Abstract
The maturation and meiotic competence of human oocyte requires both healthy cytoplasmic and nuclear compartments. Germinal vesicle (GV) transfer techniques have represented useful tools for studying the interaction between the nucleus and the cytoplasm in oocyte maturation process in mammals. This report summarizes an update on the recent findings on GV transfer pertaining to improving meiotic resumption and ability of immature oocytes to mature. It also addresses mitochondrial DNA heteroplasmy as a challenge in GV transfer technology. Altogether, data to date indicate that GV transfer could improve the quality of human oocytes especially in women with advanced maternal age who usually have high rates of spindle abnormality and chromosomal misalignment. Although experimental, this technique represents a viable therapeutic option for women with diminished ovarian reserve who do not produce mature oocytes or good embryos during IVF treatment.
Keywords: Germinal vesicle, Meiotic spindle, Meiotic maturation, Oocyte, Nucleus, Cytoplasm
Introduction
The human ovary contains a large pool of inactive germ cells that reside in primordial follicles and two categories of oocytes. One category of oocytes remain arrested in the diplotene stage and the other category of oocytes mature to metaphase II in preovulatory follicles [1]. Interestingly, pre-pubertal human ovaries contain a high proportion of morphologically abnormal non-growing follicles, and follicles of pre-pubertal ovaries have reduced capacity for in vitro growth [2]. Oocyte maturation is a complex process involving both the advancement of the meiotic cycle and the cytoplasmic reprogramming [1, 3–6]; such as increase in the number of mitochondria and accumulation of ribosomes [1, 7]. This maturation process leads to the release of a mature metaphase II oocyte that is competent to fertilize and form an embryo. Both the nuclear and cytoplasmic compartments have been shown to be responsible for poor oocyte quality by contributing to meiotic defects and subsequent impaired embryo development [5, 8–11].
There is a critical need for a better definition of oocyte quality in assisted reproductive technology [12]. Human oocytes are arrested at the prophase/germinal vesicle (GV) stage before birth and do not resume meiotic maturation until ovulatory events at puberty. Additionally, the aberrant meiotic spindle, especially the aberrant metaphase I spindle, may cause meiotic errors and oocyte aneuploidy leading to spontaneous abortion and subfertility [13], especially in women with diminished ovarian reserve (DOR). Recent data demonstrated that there is an influence of maternal age on the actin cytoskeleton in human oocytes [14]. Additionally, abnormal oocyte spindle morphology is associated with human female infertility, and advanced maternal age has been attributed to spindle abnormality such as abnormal chromosome alignment and a microtubule matrix that compromises the meiotic spindle [15]. In older women with or without DOR, these significant alterations in the regulatory mechanisms responsible for assembly of the meiotic spindle in the cytoplasm of the oocyte lead to high prevalence of aneuploidy [15].
Summary of GV transfer technique protocol
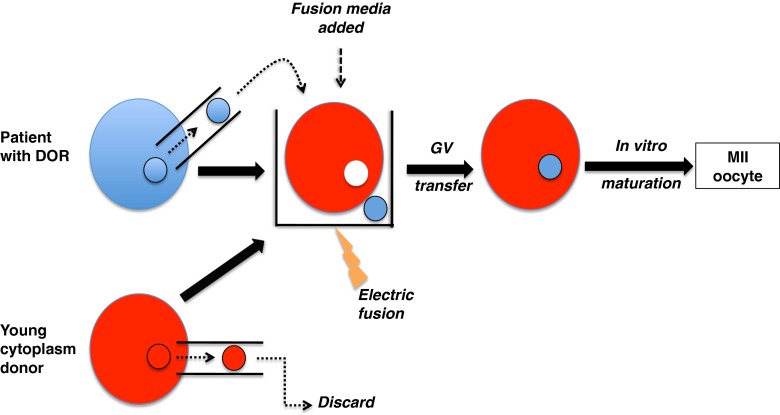
GV transfer techniques have represented useful tools for studying the interaction between the nucleus and the cytoplasm in oocyte maturation process in mammals [1, 3, 7, 8, 11, 13, 16–18]. GV can be easily observed under microscope and the removal of GV is less invasive compared to chromosome of matured oocytes as their genetic materials are protected by the nuclear membrane. Oocytes are typically exposed to modified human tubal fluid medium with 10% FCS supplemented with 7.5 μ/ml cytochalasin B (CB, Sigma, C6762) for 15 min at room temperature in order to disrupt microfilament and increase plasma membrane flexibility before manipulation. Using micromanipulators, a slot is made on the zona pellucida of the oocyte by applying a sharp-tipped pipette to penetrate and grind the zona against the wall of the holding pipette. This allows an enucleation pipette to pass through the zona slot and approach the GV before gently applying negative pressure to aspirate the GV. Once a GV is separated from the cytoplasm, it gets transferred into the perivitelline space of an enucleated recipient GV oocyte. The membrane fusion between GV and cytoplasm is initiated by placing the reconstructed oocyte into a fusion medium (0.3 M mannitol, 0.1 mM CaCl2, and 0.05 mM MgSO4) between platinum electrodes alignment in response to AC (6–8 V) for 5–10 s before electrical pulse (1.8-2.5 kV/cm DC for 50 μs). The formed complex is then rinsed and then incubated in medium at 5% CO2 and 37 °C. Membrane fusion usually occurs 30 min after electric pulse (Fig. 1). Finally, reconstructed GV stage oocytes need to be matured in vitro and then fertilized.
Fig. 1.
Schematic diagram of GV transfer between a woman with diminished ovarian reserve (DOR) and a young donor
GV transfer technique produces functionally competent oocytes and is not species-specific
GV transfer technique by itself does not impact oocyte fertilization. For example, mouse oocytes reconstructed by GV transfer developed to blastocyst stage [8]. In that study, reconstructed oocytes matured and, following artificial activation, consistently developed a pronucleus with a haploid karyotype [8]. These observations demonstrate that the nucleus of a mouse oocyte subjected to sequential nuclear transfer at GV and pronucleus stages is capable of maturing meiotically, activating normally and supporting embryonic development to hatching blastocyst stage. On the other hand, other findings suggested that the cytoplasm from young mice could not rescue aging-associated chromosome misalignment in meiosis of GV from aged mice; and that the behavior of chromosome alignment over metaphase spindle is predominantly determined by GV material [19].
Using CB6F1 mouse oocytes, we previously reported that metaphase II nuclei that form following GV transfer are functionally competent to support embryonic development to the blastocyst stage [8]. In subsequent studies, we demonstrated that, when transferred to the uterus of a pseudopregnant foster mouse at the 2-cell stage, embryos that develop from metaphase II nuclei post GV-transfer do continue to develop and result in live birth offsprings [9]. Moreover, the live birth rate observed for these embryos, approached that noted previously when non-GV transferred oocytes were denuded, fertilized and transferred as 2-cell embryos to the uteri of foster mice (12% vs. 18%; respectively) [20].
GV cytoplasmic factors regulating the progression of the first and the second meioses are not species-specific in mammalian oocytes. Oocytes reconstructed by transferring mouse GV into rabbit GV-stage cytoplasts can mature to MII stage and can further cleave and develop to eight-cell stage after ICSI. Additionally the maturation rate was not significantly different with that of control mouse oocytes or control rabbit oocytes; further indicating that the technique of GV transfer per se does not affect oocyte maturation.
GV transfer using human oocytes
Before the start of nuclear maturation, the oocyte contains two sets of chromosomes (4N) with intact nuclear membrane known as GV. Induced by the preovulatory LH surge, the oocyte resumes first meiotic division, extrudes first polar body and gets arrested at the metaphase of second meiotic division with two sets of chromosomes (2N). For a successful fertilization to occur, these maturation events need to be completed in an orderly manner. Oocytes from older women with DOR have been known to have higher rates of aneuploidy [15, 21]. Thus, these abnormalities may be avoided by replacement of compromised cytoplasm with healthy cytoplasm through GV transfer before the start of chromosome segregation. This strategy in a preclinical study has been shown to overcome aneuploidy in reconstructed human oocytes carrying chromosomes from aged, and cytoplasm from young individuals with a euploidy rate of 80% [17].
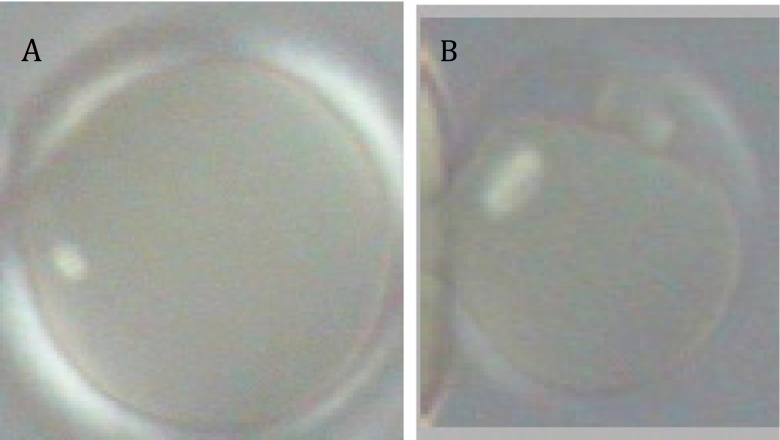
Studies have been performed on discarded immature oocytes of women who underwent IVF. In one study, 4 out of 7 human oocytes denuded of cumulus cells, reconstructed by GV of old oocytes (from women aged > 38) with cytoplasm of young oocytes (from women aged <31), displayed normal polar body (PB) extrusion and second meiotic chromosome complement [17]. Additionally, GV transfer between mouse and human oocytes was performed in order to construct xeno-oocytes. Thus, human GV/mouse cytoplasm and mouse GV/human cytoplasm oocytes were reconstructed in order to evaluate the origin of the cytoplasm on meiosis resumption. Mouse GV/human cytoplasm xeno-oocytes displayed significantly reduced meiotic maturation ability with < 50% of these xeno-oocytes extruding the first PB. Interestingly, all the human GV/mouse cytoplasm xeno-oocytes matured to metaphase II and extruded the first PB (Fig. 2). Nine pairs of oocytes reconstructed by reciprocal exchange of GV between human and mouse were successfully prepared for cytogenetic analysis. Five out of nine human GV/mouse cytoplasm xeno-oocytes had normal 23 sets of chromosomes. In contrast, none of nine mouse GV/human cytoplasm xeno-oocytes had normal number of chromosomes. Additionally, precocious chromatids division was found in six mouse GV/human cytoplasm xeno-oocytes while none of the human GV/mouse cytoplasm xeno-oocytes displayed similar findings. Because mouse oocytes are known to fully mature while human oocytes are known to poorly mature in vitro, these findings indicate that the origin of the cytoplasm determined meiosis behavior.
Fig. 2.
Birefringent spindle in metaphase II xeno-oocytes of mouse GV/human cytoplasm (a) and human GV/mouse cytoplasm (b). Only xeno-oocyte formed by human GV/mouse cytoplasm shows extrusion of the first PB and normal meiotic spindle
Mitochondrial DNA heteroplasmy: a problem to consider during GV transfer
Mitochondria, the source of energy in animal cells, are inherited exclusively from mothers and contain their own DNA. There is accumulating evidence demonstrating that ovarian aging is accompanied by both qualitative and quantitative alterations of mitochondria [22]. The distribution of mitochondrial DNA macro-haplogroups in patients with DOR differ significantly from that of patients with normal ovarian reserve [23]. Additionally, maternally transmitted mitochondrial DNA mutations may have a role in aggravating aspects of human aging [24]. Pathogenic mitochondrial DNA mutations are found in 0.5% of the population [25] and are a frequent cause of maternally inherited human disease affecting at least 1 in 6500 of the population [26]. During GV transfer, the mitochondria adjacent to the GV are likely to be carried over into the reconstructed oocytes thus creating mitochondrial DNA heteroplasmy (mixture of two different mitochondrial DNA). In patients with DOR, the reconstructed oocytes will contain mitochondrial DNA from the patients with DOR, although the majority of mitochondria are derived from the young cytoplasm donor. The effect of mitochondrial heteroplasmy on meiosis resumption in human reconstructed oocyte is still not clear although a study in mice demonstrated that mitochondrial heteroplasmy of reconstituted oocytes did not influence their in vitro maturation and preimplantation development [27].
Another problem arises if the patient with DOR has mutated mitochondrial DNA—a reason for many genetic diseases such as Leigh syndrome and MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes) [28]. Interestingly, the “degree” (percentage carried with the GV) of mitochondrial DNA heteroplasmy, if mutated mitochondrial DNA are transported with the GV, in the reconstructed oocytes could have implications on embryo development and ultimately the offspring [29]. However, the level of “acceptable” mitochondrial DNA heteroplasmy in embryos created by GV transfer depends on the mitochondrial disorder of interest [29].
In order to reduce the risk of transmission of mutated mitochondrial DNA into the reconstructed oocytes, it is necessary to remove mitochondrial DNA completely from the patient with DOR when GV transfer is performed. However, this remains a challenge nowadays in GV transfer technology. Thus there is a need for new methods in which the injected GV contains no residual ooplasm into enucleated GV stage oocyte. GV transfer represents a technology that can overcome chromosome abnormalities in oocytes from patients with DOR and holds the promise for prevention of mitochondrial disorders by replacement of mutated mitochondrial DNA with wild-type haplotypes.
Conclusion
GV transfer provides a unique model for future studies designed to identify the cytoplasmic factors that could restore resumption of meiosis in arrested oocytes or oocytes not responsive to gonadotropins. The abnormal assembly of meiotic spindle is believed to be a major mechanism for aneuploidy, a major cause for age-related infertility in women. GV transfer may provide a potential therapeutic option for women who suffer from age-related infertility by transferring the GV from an older woman’s oocyte into a donated cytoplasm from a young woman. It is only by considering all aspects of maturation, particularly the cytoplasm, that the production of in vitro mature human oocytes with live-birth producing potential will be achieved. GV transfer represents a unique technology that can overcome chromosomal abnormalities in oocytes from aged individuals and holds the promise for prevention of aneuploidy in women with diminished ovarian reserve.
Acknowledgments
Disclosure
None.
Footnotes
Capsule
Germinal vesicle transfer could improve meiotic errors such as spindle abnormalities and chromosomal misalignment.
References
- 1.Fulka J, Jr, First NL, Moor RM. Nuclear and cytoplasmic determinants involved in the regulation of mammalian oocyte maturation. Mol Hum Reprod. 1998;4:41–49. doi: 10.1093/molehr/4.1.41. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod (Oxf Engl) 2014;29:97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Wang CW, Grifo JA, Krey LC, Zhang J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum Reprod (Oxf Engl) 1999;14:2357–2361. doi: 10.1093/humrep/14.9.2357. [DOI] [PubMed] [Google Scholar]
- 4.Karnikova L, Urban F, Moor R, Fulka J., Jr Mouse oocyte maturation: the effect of modified nucleocytoplasmic ratio. Reprod Nutr Dev. 1998;38:665–670. doi: 10.1051/rnd:19980608. [DOI] [PubMed] [Google Scholar]
- 5.Moor RM, Dai Y, Lee C, Fulka J., Jr Oocyte maturation and embryonic failure. Hum Reprod Update. 1998;4:223–236. doi: 10.1093/humupd/4.3.223. [DOI] [PubMed] [Google Scholar]
- 6.Inoue A, Nakajima R, Nagata M, Aoki F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Hum Reprod (Oxf Engl) 2008;23:1377–1384. doi: 10.1093/humrep/den096. [DOI] [PubMed] [Google Scholar]
- 7.Heacox AE, Schroeder PC. A light- and electron-microscopic investigation of gametogenesis in Typosyllis pulchra. (Berkeley and Berkeley) (Polychaeta: Syllidae). II. Oogenesis. Cell Tissue Res. 1981;218:641–658. doi: 10.1007/BF00210121. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Zhang J, Krey LC, Grifo JA. In-vitro development of mouse zygotes following reconstruction by sequential transfer of germinal vesicles and haploid pronuclei. Hum Reprod (Oxf Engl) 2000;15:1997–2002. doi: 10.1093/humrep/15.9.1997. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Chang HC, Zhang J, Grifo J, Krey LC. Metaphase II nuclei generated by germinal vesicle transfer in mouse oocytes support embryonic development to term. Hum Reprod (Oxf Engl) 2003;18:1903–1907. doi: 10.1093/humrep/deg372. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Keefe DL. Nuclear origin of aging-associated meiotic defects in senescence-accelerated mice. Biol Reprod. 2004;71:1724–1729. doi: 10.1095/biolreprod.104.028985. [DOI] [PubMed] [Google Scholar]
- 11.Fulka J, Jr, Loi P, Ledda S, Moor RM, Fulka J. Nucleus transfer in mammals: how the oocyte cytoplasm modifies the transferred nucleus. Theriogenology. 2001;55:1373–1380. doi: 10.1016/S0093-691X(01)00488-5. [DOI] [PubMed] [Google Scholar]
- 12.Coticchio G, Guglielmo MC, Dal Canto M, Fadini R, Mignini Renzini M, De Ponti E, et al. Mechanistic foundations of the metaphase II spindle of human oocytes matured in vivo and in vitro. Hum Reprod (Oxf Engl) 2013;28:3271–3282. doi: 10.1093/humrep/det381. [DOI] [PubMed] [Google Scholar]
- 13.Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 2012;86:1–7. doi: 10.1095/biolreprod.111.094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coticchio G, Guglielmo MC, Albertini DF, Dal Canto M, Mignini Renzini M, De Ponti E, et al. Contributions of the actin cytoskeleton to the emergence of polarity during maturation in human oocytes. Mol Hum Reprod. 2014;20:200–207. doi: 10.1093/molehr/gat085. [DOI] [PubMed] [Google Scholar]
- 15.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod (Oxf Engl) 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 16.Li GP, Chen DY, Lian L, Sun QY, Wang MK, Song XF, et al. Mouse-rabbit germinal vesicle transfer reveals that factors regulating oocyte meiotic progression are not species-specific in mammals. J Exp Zool. 2001;289:322–329. doi: 10.1002/1097-010X(20010415/30)289:5<322::AID-JEZ6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wang CW, Krey L, Liu H, Meng L, Blaszczyk A, et al. In vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer. Fertil Steril. 1999;71:726–731. doi: 10.1016/S0015-0282(98)00549-4. [DOI] [PubMed] [Google Scholar]
- 18.Dekel N, Beers WH. Rat oocyte maturation in vitro: relief of cyclic AMP inhibition by gonadotropins. Proc Natl Acad Sci U S A. 1978;75:4369–4373. doi: 10.1073/pnas.75.9.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui LB, Huang XY, Sun FZ. Transfer of germinal vesicle to ooplasm of young mice could not rescue ageing-associated chromosome misalignment in meiosis of oocytes from aged mice. Hum Reprod (Oxf Engl) 2005;20:1624–1631. doi: 10.1093/humrep/deh826. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Krey LC, Zhang J, Grifo JA. Ooplasmic influence on nuclear function during the metaphase II-interphase transition in mouse oocytes. Biol Reprod. 2001;65:1794–1799. doi: 10.1095/biolreprod65.6.1794. [DOI] [PubMed] [Google Scholar]
- 21.Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8:45–58. doi: 10.1016/S1472-6483(10)60497-X. [DOI] [PubMed] [Google Scholar]
- 22.Kushnir VA, Ludaway T, Russ RB, Fields EJ, Koczor C, Lewis W. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J Assist Reprod Genet. 2012;29:637–642. doi: 10.1007/s10815-012-9771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May-Panloup P, Desquiret V, Moriniere C, Ferre-L’Hotellier V, Lemerle S, Boucret L, et al. Mitochondrial macro-haplogroup JT may play a protective role in ovarian ageing. Mitochondrion. 2014;18c:1–6. doi: 10.1016/j.mito.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Ross JM, Stewart JB, Hagstrom E, Brene S, Mourier A, Coppotelli G, et al. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature. 2013;501:412–415. doi: 10.1038/nature12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet. 2008;83:254–260. doi: 10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, et al. Prevalence of mitochondrial DNA disease in adults. Ann Neurol. 2008;63:35–39. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Sato K. Mitochondrial behavior and localization in reconstituted oocytes derived from germinal vesicle transfer. Hum Cell. 2008;21:7–11. doi: 10.1111/j.1749-0774.2007.00044.x. [DOI] [PubMed] [Google Scholar]
- 28.Leng Y, Liu Y, Fang X, Li Y, Yu L, Yuan Y, Wang Z (2014) The mitochondrial DNA 10197 G > A mutation causes MELAS/Leigh overlap syndrome presenting with acute auditory agnosia. Mitochondrial DNA [DOI] [PubMed]
- 29.Yabuuchi A, Beyhan Z, Kagawa N, Mori C, Ezoe K, Kato K, et al. Prevention of mitochondrial disease inheritance by assisted reproductive technologies: prospects and challenges. Biochim Biophys Acta. 2012;1820:637–642. doi: 10.1016/j.bbagen.2011.10.014. [DOI] [PubMed] [Google Scholar]