopy probe, and the probe should be disinfected between each animal using sterile water.
Representative Results
Implanting ECM-mimic gel plugs with C.M. from fast-growing angiogenic tumor-generating cell line U-87 MG, results in extensive angiogenesis. This vasculature can be extensively explored by using two complementary imaging methods.
Microbubbles contrast-enhanced US imaging reveals extensive recruitment of functional blood vessels into the plug by illustrating extensive blood flow within U-87 MG C.M. loaded plugs, as opposed to undetectable blood flow within plugs containing C.M. from dormant tumor-generating cells (Figure 1).
The morphology of these newly-formed blood vessels is emphasized by fibered confocal endomicroscopy (Figure 2). Blood vessels within plugs loaded with U-87 MG C.M. exhibited morphology typical to the enhanced permeability and retention (EPR) effect for macromolecules such as Dextran-FITC at 70 kDa size used here. Blood vessels were enlarged and highly tangled, with non-continuous and sluggish flow (Figure 2A, C). Also, leakage of blood was observed to the surrounding environment, as shown by high fluorescent signal outside the blood vessels (Figure 2B).
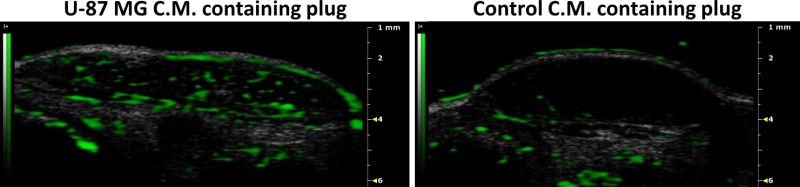 Figure 1: Microbubbles contrast-enhanced US imaging. Following intravenous administration of microbubbles and their destruction, U-87 MG C.M. containing plugs exhibit increased vascularization (in green). Plugs containing C.M. from U-87 MG-derived dormant tumor-generating cells were used as control. Please click here to view a larger version of this figure.
Figure 1: Microbubbles contrast-enhanced US imaging. Following intravenous administration of microbubbles and their destruction, U-87 MG C.M. containing plugs exhibit increased vascularization (in green). Plugs containing C.M. from U-87 MG-derived dormant tumor-generating cells were used as control. Please click here to view a larger version of this figure.
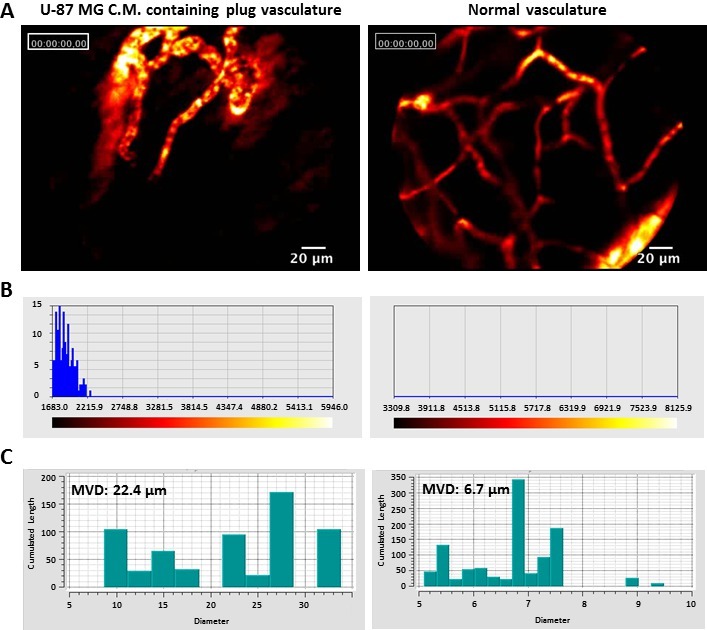 Figure 2: Fiber confocal microscopy imaging. (A) Intravenous administration of 70 KDa Dextran-FITC, enables visualization of blood flow and blood vessels morphology. U-87 MG C.M. containing plugs vasculature consists of leaky, enlarged vessels with blunts ends. Blood vessels leakage is exhibited by fluorescent signal outside the vasculature. Normal vasculature in mouse abdomen was used as control. (B) Fluorescent signal intensity in areas adjacent to blood vessels. The fluorescent signal is exemplified by a spectrum from red to white. Dark red represents low signal intensity, while white represents high fluorescent signal. U-87 MG C.M. containing plugs show a clear fluorescent signal outside the vessels, while no signal is shown outside normal vessels. (C) Mean Vessel Diameter (MVD) of blood vessels within U-87 MG C.M. containing plugs (22.4 µm) is significantly higher compared to the MVD of normal vessels (6.7 µm). Please click here to view a larger version of this figure.
Figure 2: Fiber confocal microscopy imaging. (A) Intravenous administration of 70 KDa Dextran-FITC, enables visualization of blood flow and blood vessels morphology. U-87 MG C.M. containing plugs vasculature consists of leaky, enlarged vessels with blunts ends. Blood vessels leakage is exhibited by fluorescent signal outside the vasculature. Normal vasculature in mouse abdomen was used as control. (B) Fluorescent signal intensity in areas adjacent to blood vessels. The fluorescent signal is exemplified by a spectrum from red to white. Dark red represents low signal intensity, while white represents high fluorescent signal. U-87 MG C.M. containing plugs show a clear fluorescent signal outside the vessels, while no signal is shown outside normal vessels. (C) Mean Vessel Diameter (MVD) of blood vessels within U-87 MG C.M. containing plugs (22.4 µm) is significantly higher compared to the MVD of normal vessels (6.7 µm). Please click here to view a larger version of this figure.
Discussion
Combining complementary imaging methods allows the acquirement of information on different angiogenesis components' microvessel density, functionality and morphology. Loading C.M. on ECM-mimic gel plugs generates a tumor microenvironment, which is separated from other cell populations, like endothelial cells, that are recruited and extravasate into the plug.
By performing, in parallel, microbubbles contrast-enhanced US imaging and fibered confocal endomicroscopy imaging, we can conclude that a pool of factors secreted by fast-growing angiogenic tumors generating cells, without the cancer cells themselves, can not only recruit blood vessels, but also form functional vasculature with EPR typical characteristics. Moreover, this method allows the separation between the impact of the secreted factors and cell-to-cell interaction in tumor microenvironment.
Information obtained from the US and fibered confocal endomicroscopy is however subjected to the limitation of the imaging technique. For instance, while using the fibered confocal endomicroscopy imaging, it is impossible to image a single specific blood vessel over time, or even the same area at the plug.
The ECM-mimic gel plug assay is relatively easy to perform, and this is sustained when loading C.M. with the ECM-mimic gel. However, a critical issue that should be taken under consideration is the C.M. to ECM-mimic gel proportion. High volumes of C.M. might prevent the ECM-mimic gel from solidifying. Therefore, C.M. amount should not exceed approximately 13% of the ECM-mimic gel.
This method can be adjusted for different experimental systems and applications. For example, it can be used to assess anti-angiogenic drugs by loading C.M. from pre- and post-treated cells. Also, other cell lines may be used under these experiment settings. However, calibration is required in order to apply the optimal C.M. concentration. Moreover, the ideal time point for imaging should be optimized according to each model system.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The Satchi-Fainaro research laboratory is partially supported by The Association for International Cancer Research (AICR), German-Israel Foundation (GIF), THE ISRAEL SCIENCE FOUNDATION (Grant No. 1309/10), Swiss Bridge Award, and by grants from the Israeli National Nanotechnology Initiative (INNI), Focal Technology Area (FTA) program: Nanomedicine for Personalized Theranostics, and by The Leona M. and Harry B. Helmsley Nanotechnology Research Fund.
References
- Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Passaniti A, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- Norrby K. In vivo models of angiogenesis. J Cell Mol Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- Johns A, Freay AD, Fraser W, Korach KS, Rubanyi GM. Disruption of estrogen receptor gene prevents 17 beta estradiol-induced angiogenesis in transgenic mice. Endocrinology. 1996;137:4511–4513. doi: 10.1210/endo.137.10.8828515. [DOI] [PubMed] [Google Scholar]
- Adini A, et al. Matrigel cytometry: a novel method for quantifying angiogenesis in vivo. J Immunol Methods. 2009;342:78–81. doi: 10.1016/j.jim.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- Kuliszewski MA, et al. Molecular imaging of endothelial progenitor cell engraftment using contrast-enhanced ultrasound and targeted microbubbles. Cardiovasc Res. 2009;83:653–662. doi: 10.1093/cvr/cvp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger SM, et al. Ultrasound assessment of angiogenesis in a matrigel model in rats. Ultrasound Med Biol. 2006;32:673–681. doi: 10.1016/j.ultrasmedbio.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent. Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Segal E, Satchi-Fainaro R. Design and development of polymer conjugates as anti-angiogenic agents. Adv Drug Deliv Rev. 2009;61:1159–1176. doi: 10.1016/j.addr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Satchi-Fainaro R, et al. Prospective identification of glioblastoma cells generating dormant tumors. PLoS One. 2012;7:44395. doi: 10.1371/journal.pone.0044395. [DOI] [PMC free article] [PubMed] [Google Scholar]


