Abstract
Purpose
The Fas-Fas Ligand interaction is one of the essential events for the induction of apoptosis whereas the exact role of their soluble forms in the reproductive system is still not fully understood. Also oxidative stress in the pathogenesis of infertility causing diseases in women and has been suggested as one of the important factors that negatively affect IVF outcome. In this study, our aim was to evaluate serum and follicular fluid levels of soluble Fas soluble Fas Ligand, malondialdehyde, superoxide dismutase and total antioxidant capacity in patients undergoing IVF and compared with controls.
Methods
This study included 109 patients. Patients were classified as unexplained infertility (N = 31), PCOS (N = 19), tubal factor (N = 9) and endometriosis (N = 10) and compared with male factor infertility (N = 40) that was the control group. sFas and sFasL levels were measured by immunoassay method. MDA, SOD and TAC levels were measured by colorimetric method.
Results
Patients with unexplained infertility, PCOS and tubal factor had significantly lower sFas levels compared with their controls (respectively, p < 0.01, p < 0.05, p < 0.05). However, SOD activity in unexplained infertility, PCOS and endometriosisgroupswere significantly higher than control group (p < 0.01).Decreased follicular fluid TAC levels were found in all patient groups compared with controls (respectively, p < 0.01, p < 0.05, p < 0.01, p < 0.01).Patients with tubal factor had significantly higher serum sFasL (p < 0.05), but lower follicular fluid sFasL levels (p < 0.05) compared with unexplained infertility. Tubal factor and endometriosis groups had lowerfollicular fluid TAC levels compared to unexplained infertility and PCOSgroups (p < 0.01).
Conclusion(s)
In this study, serum and follicular fluid sFas levels were decreased and antioxidant activity was impaired in infertility, possibly implying increased apoptosis. Especially in unexplained infertility group changes in this parametres more remarkable.
Keywords: sFas, sFasL, Oxidative stress, Antioxidant, IVF
Introduction
Apoptosis or programmed cell death is the co-ordinated collapse of cell with protein degradation, DNA fragmentation, followed by rapid engulfment through neighboring cells [1]. Apoptotic changes in follicular fluid or in follicular cells can influence IVF outcome. Follicular fluid provides an important microenvironment for the proper development of oocytes. It contains a complex mixture of proteins and other factors, which can influence their subsequent developmental potential [2, 3]. Fas and Fas ligand (FasL) are membrane proteins that exist in both transmembrane and soluble forms. The former triggers apoptosis when bound by FasL, whereas the latter inhibits Fas mediated apoptosis by preventing death signal transduction [4]. Soluble Fas (sFas) is detected in human serum and fluids of the reproductive system, including seminal plasma, oviductal fluid and follicular fluid [5]. Presence of apoptotic and proliferative factors in both follicular fluid and serum samples of women undergoing IVF cycles further confirms apoptosis as a regulatory mechanism for oocyte maturation and survival [6].
Study of oxidative stress in reproductive diseases is also growing science. Recently, presence of various oxidant/antioxidant systems in a number of reproductive tissues has sparked an interest in studying the relationship of oxidative stress parameters with different female infertility [7]. Strong evidence implicated oxidative stress in the pathogenesis of infertility causing diseases in women has been suggested as one of the most important factors that negatively affect artificial reproduction technique outcome [8–10]. The presence of reactive oxygen species (ROS) in reproductive tissues, peritoneal fluid in endometriosis patients and follicular fluid of patients undergoing IVF has been documented [7, 11]. Antioxidant enzymes such as superoxide dismutase could be beneficial in enchancing implantation and maintaining pregnancy by antagonizing the harmful oxygen free radicals [12].
Superoxide dismutase enzyme is believed to play a major role in the first line of antioxidant defence by catalyzing the dismutation of superoxide anion radicals to form hydrogen peroxide (H2O2) and molecular oxygen [13]. Lipid peroxidation (LPO) is a normal phenomenon that occurs continuously at low levels of in all humans [8]. Uncontrolled peroxidation can damage enzymes, lipids, proteins and cell membranes and results in cell injury and death [14]. One of the several consequences of ROS production in the ovary is damage to the plasma membranes through LPO of polyunsaturated fatty acids [15–17]. ROS levels and LPO in follicular fluid of the developing oocyte have been suggested as markers of embryo quality in IVF [17]. Oyawoye et al. assessed the stability and role of non-enzymatic antioxidants in the follicular fluid (FF) of women undergoing IVF-embryo transfer by measuring antioxidant consumption and total antioxidant capacity (TAC) in follicular fluid. The mean TAC in follicular fluid from follicles containing oocytes did not differ from that of empty follicles. Fertilized oocytes had significantly higher TAC in their follicular fluid than non-fertilized ones [12].
In this study, we aimed to examine serum and FF levels of sFas and soluble Fas Ligand (sFasL) as a apoptotic markers, lipid peroxidation (MDA), superoxide dismutase (SOD) and total antioxidant capacity (TAC) as a oxidative stress markers in patients undergoing IVF and compared with controls.
Materials and methods
The study performed with patients attending Turgut Ozal University IVF Clinic between April 2011 and June 2012. A total of 109 patients were enrolled in this study. The inclusion criteria were being <37 years old, having basal FSH levels <12 IU/I and diagnosis of unexplained infertility (N = 31), polycystic ovarian syndrome (PCOS) (N = 19), tubal factor (N = 10), endometriosis (stage I and II) (N = 9) and male factor infertility (control group) (N = 40). Exclusion criteria were poor ovarian response, hypogonodotropic hypogonodism and severe endometriosis according to the revised American Fertility Society stage III and IV. All patients were informed about the study and signed a written informed consent. Consent forms and protocols were approved by the Local Ethic Committee.
Controlled ovarian stimulation protocol
All IVF patients underwent controlled ovarian stimulation by recombinant human follicule stimulating hormone (rec FSH) or human menopausal gonadotropin (hMG) daily for 7–8 consecutive days and follicular development was monitored by serial transvaginal ultrasonography and serum E2 concentrations. Two hundred fifty microgram of recombinant human chorionic gonadotropin (hCG, Ovitrel; Serono) injection was given to trigger the final stages of oocyte maturation and ultrason-guided oocyte pick-up was performed 35–36 h later.
The intracytoplasmic sperm injection (ICSI) procedure was performed 4–6 h after oocyte aspiration for all mature oocytes. Fertilization and cleavage was assessed daily and the embryos were classified according to their morphological appearance. Embryos were transfered at day 2, 3 or 5. The luteal phase was supported with a daily 8 % of progesterone gel (Crinone; Serono) starting on the day of oocytr retrieval. Pregnancy was assesed 12 days after embriyo transfer by analyzing the serum β-hCG. Clinical pregnancy was was defined by the presence of gestational sac by transvaginal ultarasonography examination after 5–7 weeks embriyo transfer.
Serum and follicular fluid collection and analysis of markers
Blood samples were taken just before oocyte pick-up. FF samples were aspirated from mature follicles (≥14 mm diameter) at the time of transvaginal ultrason-guided oocyte pick-up. Blood and FFwere processed immediately after collection. Blood samples were centrifuged at 4000 rpm 10 min and supernatant was used for total cholesterol, HDL cholesterol, VLDL cholesterol and triglyserid levels analysis and then aliquoted and stored at -70 °C until assayed for serum sFas, sFasLigand, MDA, SOD and TAC measurements. All FFbelonging to mature follicles were collected and pooled every patient. FF was centrifuged at 3000 rpm for 7 min to separate cellular cantents and debris and supernatant was aliquoted and stored at −70 °C until assayed.
Total cholesterol, HDL-cholesterol, VLDL cholesterol and triglyserid were measured spectrophotometrically on the Roche Cobas Integra 400 analyzer by using commercial kit (Roche, Mannheim, Germany). Concentration of serum LDL-cholesterol was calculated by the standard Friedewald formula. Serum FSH, E2 and TSH concentrations were measured using Siemens enzyme immunometric assay kit on the ADVIA centeaur CP analyzer.
sFas measurement
sFas levels in serum and follicular fluid were measured by using eBioscience human sAPO-1/Fas ELISA kit. Standart/serum sample follicular fluid sample (100 μL) were pipetted into each well of an assay plate. Fifty microliter biotin-conjugate was added to each well and then incubated for 1 h at 37 °C. Each well was aspirated and washed by 200 μL by wash buffer at three times. One hundred microliter Streptavidin-HRP was added to each well, then incubated for 1 h at 37 37 °C. Each well was aspirated and washed by 200 μL by wash buffer at three times. One hundred microliter TMB Substrate was added to each well, then incubated for about 10 min at room temperature. After stopping the reaction, optical density at 450 nm of each well was measured using Standard microplate reader, and a blank well was set as zero. According to the standard concentrations and corresponding optical density values, the standard curve was drawn. The corresponding sample concentrations were then determined by comparing the optical density values of samples to the standard curve. The intra-assay CV is 3.8 %.
sFas ligand measurement
sFas Ligand levels in serum and follicular fluid were measured by using eBioscience human sFasL ELISA kit. Standart/serum sample/follicular fluid sample (100 μL) were pipetted into each well of an assay plate. Fifty microliter biotin-conjugate was added to each well and then incubated for 2 h at room temperature. Each well was aspirated and washed by 200 μL by wash buffer at three times. One hundred microliter Streptavidin-HRP was added to each well, then incubated for 1 h at room temperature on a microplate shaker set at 100 rpm. Each well was aspirated and washed by 200 μL by wash buffer at three times. One hundred microliter TMB Substrate was added to each well, then incubated for about 10 min at room temperature. After stopping the reaction, optical density at 450 nm of each well was measured using Standard microplate reader, and a blank well was set as zero. According to the standard concentrations and corresponding optical density values, the standard curve was drawn. The corresponding sample concentrations were then determined by comparing the optical density values of samples to the standard curve. The intra-assay CV is 4.6 %.
MDA measurement
Bioxytech MDA-586 Spectrometric Assay kit for malondialdehyde is widely used as indicator of lipid peroxidation. The MDA-586 method is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole(NMPI) with MDA at 45 37 °C to form an colored carbocyanine dye with a maximum absoryion at 586 nm. In brief, 10 μL probucal was added each assay tube. Standart/serum sample/ follicular fluid sample (200 μL) were pipetted into each assay tube. Six hundred fory microliter diluted NMPI was added to each tube. After vortexing each tube, 150 μL concentrated hydrochloric acid was added and incubated at 45 °C for 60 min. Turbid samples were centrifuged at 10.000 g for 10 min to obtain a clear supernatant. The clear supernatant was transfered to a cuvette and the absorbance was measured at 586 nm against reference blank. The MDA is expressed as μM. The intra-assay CV is 3.9 %.
SOD measurement
Cayman’s Superoxide Dismutase Assay Kit utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. Ten microliter standart/serum sample/ follicular fluid sample and 200 μL diluted Radical Detector were added each well. Reactions were initiated by adding 20 μL of diluted Xanthine Oxidase to all the wells. The plate was incubated on a shaker for 20 min at room temperature. Absorbance was read at 440–460 nm using a plate reader. SOD activity of the samples was calculated the equation obtained from the linear regression of the standart curve substituting the linearized rate for each sample. The intra-assay CV is 3.9 %.
TAC measurement
Serum and follicular fluid total antioxidant capacity (TAC) levels were determinedspectrophotometrically according to 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) decolorization assay by using Rel Assay Diagnostics, Total Antioxidant Status Assay kit. Thirty microliter standart/serum / follicular fluid sample were added in eppendorf tube. Five hundred microliter assay buffer (Reagent 1) were added and first absorbance was mesured at 660 nm. After measurement of first absorbance 75 μL colored ABTS Radical solution (Reagent 2) were added, incubated 10 min at room temperature and second absorbance was measured at 660 nm. The intra-assay CV is 4.4 %.
Statistical analysis
A minimum sample of n = 105 was calculated to detect a difference of approximately 40 % between controls and study group patients with power of 0.9 and alpha at 0.05. All results are expressed as means ± standard error. Statistical analysis were performed by using SPSS packed programme (version 18 software, SPSS Inc. Chicago, Illionis, USA). One way analysis of variance was used in significance of differences between the groups. Student’t t-test was used to compare the results between independent groups. The relationship between variables with the Pearson coefficient is viewed. All statistical analysis the value of p < 0.05 was considered statistically significantand Pearson chi-squared test for categorical variables,where applicable.
Results
Clinical characteristics of study group are shown in Table 1. There were no significant differences in age, body mass index (BMI), duration of infertility, basal serum FSH, E2and TSH levels. There was not found any significant difference between total patient group and control group according to serum lipids. Cycle characteristics and treatment outcomes of the study group are shown in Table 2. Total dose of gonadotropinused, E2 levels on the day of HCG administration, endometrium thickness, number of oocyte retrieved,number of mature oocyte, fertilization rate,number of embryo transfered were similar between total patient group and control group. In the present study, cycle characteristics and clinical pregnancy rates showed no significantly difference between groups.
Table 1.
Clinical characteristics of study group
| Control group N = 40 | Total patient group N = 69 | p value | |
|---|---|---|---|
| Age | 29.3 ± 3.0 | 30.6 ± 3.5 | p > 0.05 |
| Duration of infertility (years) | 4.0 ± 2.7 | 5.3 ± 2.3 | p > 0.05 |
| BMI (kg/m2) | 23.8 ± 3.1 | 23.4 ± 2.8 | p > 0.05 |
| FSH (mIU/mL) | 7.9 ± 2.6 | 7.7 ± 2.2 | p > 0.05 |
| E2 (pg/mL) | 51.0 ± 24.3 | 56.4 ± 31.9 | p > 0.05 |
| TSH (uIU/mL) | 2.3 ± 1.1 | 3.3 ± 5.6 | p > 0.05 |
| T-Cholesterol(mg/dL) | 160.1 ± 25.1 | 160.2 ± 23.1 | p > 0.05 |
| HDL-Cholesterol(mg/dL) | 59.6 ± 18.1 | 56.2 ± 14.3 | p > 0.05 |
| LDL-Cholesterol (mg/dL) | 84.3 ± 23.23 | 91.7 ± 22.1 | p > 0.05 |
| VLDL-Cholesterol(mg/dL) | 16.1 ± 8.1 | 18.5 ± 9.6 | p > 0.05 |
| Triglyceride (mg/dL) | 82.2 ± 40.63 | 91.3 ± 46.7 | p > 0.05 |
Data were expressed as mean ± standard error
Table 2.
Cycle characteristics and treatment outcomes of the study group
| Control group n = 40 | Total patient Group n = 69 | p value | |
|---|---|---|---|
| Total dose of gonadotropin used (IU) | 1793.7 ± 600.7 | 2046.7 ± 833.4 | p > 0.05 |
| E2 levels on the day of HCG administration (pg/mL) | 1727.9 ± 1115.3 | 1987.8 ± 1021.7 | p > 0.05 |
| Endometrium thickness (mm) | 9.4 ± 2.4 | 9.7 ± 2.6 | p > 0.05 |
| No of oocyte retrieved | 9.3 ± 4.3 | 8.7 ± 4.1 | p > 0.05 |
| No of mature oocyte | 6.8 ± 3.3 | 6.4 ± 3.3 | p > 0.05 |
| Fertilization rate (%) | 79.4 ± 17.4 | 82.7 ± 19.6 | p > 0.05 |
| No of embryo transfered | 1.3 ± 0.6 | 1.2 ± 0.4 | p > 0.05 |
| Clinical pregnancy rate (%) | 50 (20/40) | 36.2 (25/69) | p > 0.05 |
Data were expressed as mean ± standard error
Our data showed that there was not found any significant difference between control group and total patient group in the comparison of serum/follicular fluid sFas, sFasL and serum SOD levels (p > 0.05) (Table 3). Both serum MDA and TAC levels were higher (respectively, p < 0.05, p < 0.01), follicular fluid MDA and TAC levels were lower in patient group (p < 0.01). Follicular fluid SOD levels in patient group were significantly higher when compared to control group (p < 0.05).
Table 3.
Comparison of serum and follicular fluid levels of sFas, sFas Ligand, MDA, SOD and TAC in the study group
| Control group (n = 40) | Total patient group (n = 69) | |
|---|---|---|
| Serum sFas (pg/mL) | 2.90 ± 0.91 | 2.86 ± 0.61 |
| Follicular fluid sFas (pg/mL) | 2.70 ± 0.61 | 2.54 ± 0.64 |
| Serum FasL (ng/mL) | 3.50 ± 1.34 | 3.71 ± 1.57 |
| Follicular fluid FasL (ng/mL) | 3.28 ± 1.40 | 3.36 ± 1.64 |
| Serum MDA (μM) | 3.99 ± 0.72 | 4.64 ± 1.68* |
| Follicular fluid MDA (μM) | 3.47 ± 0.30 | 3.26 ± 0.28** |
| Serum SOD (U/mL) | 3.47 ± 0.68 | 3.57 ± 0.94 |
| Follicular fluid SOD (U/mL) | 3.14 ± 0.91 | 4.55 ± 1.46* |
| Serum TAC (mmol/L) | 1.50 ± 0.48 | 1.85 ± 0.60** |
| Follicular fluid TAC (mmol/L) | 1.31 ± 0.63 | 0.82 ± 0.23** |
Data were expressed as mean ± standard error
*p < 0.05, ** p < 0.01
We found positive correlations between sFas in FF and and the number of oocytes retrieved, fertilization rate and number of total embryos in unexplained infertility women. There was observed positive correlation between MDA in serum and number of total embryos in this patient group (Table 4).
Table 4.
Correlation coefficients between different cycle parameters and serum/follicular fluid concentrations of measured parameters in women with unexplained infertility
| No. of oocytes retrieved | Fertilization rate(%) | No. of total embryos | |
|---|---|---|---|
| Serum sFas | 0.222 | 0.067 | 0.074 |
| Follicular fluid sFas | 0.483** | 0.453** | 0.493** |
| Serum sFasL | −0.203 | −0.078 | −0.135 |
| Follicular fluid sFasL | −0.028 | −0.080 | −0.048 |
| Serum MDA | 0.234 | 0.302 | 0.385* |
| Follicular fluid MDA | 0.186 | 0.114 | 0.156 |
| Serum SOD | −0.062 | −0.102 | −0.201 |
| Follicular fluid SOD | −0.185 | −0.124 | −0.167 |
| Serum TAC | 0.218 | 0.173 | 0.193 |
| Follicular fluid TAC | −0.283 | −0.310 | −0.228 |
* Correlation is significant for p < 0.05
** Correlation is significant for p < 0.01
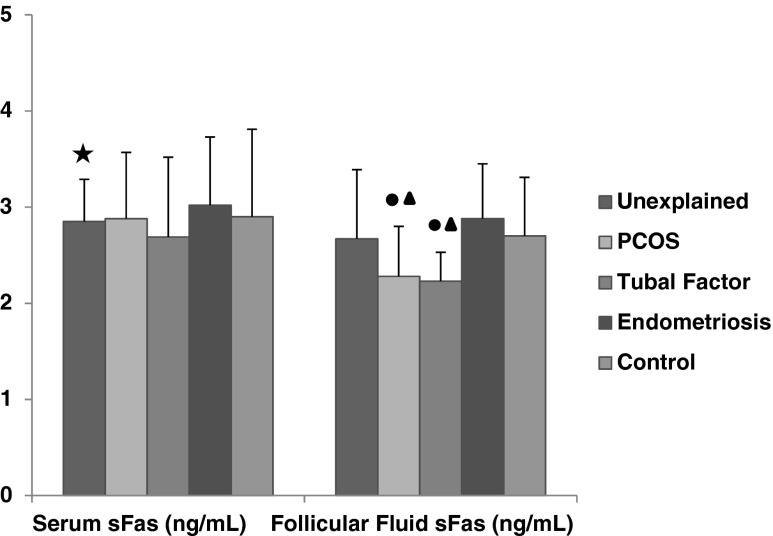
Serum sFas levels of unexplained group (2.85 ± 0.44 pg/mL) and follicular fluid sFas levels in patients with PCOS (2.28 ± 0.52 pg/mL) and tubal factor (2.23 ± 0.30 pg/mL) had significantly lower than control group (for serum 2.90 ± 1.01 pg/mL, for FF 2.70 ± 0.61 pg/mL) (p < 0.01, p < 0.05, p < 0.05 respectively). In patients with endometriosis, follicular fluid sFas levels (2.88 ± 0.57 pg/mL) were higher when compared to tubal factor (2.23 ± 0.30 pg/mL) and PCOS group (2.28 ± 0.52 pg/mL) (p < 0.01, p < 0.01, respectively) (Fig. 1).
Fig. 1.
The comparison of serum and follicular fluid sFas concentrations between patients groups and control group. Black star a significant difference from control group (p < 0.01). Black circle a significant difference from control group(p < 0.05). Black up-pointing triangle a significant difference fromendometriosis group (p < 0.01)
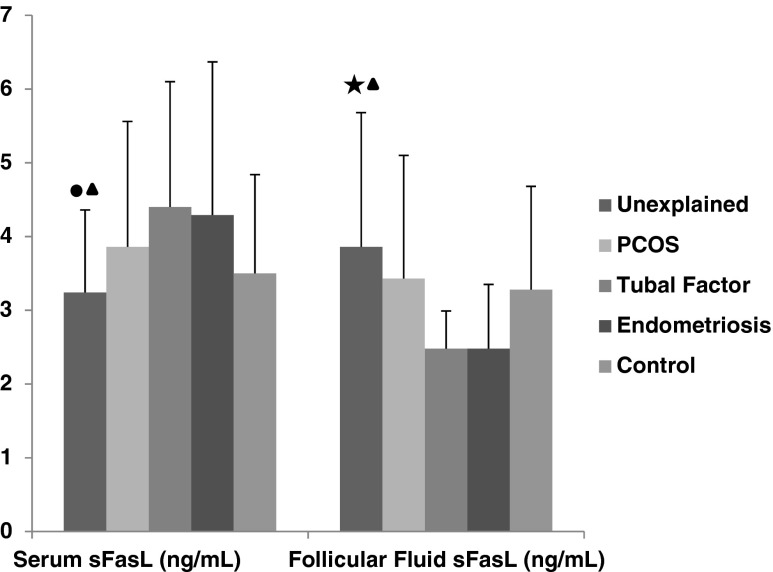
Tubal factor group had significantly higher serum sFasL (4.40 ± 1.70 ng/mL) but lower follicular fluid sFasL (2.48 ± 0.51 ng/mL) when compared to unexplained group (serum 3.24 ± 1.11 ng/mL, follicular fluid 3.86 ± 1.82 ng/mL) (p < 0.05, p < 0.05, respectively). Moreover, serum sFasL (3.24 ± 1.12 ng/mL) was found lower but follicular fluid sFasL levels (3.87 ± 1.83 ng/mL) were higher in unexplained group when compared to endometriosis group (for serum 4.29 ± 2.08 ng/mL, for FF2.48 ± 0.87 ng/mL) (p < 0.05) (Fig. 2).
Fig. 2.
The comparison of serum and follicular fluid sFas L concentrations between patients groups and control group. Black star a significant difference from tubal group (p < 0.01). Black circle a significant difference from tubal group (p < 0.05). Black up-pointing triangle a significant difference from endometriosis group (p < 0.05)
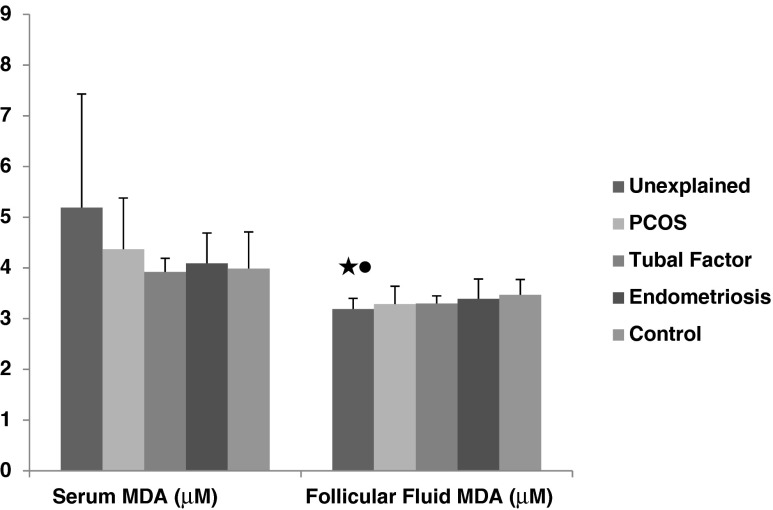
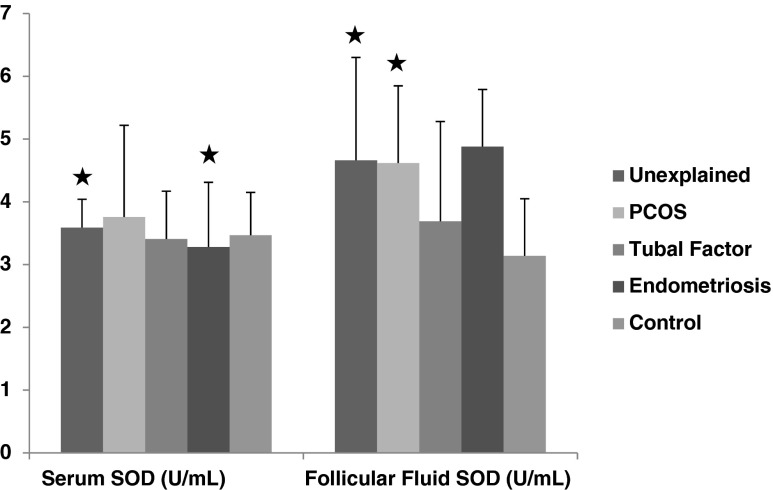
In patients with unexplained infertility had lower follicular fluid MDA levels (3.19 ± 0.21 μM) than control group (3.47 ± 0.30 μM) and endometriosis (3.39 ± 0.39 μM) (p < 0.01, p < 0.05, respectively) (Fig. 3). Serum and follicular fluid SOD levels (for serum 3.59 ± 0.45 U/mL, for FF 4.66 ± 1.64 U/mL) in unexplained infertility was found significantly higher than control group (for serum 3.47 ± 0.68 U/mL, for FF 3.14 ± 0.91 U/mL) (p < 0.01, p < 0.01, respectively). Similarly follicular fluid SOD levelsin PCOS (4.62 ± 1.23 U/mL) and endometriosis (4.88 ± 0.91 U/mL) were higher than control group (PKOS 3.14 ± 0.90 U/mL, endometriosis 3.14 ± 0.90 U/mL) (p < 0.01, p < 0.01, respectively) (Fig. 4).
Fig. 3.
The comparison of serum and follicular fluid MDA concentrations between patients groups and control group. Black star a significant difference from control group (p < 0.01). Black circle a significant difference fromendometriosis group (p < 0.05)
Fig. 4.
The comparison of serum and follicular fluid SOD concentrations between patients groups and control group. Black star a significant difference from control group (p < 0.01)
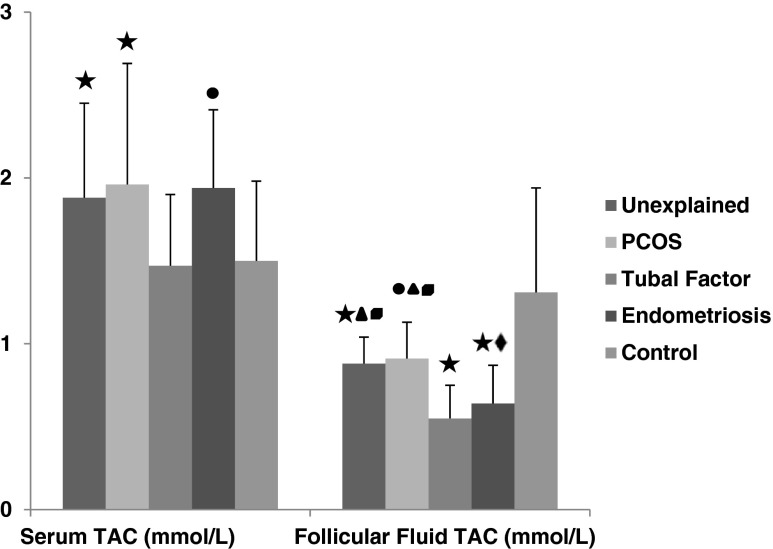
Signicant decrease of follicular fluid TAC levels were observed in unexplained(0.88 ± 0.16 mmol/L), PCOS 0.91 ± 0.22 mmol/L), tubal factor (0.55 ± 0.20 mmol/L) and endometriosis (0.64 ± 0.23 mmol/L) group when compared to controls (1.31 ± 0.63 mmol/L) (p < 0.01, p < 0.05, p < 0.01, p < 0.01, respectively) but serum TAC levels were higher in unexplained (1.88 ± 0.57 mmol/L), PCOS (1.96 ± 0.73 mmol/L) endometriosis (1.94 ± 0.47 mmol/L) (p < 0.01, p < 0.01, p < 0.05, respectively) than control (1.50 ± 0.48 mmol/L) (Fig. 5). There was not found any significant difference in serum TAC levels of tubal factor (1.47 ± 0.43 mmol/L) and control group (1.50 ± 0.48 mmol/L) . In patients with tubal factor, levels of follicular fluid TAC were lower (0.55 ± 0.20 mmol/L) than PCOS (0.91 ± 0.22 mmol/L) and unexplained infertility group (0.88 ± 0.16 mmol/L) (p < 0.05, p < 0.01, respectively). Similarly serum TAC levels in tubal factor (1.47 ± 0.43 mmol/L) were lower than endometriosis group (1.94 ± 0.47 mmol/L) (p < 0.01). PCOS (0.91 ± 0.22 mmol/L) and unexplained infertility (0.88 ± 0.16 mmol/L) had higher follicular fluid TAC levels when compared to endometriosis (0.64 ± 0.23 mmol/L) group (p < 0.01) (Fig. 5).
Fig. 5.
The comparison of serum and follicular fluid TAC concentrations between patients groups and control group. Black star a significant difference from control group (p < 0.01). Black circle a significant difference from control group (p < 0.05). Black up-pointing triangle a significant difference from tubal group (p < 0.01). Black diamond a significant difference from tubal group (p < 0.05). Black square a significant difference from endometriosis group (p < 0.01)
Discussion
In this study, we examined serum and FF levels of sFas and sFas Ligand as a apoptotic markers, lipid peroxidation, superoxide dismutase and total antioxidant capacity as a oxidative stress markers in patients undergoing IVF and compared with controls.
Apoptosis has emerged as a major mode of cell death in living tissues, regulating homeostasis. It is involved in many processes of reproductive physiology, e.g. follicular atresia, implantation and endometrium proliferation. It is coordinated by a number of molecules including Fas, Fas ligand, soluble (s)Fas, and bcl-2. Fas is activated by its natural ligand, FasL, which exists in a soluble and membranal form [18]. Fas mediated apoptosis requires the balancing of receptor-ligand interaction that can be modulated by the sFas that acts as a functional antagonist of FasL mediated apoptosis [19]. There are limited data about soluble apoptotic markers in IVF patients. Sarandakou et al. found diminished serum sFas levels in women undergoing IVF compare with those of healthy female controls [6]. They think that low levels in this apoptotic marker imply increased apoptosis and possibly a down-regulation of the immune system of patients. Onalan et al. reported that low levels of serum sFas in patients with PCOS compared to male factor infertility group [20]. But they didn’t found any significant difference in terms ofFF of sFas and sFasL, serum sFasL in these groups. They observed increased serum levels of sFas and decreased FF levels of sFasL observed in IVF patients receiving metformin therapy compared to those on placebo. They concluded that Metformin therapy has antiapoptotic effect in PCOS patients. In another study by the same authors, similar results were found in infertility patients due to endometriosis [21]. There was found decreased serum sFas levels in endometriosis group compared to male factor infertility group. They suggests that low levels of serum sFas may be associated with increased apoptosis in endometriosis. In contrast, there is study mentioning that serum and FF sFas levels in IVF patients varies with different patients’ diagnosis [19]. They reported that high concentration of sFas in serum and FF in patients with uterine causes of infertility compared with those having other causes of infertility. In our study, we found serum sFas levels in unexplained infertility patients, FF sFas levels in PCOS and tubal patients lower than those of control group. Moreover, endometriosis group FF sFas levels were higher than those of tubal and PCOS patients. Our findings suggest that low levels of sFas may be associated with increase of apoptotic activity in IVF patients with different causes of infertility. In a study about serum sFasL levels in women with endometriosis, elevated sFasL levels were found in patients compare with fertile women without endometriosis [22]. They think that women with endometriosis is susceptible to apoptosis more thanwomen without disease. In our study, we found significant difference between tubal and unexplained infertility, between endometriosis and unexplained infertility patients according to sFasL in serum and FF. Interestingly, we observed high serum sFasL but low FF sFasL concentrations in infertility patients with tubal and endometriosis compared to those of unexplained infertility patients, not controls.
In our study, we observed that FF sFas concentrations in patients with unexplained infertility positively correlated with the number of oocytes retrieved, fertilization rate and number of total embryos. However, Abdelmeged et al. found low levels of sFas in serum were associated with high numbers of fertilized oocytes.
Apoptosis can be initiated by oxidants [23, 24]. Following an apoptotic signal, cells sustain progressive lipid peroxidation. Thus, reactive oxygen species (ROS) have been implicated in the induction of apoptosis [25, 26] . Normally, there is equilibrium between ROS production and the ability of the antioxidants to scavenge their excessive production. High ROS production or impaired antioxidant mechanisms result in oxidative stress targeting cellular components, including cell membranes and nucleic acids [27]. One of the several concequences of ROS production in the ovary is damage to the plasma membranes through LPO of polyunsaturated fatty acids [17, 28]. In a study by Pasqualotto et al., FF lipid peroxidation in women undergoing IVF was positively correlated with the pregnancy rate [15]. They suggest that lipid peroxidation may be good marker of metabolic activity within the follicle. There is inconsistent data about lipid peroxidation in IVF patients. In a study by Chattopadhayay et al., increased MDA concentrations, as an index of lipid peroxidation were observed [29]. In contrast to this study, Prieto et al. found no difference between women with endometriosis compared with control group [30]. In our study, women undergoing IVF had higher levels of serum MDA, but lower levels of FF MDA than those of controls. The highest value in FF MDA levels was in endometriosis group, the lowest value in FF MDA levels was in unexplained group. We assumed that there was oxidant-antioxidant imbalance in IVF patients, but serum values were not parallel with the follicular fluid values.
Antioxidants are scavengers that detoxify excess ROS, which helps maintain the body’s delicate oxidant/antioxidant balance. Enzymatic antioxidants such as superoxide dismutase and glutathione oxidase as well as nonenzymatic antioxidants including vitamin A, vitamin E, zinc, and selenium are essential inmaintaining adequate levels of ROS in the cell by disposingand removing excess free radicals [31]. Strong evidence implicated oxidative stress in the pathogenesis of infertility causing diseases in women [8–10, 32]. Sabatini et al. demonstrated the presence of SOD activity in FF and serum from women undergoing IVF. SOD activity was in FF higher than in serum [33]. In prospective cohort study by Younis et al.,in patients including PCOS, endometriosis and unexplained infertility, antioxidant enzyme’s activities were measured [34]. There was statistically difference between pregnancy positive group and pregnancy negative group according to serum SOD activities. Higher SOD activities were found in pregnancy positive group than those of pregnancy negative group. Ovarian stimulation caused a significant increase in serum SOD activities in women undergoing IVF. Similarly, another study showed that good correlation between FF SOD activitiesand fertilization rate [28]. In this study, we found elevated FF SOD activities in infertility patients compare with controls. In PCOS and endometriosis patients, FF SOD activities were increased compared to control group. Specially, increased serum and FF SOD activities were found in patients with unexplained infertility in our study. It is possible that this increase can be to maintain oxidant-antioxidant balance in IVF patients.
The imbalance that leads to oxidative stress may be due to decreased TAC as well as to increased ROS levels [35–38]. The sum of levels of non-enzymatic antioxidants called as total antioxidant activity or TAC may be indicative of the extent of oxidative stress. Pasqualotto et al. reported that decline in FF TAC concentration in nonpregnant women compare with pregnant women undergoing IVF [28]. In earlier study by the same authors, they found lower serum TAC concentrations than FF TAC concentrations in IVF patients. Some authors suggested that impaired embryo development may be associated with an elevated ROS production and for neutralizethem reduction of TAC concentrations [39]. Appasamy et al. were found no significant relationship between plasma or FF TAC levels and the underlying etiology of infertility in IVF patients [40]. Our results conflict with their findings. In this study, signicant decrease in FF TAC levelswere observed in IVF patients compared with controls. Surprisingly, there was found elevation in serum TAC levels between patients and control group. In infertility groups except tubal, serum TAC levels higher than those of controls. FF TAC levels are lower in all patients compared to control group. FF TAC concentrations in tubal and endometriosis infertility patients, serum TAC concentrations in tubal infertility patients were lowest in patient groups. Finally, our results indicate that presence of oxidative stress in FF of patients with different causes of infertility.
These findings support the idea that serum and follicular fluid sFas levels were decreased and antioxidant activity was impaired in infertility, possibly implying increased apoptosis. Especially in unexplained infertility group changes in this parameters more remarkable. In spite of small number of participants, the present study is one of the limited number of studies evaluating apoptosis in infertility patients with different diagnosis. Larger studies are warranted to explore possible association between apoptosis and infertility.
Acknowledgments
The work was supported by Gazi University Research Foundation (Project no. 02/2011-36). Our sincere thanks are due to Assoc. Prof. İ.Safa Gürcan, Department of Biometry, Veterinary Faculty, Ankara University for the assistance in data analysis.
Conflict of interest
No conflict of interest.
Footnotes
Capsule Decreased serum/follicular fluid sFas and impaired antioxidant activity implies possible increased apoptosis in infertility. Especially in unexplained infertility, changes in this parameters are more remarkable.
References
- 1.von Rango U, Classen-Linke I, Krusche CA, Beier HM. The receptive endometrium is characterized by apoptosis in the glands. Hum Reprod. 1998;13:3177–3189. doi: 10.1093/humrep/13.11.3177. [DOI] [PubMed] [Google Scholar]
- 2.Malamitsi-Puchner A, Sarandakou A, Baka S, et al. Concentrations of angiogenic factors in follicular fluid and oocyte-cumulus complex culture medium from women undergoing in vitro fertilization. Association with oocyte maturity and fertilization. Fertil Steril. 2001;76:98–101. doi: 10.1016/S0015-0282(01)01854-4. [DOI] [PubMed] [Google Scholar]
- 3.Malamitsi-Puchner A, Sarandakou A, Baka S, Hassiakos D, Kouskouni E, Creatsas G. In vitro fertilization: angiogenic, proliferative and apoptotic factors in follicular fluid. Ann NY Acad Sci. 2003;997:124–128. doi: 10.1196/annals.1290.043. [DOI] [PubMed] [Google Scholar]
- 4.Ueno T, Toi M, Tominaga T. Circulating soluble Fas concentration in breast cancer patients. Clin Cancer Res. 1999;5:3529–3533. [PubMed] [Google Scholar]
- 5.Srivastava M, Lippes J, Pichorova R, De los Santos, Anderson DJ. Soluble Fas (sFas) and soluble Fas ligand (sFasL) in human reproductive tract fluids. In: Proceedings of the 45th Annual Meeting of the Society for the Gynecologic Investigation, Chicago; 1998. p.T203[abstract].
- 6.Sarandakou A, Malamitsi-Puchner A, Baka S, Rizos D, Hassiakos D, Creatsas G. Apoptosis and proliferation factors in serum and follicular fluid from women undergoing in vitro fertilization. Fertil Steril. 2003;79:634–636. doi: 10.1016/S0015-0282(02)04802-1. [DOI] [PubMed] [Google Scholar]
- 7.Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Semin Reprod Endocrinol. 1998;16(4):263–273. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86(3):503–512. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 10.Marsillach J, Checa MA, Pedro-Botet J, Carreras R, Joven J, Camps J. Paraoxonase-1 in female infertility: a possible role against oxidative stress-induced inflammation. Fertil Steril. 2010;94(3):1132–1134. doi: 10.1016/j.fertnstert.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Attaran M, Pasqualotto E, Falcone T, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45(5):314–320. [PubMed] [Google Scholar]
- 12.Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18(11):2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- 13.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244(22):6056–6063. [PubMed] [Google Scholar]
- 14.Agarwal A, Said TM. Oxidative stres, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95(4):503–537. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- 15.Pasqualotto EB, Agarwal A, Sharma RK, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81(4):973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Wiener-Megnazi Z, Vardi L, Lissak A, et al. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Das S, Chattopadhyay R, Ghosh S, et al. Reactive oxygen species level in follicular fluid-embryo quality marker in IVF? Hum Reprod. 2006;21(9):2403–2407. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- 18.Mor G, Straszewski S, Kamsteeg The Fas/Fas ligand system in reproduction: survival and apoptosis. The Sci World J. 2002;2:1828–1842. doi: 10.1100/tsw.2002.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelmeged AN, Yasser IA, Elmoghazi DE, Eissa MK. Evaluation of sFas in serum and follicular fluid during ovarian stimulation for assisted reproduction. Middle East Fertil Soc J. 2011;16(1):50–55. doi: 10.1016/j.mefs.2010.12.012. [DOI] [Google Scholar]
- 20.Onalan G, Selam B, Baran Y, et al. Serum and follicular fluid levels of soluble Fas, soluble Fas ligand and apoptosis of luteinized granulosa cells in PCOS patients undergoing IVF. Hum Reprod. 2005;20(9):2391–2395. doi: 10.1093/humrep/dei068. [DOI] [PubMed] [Google Scholar]
- 21.Onalan G, Selam B, Onalan R, Geyhan T, Cincik M, Pabuccu R. Serum and follicular fluid levels of soluble Fas and soluble Fas ligand in IVF cycles. Eur J Obstet Gynecol Reprod Biol. 2006;125:85–91. doi: 10.1016/j.ejogrb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Velasco JA, Mulayim N, Kayisli UA, Arici A. Elevated soluble Fas ligand levels may suggest a role for apoptosis in women with endometriosis. Fertil Steril. 2002;78(4):855–859. doi: 10.1016/S0015-0282(02)03320-4. [DOI] [PubMed] [Google Scholar]
- 23.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- 24.Bojes HK, Datta K, Xu J, et al. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem J. 1997;325:115–119. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimmeler S, Haendeler J, Sause A, Zeiher AM. Nitric oxide inhibits APO-1/Fas-mediated cell death. Cell Growth Differ. 1998;9:415–422. [PubMed] [Google Scholar]
- 26.Tamarit J, Cabiscol E, Ros J. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem. 1998;273:3027–3032. doi: 10.1074/jbc.273.5.3027. [DOI] [PubMed] [Google Scholar]
- 27.Hunt CR, Sim JE, Sullivan SJ, et al. Genomic instability and catalase gene amplifcation induced by chronic exposure tooxidative stress. Cancer Res. 1998;58:3986–3992. [PubMed] [Google Scholar]
- 28.Pasqualotto EB, Lara LV, Salvador M, Sobreiro BP, Borges E, Pasqualotto FF. The role of enzymatic antioxidants detected in the follicular fluid and semen of infertile couples undergoing assisted reproduction. Hum Fertil (Camb) 2009;12(3):166–171. doi: 10.1080/14647270903207941. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN, Chaudhury K. Effect of follicular fluid oxidative stres on meiotic spindle formation in infertile women with polycyctic ovarian syndrome. Gynecol Obstet Invest. 2010;69:197–202. doi: 10.1159/000270900. [DOI] [PubMed] [Google Scholar]
- 30.Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril. 2012;98(1):126–130. doi: 10.1016/j.fertnstert.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(8):1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- 32.Chandra A, Surti N, Kesavan S, Agarwal A. Significance of oxidative stress in human reproduction. Arch Med Sci. 2009;5(1A):S28–S42. [Google Scholar]
- 33.Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril. 1999;72(6):1027–1034. doi: 10.1016/S0015-0282(99)00411-2. [DOI] [PubMed] [Google Scholar]
- 34.Younis A, Clower C, Nelsen D, et al. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. Assist Reprod Genet. 2012;29(10):1083–1089. doi: 10.1007/s10815-012-9831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Foote RH, Simkin M. Development of rabbit zygotes cultured in protein-free medium with catalase, taurine, or superoxide dismutase. Biol Reprod. 1993;49:33–37. doi: 10.1095/biolreprod49.1.33. [DOI] [PubMed] [Google Scholar]
- 36.Gönenç A, Hacışevki A, Tavil Y, Çengel A, Torun M. Oxidative stress in patients with essential hypertension: a comparison of dippers and non-dippers. Eur J Int Med. 2013;24:139–144. doi: 10.1016/j.ejim.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Gönenç A, Atak Y, Orman MN, Şimşek B. Lipid peroxidation and antioxidant systems in hemodialyzed patients. Dial Transplant. 2002;31:88–96. [Google Scholar]
- 38.Gönenç A, Hacışevki A, Griffiths HR, Torun M, Bakkaloğlu B, Şimşek B. Free radical reaction products and antioxidant capacity in beating heart coronary artery surgery compared to conventional bypass. Biochem Moscow. 2011;76:677–685. doi: 10.1134/S0006297911060083. [DOI] [PubMed] [Google Scholar]
- 39.Bedaiwy M, Agarwal A, Said TM, et al. Role of total antioxidant capacity in the differential growth of human embryos in vitro. Fertil Steril. 2006;86(2):304–309. doi: 10.1016/j.fertnstert.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Appasamy M, Jauniaux E, Serhal P, Al-Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil Steril. 2008;89(4):912–921. doi: 10.1016/j.fertnstert.2007.04.034. [DOI] [PubMed] [Google Scholar]