Abstract
Purpose
Does transfer of supernatant embryo culture fluid (stimulation of endometrial embryo transfer - SEET) prior to vitrified warmed blastocyst transfer result in better clinical pregnancy and live birth rates than direct vitrified warmed blastocyst transfer?
Methods
This randomized controlled trial compared SEET group and direct transfer group (control) in 60 women undergoing vitrified warmed blastocyst transfers. The duration of the study was 3 years. The patients were undergoing vitrified warmed blastocyst transfer at university level infertility centre. Sixty women were randomized to SEET (n = 30) or control (n = 30).
Results
Data was available for analysis from all the 30 women in the SEET group and 30 women in the control group. There were no drop outs in the trial. The implantation rate was significantly lower in the SEET group compared to the control group (27 vs. 44 %, P = 0.018). The clinical pregnancy rates were similar in both the groups (47 vs. 53 %) but the live birth rate was also significantly lower in SEET group (23 vs. 50 %, P = 0.03).
Limitations
The sample size based on clinical pregnancy rates was small and hence not adequately powered to detect differences in live birth rates. Lack of blinding leading to possible bias cannot be ruled out.
Conclusion
There was no evidence of an improvement in clinical pregnancy rate following SEET in vitrified warmed blastocyst transfer compared to direct transfer.
Keywords: SEET, Supernatant embryo culture medium, Blastocyst transfer
Introduction
Successful implantation of embryos in the uterine cavity is the end result of a synchronized sequence of complex physiological events [1]. This coordinated development of the embryo and uterine endometrium, ultimately leading to an optimal environment for implantation, is possible due to the existence of a communication link between the maternal tissue and embryo, commonly known as “cross-talk”.
There is evidence to suggest that this “cross talk” is mainly carried out through secretion of various factors by the developing embryo [2]. They include interleukins [3], immunosuppressive factors [4] and growth factors which have been isolated from the culture fluid surrounding the embryo during in-vitro culture. These factors produced by the embryo induce endometrial expression [5] of integrins, leukemia inhibitory factor and other factors which possibly facilitate implantation.
Currently in Assisted Reproductive Technology (ART) practice, blastocyst stage transfer compared to cleavage stage transfer is associated with higher live birth rates [6]. Worldwide following blastocyst transfer, the implantation rate has been around 4–55 % and the rate has remained stagnant in the past decade [6]. A proposed reason for this lower implantation rate is the absence of cross-talk till the embryo is transferred [7].
With an intention to introduce cross talk prior to a delayed embryo transfer, which occurs in day 5 blastocyst transfers, Goto et al. tried a novel method called stimulation of endometrial embryo transfer (SEET). In this trial supernatant embryo culture fluid from the fresh embryo cycle, was transferred prior to a frozen blastocyst transfer in poor prognosis patients resulting in a significantly higher pregnancy rates [7]. However a similar study conducted in patients undergoing cleavage stage embryo transfer, did not show any benefits [8].
Due to conflicting nature of the results, we decided to study the effectiveness of the SEET protocol following transfer of vitrified-warmed blastocysts.
Materials and methods
We decided to evaluate whether transferring the supernatant embryo culture prior to warmed blastocyst transfer resulted in better clinical outcomes when compared to direct vitrified warmed blastocyst transfer. To test this hypothesis, we planned a randomized controlled trial and invited all consecutive women who were due for a fresh blastocyst transfer and had supernumerary embryos available for cryopreservation. For eligible women who agreed to participate in the trial, we cryopreserved the supernatant culture medium along with the supernumerary blastocysts. The study duration was three years (Sep 2011 to June 2014).
Women entering the trial were randomly distributed, using a computer generated randomization sequence (blocks of 6), into two groups – the study (SEET) and control group. The randomization sequence was generated by a statistician from the institutional biostatistics department. Allocation concealment was achieved by using consecutively numbered opaque sealed envelope. Once the women were planned for vitrified thawed transfer, the envelope was opened and group allotment was done by the investigators. In women allotted to the SEET group thawed supernatant embryo culture fluid was injected into the uterine cavity two days prior to the planned blastocyst transfer. In the control group, direct vitrified warmed blastocyst transfer was planned. The study was done at university level infertility centre in India and was approved by the institutional review board. The trial was registered with the clinical trial registry of India (CTRI/2013/01/003280).
ART protocol: Prior to the study cycle, all patients underwent a fresh ART cycle using either the agonist or antagonist protocol. Controlled ovarian hyperstimulation (COH) was done using recombinant gonadotrophins and oocyte retrieval was planned 35 h after human chorionic gonadotrophin (hCG) trigger administration. Fertilization was achieved either by In Vitro Fertilization (IVF) or Intra Cytoplasmic Sperm Injection (ICSI). Fertilized oocytes were transferred into cleavage medium (SAGE cleavage medium, Trumbull, Connecticut, USA), incubated and observed for cleavage on day 3.
On day 3, if four or more grade 1 embryos (8 cells stage with no or minimal fragmentation) were obtained, they were transferred into blastocyst medium (SAGE blastocyst medium, Trumbull, Connecticut, USA) and cultured till day 5. Fresh blastocyst transfer was performed on day 5, and supernumerary good quality blastocysts were chosen for vitrification. Embryos were graded according to Gardner’s grading system and embryos with a score of 3AA or more were considered good quality [9]. These blastocysts were cryopreserved on day 5 and day 6.
Vitrification and warming protocol
Supernumerary blastocysts were vitrified using the solid surface method. A pre-cooled metal block (Cryologic, Victoria, Australia), fibre plugs (Cryologic, Victoria, Australia) and in house prepared equilibrium and vitrification solution were used. The equilibrium solution was composed of ethylene glycol and dimethyl sulphoxide in HEPES HTF medium (Cryobase, Cook IVF, Queensland, Australia) [10].
Warming of vitrified blastocysts was done using filter sterilized trehalose solution and blastocyst medium (SAGE Blastocyst medium, Trumbull, Connecticut, USA). Survival was assessed by the percentage of viable trophoectodermal and inner cell mass cells together with the degree of re-expansion of the blastocoel cavity. Assisted hatching using laser was performed prior to transfer.
After transfer of embryos in fresh cycle, for women who had supernumerary blastocysts and who were willing to participate in the trial, the supernatant culture medium was transferred into a sterile Eppendorf container and frozen at −80 °C.
Transfer of blastocysts /supernatant culture fluid and assessment of outcome
Endometrial preparation for vitrified thawed blastocyst transfer was done by starting step-wise increment of estradiol valerate (Progynova, Schering AG, Germany; 2 mg once daily from Day 1–5, 2 mg daily twice from Day6-9 and daily three times from Day 10–15). On day 15 a transvaginal ultrasound was done to check for endometrial thickness and to rule out follicular activity. Micronized progesterone (Orgagest vaginal pessaries, Schering AG, Germany; 400 mgs daily twice) was started once endometrial thickness of greater than 7 mm was documented.
In the SEET group, 10–15 μl of thawed supernatant embryo culture medium was injected into the uterine cavity transcervically, using an embryo transfer catheter placed just beyond the internal os, two days prior to the vitrified thawed blastocyst transfer. For both the groups, the vitrified thawed blastocyst transfer was done on day 6 after initiation of progesterone.
After pre transfer counseling, between one to three surviving blastocysts were transferred. A serum beta hCG was done on the 12th day following embryo transfer. Women with a positive pregnancy test were advised to continue luteal support and a transvaginal ultrasound was carried out ten days later to confirm clinical pregnancy and viability. Once confirmed, antenatal care was provided and women were followed up till delivery. Hormonal supplementation was stopped at 12 weeks of gestation.
Clinical pregnancy rate was defined as clinical pregnancy (ultrasound visualization of gestational sac) per embryo transfer. Live birth rate was defined as number of deliveries with at least one live born baby per embryo transfer.
All enrolled women underwent the study cycle only once. The primary outcome was the clinical pregnancy rate while secondary outcomes were implantation rate, multiple pregnancy rates, miscarriage and live birth rates. It was an open label trial.
The vitrified blastocyst embryo pregnancy rate per transfer in our centre was 40 %. (Goto et al. in their study demonstrated a doubling of the clinical pregnancy rate following SEET transfer (48 to 87 %). Hypothesizing a doubling of the clinical pregnancy rate (80 %) following SEET transfer, a sample size of 30 women in each arm (80 % power and alpha 0.05 for a two sided test) was calculated.
Data obtained was analyzed using SPSS version 15 (Chicago, IL). Independent t test was used for analyzing continuous data. χ2 test and Fishers exact test was used for categorical data. The differences were considered to be significant if P <0.05. Analysis was done on an intention- to- treat basis.
Results
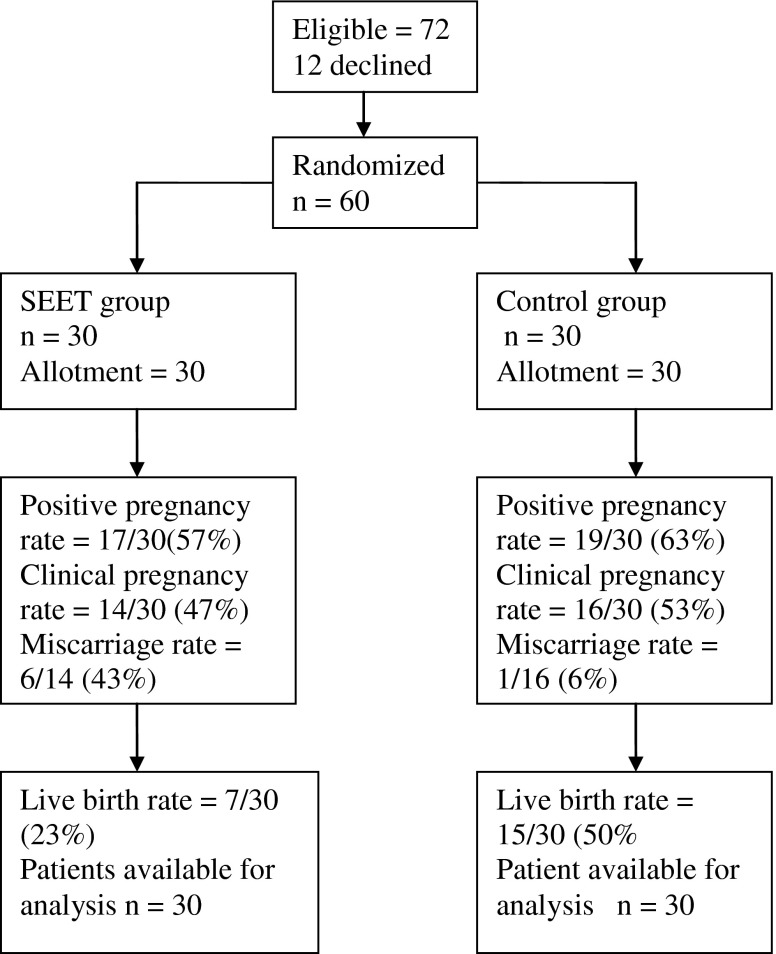
Seventy two eligible women agreed to participate in the trial. Prior to randomisation 12 women opted out of the trial. The sixty women, who were included in the trial, had either an unsuccessful fresh blastocyst transfers earlier or did not undergo a fresh transfer due to the risk of ovarian hyperstimulation (OHSS). The group also included women who had a successful outcome previously and were interested in having another child. All the women who entered the trial were available for analysis with no loss to follow up reported (30 in each group) (Fig. 1).
Fig. 1.
Consort flow chart
Baseline clinical characteristics of the SEET and control groups were analyzed and found to be similar with respect to parameters like age, body mass index (BMI), nature of infertility, duration of infertility, and previous ART attempts. There was no statistically significant difference between the two groups in the controlled ovarian stimulation protocols used, dose of gonadotrophins, estradiol levels on the day of hCG trigger, the total number of mature oocytes retrieved (in fresh cycles), and the number of fertilized oocytes (Tables 1 and 2).
Table 1.
Baseline clinical characteristics and ART cycle details of the study and control groups
| Study mean (S.D.) |
Control mean (S.D.) |
P Value | |
|---|---|---|---|
| Age (Years) | 29.9 (4.0) | 29.8 (4.3) | 0.92 |
| Body Mass Index (kg/m2) | 24.51 (3.36) | 24.49 (3.26) | 0.98 |
| Duration of infertility (years) | 7.55 (3.36) | 8.58 (3.62) | 0.26 |
| Total dose of gonadotrophins (IU) | 1914.17 (900.35) | 1792.50 (764.52) | 0.58 |
| Estradiol levels on trigger day (pg/ml) | 3282.42 (2154.68) | 2737.62 (1353.83) | 0.424 |
| Number of MII oocytes | 13.05 (5.34) | 13.53 (5.22) | 0.72 |
| Number of fertilized ooyctes | 10.59 (4.38) | 10.28 (3.64) | 0.857 |
| Number of blastocysts transferred | 2.1 (0.5) | 1.9 (0.6) | 0.186 |
| Number of good quality blastocysts transferred (≥3AA) | 1.8 (0.5) | 1.7 (0.6) | 0.502 |
Table 2.
Baseline clinical and ART cycle characteristics
| Study group | Control group | P value | ||
|---|---|---|---|---|
| Type of infertility | Primary | 14 | 19 | 0.194 |
| Secondary | 16 | 11 | ||
| Previous ART attempt | Yes | 13 | 9 | 0.284 |
| No | 17 | 21 | ||
| ART protocol | Antagonist | 13 | 10 | 0.11 |
| Long protocol | 17 | 16 | ||
| Ultralong protocol | 0 | 4 |
The mean number of blastocysts transferred in the SEET group and control group were 1.9 and 2.1 respectively and the difference was not statistically significant. The mean number of top quality blastocysts (grade 3AA and above) transferred in the SEET group (1.8) and control group (1.7) were similar.
17 (57 %) patients in the SEET group had positive pregnancy test compared to 19 (63 %) patients in the control group which was not statistically significant difference (Table 3).
Table 3.
Clinical outcomes in the study and control groups
| Study group (n = 30) | Control group (n = 30) | P value | |
|---|---|---|---|
| Positive pregnancy rate per embryo transfer | 17 (57 %) | 19 (63 %) | 0.792 |
| Clinical pregnancy rate per embryo transfer | 14 (47 %) | 16 (53 %) | 0.796 |
| *Implantation rate per embryo | 17/63 (27 %) | 25/57 (44 %) | 0.018 |
| Multiple pregnancy rate per clinical pregnancy | 3 (21 %) | 8 (50 %) | 0.095 |
| Miscarriage rate per clinical pregnancy | 6 (43 %) | 1 (6 %) | 0.103 |
| *Live birth rate per embryo transfer | 7 (23 %) | 15 (50 %) | 0.032 |
* Significant difference
Pregnancy outcomes of the two groups were compared. The clinical pregnancy rates were similar in both the groups (47 vs. 53 %; P = 0.796) (Table 3).
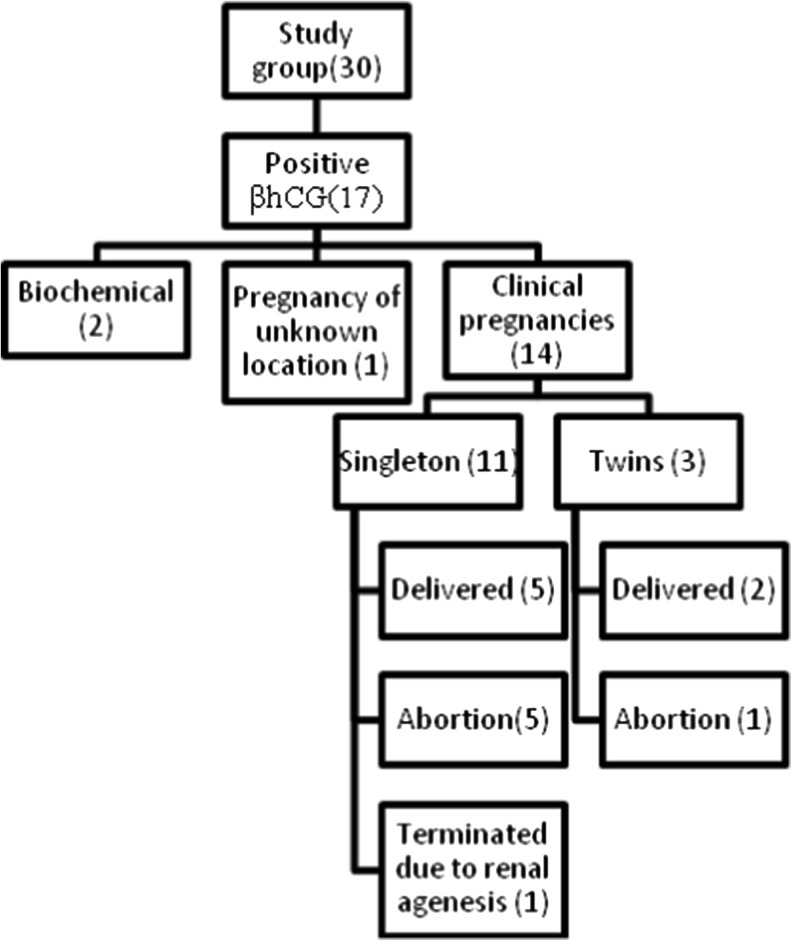
Two biochemical pregnancies were recorded in SEET group where as in the control group there were a total of three biochemical pregnancies. There was one pregnancy of unknown location in the SEET group which was managed medically (by administeri ng Inj. Methotrexate).
The implantation rate was significantly lower in the SEET group compared to the control group (27 vs. 44 %; P = 0.018).
There were 7 live births in the SEET group (23 %) and 15 live births (50 %) in the control group, the difference being statistically significant (P = 0.032). In the SEET group there were 6 miscarriages compared to 1 miscarriage in the control group (43 vs. 6 %; P = 0.103), the difference being not statistically significant. Due to a fetal anomaly (bilateral absent kidneys) there was one termination of pregnancy in the SEET group (Figs. 2 and 3).
Fig. 2.
Pregnancy outcomes in SEET group
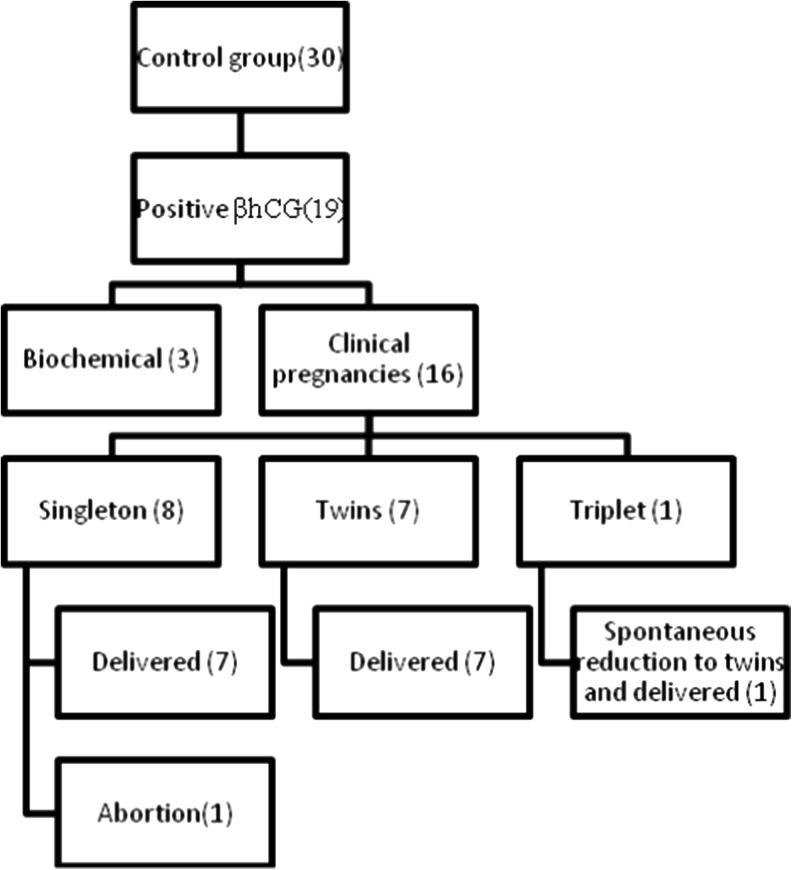
Fig. 3.
Pregnancy outcomes in control group
Multiple pregnancy rates were similar in both the groups (21 vs. 50 %; P = 0.095). In the control group, for one of the pregnancies, three gestational sacs were identified (triplet pregnancy) in the initial scan. However the pregnancy continued as a twin pregnancy due to spontaneous reduction in late first trimester and ultimately resulted in a twin delivery.
Discussion
Our trial revealed a significantly lower implantation rate and live birth rate in the group of women who had a prior transfer of supernatant embryo culture medium during the vitrified warmed blastocyst transfer cycle (SEET group) as compared to the control group which had direct embryo transfer.
Blastocyst transfers allow synchrony between the embryo and the endometrium as well as an opportunity to choose embryos with higher implantation potential. However cross talk between the embryo and the endometrium does not occur initially due to in vitro embryo culture until transfer. Goto et al. introduced this novel method of SEET as supernatant fluid is likely to contain several factors produced by the embryo, like interleukin-1 and hCG, which facilitate the cross talk and perhaps improve the implantation rate [7]. The authors conducted a randomized trial and included patients with a poor pregnancy prognosis. They found a significantly higher implantation rate (72 vs 38 %; P = 0.007) and clinical pregnancy rate (87 vs 48 %; P = 0.006) in the group of patients who had injection of embryo culture medium prior to blastocyst transfer compared to patients who had direct embryo transfer. The live birth rate was not reported and the authors suggested improvement in implantation and clinical pregnancy rates in SEET group probably due to facilitation of embryo dependent induction of endometrial receptivity. One of the limitations of the trial was the method of randomization which was based on the day of clinic visit (quasi randomization) leading to possibility of selection bias.
The same authors conducted another RCT evaluating the role of transfer of supernatant embryo culture medium in good prognosis patients who were undergoing frozen thaw embryo cycle and had no previous fresh embryo transfer (cryopreservation was done due to high risk of OHSS) [11]. To further test the embryo cross talk hypothesis, the authors decided to include an additional arm in which only culture medium was injected prior to transfer. This was done since independent effect of culture medium could not be ruled out in the previous study. The authors found a significantly higher implantation (92 vs 64 %, P = 0.03) and clinical pregnancy rates (80 vs. 56 %, P = 0.04) in the SEET group compared to the control group for patient who had good quality blastocysts. The implantation rate and pregnancy rates in patients who had only culture medium transfer prior to frozen thawed transfer was not significantly different compared to patients who had a direct embryo transfer. However as in the previous study, the authors did not report live birth rates and concluded likely hood of favorable impact of SEET in patients undergoing first time ART who have good quality blastocysts. Since all our transfer included at least one good quality blastocyst, we were unable to draw conclusions regarding the benefits of using supernatant culture medium depending upon the quality of the blastocyst transferred.
In a similar randomized trial, use of supernatant blastocyst culture medium prior to fresh blastocyst transfer was compared to direct blastocyst transfer. Similar implantation and pregnancy rates were obtained in both the groups but significantly higher live birth rates were obtained in the group which had prior transfer of supernatant culture medium (Odds ratio 4.5, CI - P = 0.001). This was mainly due to significantly higher miscarriage rate in the control group [12].
Zhu et al. evaluated the SEET effect in patients undergoing fresh cleavage stage embryos on day 3 [8]. In their randomized study, day 2 supernatant embryo culture medium was injected on day 2 for patients due for fresh embryo transfer on day 3. In the control group, direct embryo transfer was done. The authors did not find any significant benefit of SEET following fresh cleavage stage embryo transfer with the implantation rate (27 vs 22 %) and pregnancy rates (49 vs 44 %) being similar in both the groups.
Our trial revealed no significant difference in clinical pregnancy rates between the SEET group and the control group, which is similar to the findings of previous studies [8, 12]. However the implantation rate was significantly lower in the SEET group. We also found a significantly lower live birth rate following prior transfer of supernatant culture medium in the vitrified warmed blastocyst transfer group. Previous studies in a similar group of patients (frozen thawed blastocyst transfer cycles) did not report live birth rates [7, 11]. Our findings of significantly lower live birth rates in SEET group are important, since previous studies have reported either improvement in pregnancy rates or no benefit following SEET. To the best of our knowledge, this is the first trial showing a significant negative correlation following SEET in ART.
Our study included patients with previously unsuccessful fresh blastocyst transfers and those who did not undergo fresh transfer due to high risk of OHSS which is similar to study population included in trials by Goto et al. [7, 11]. The volume of supernatant fluid for uterine flushing in our trial was 10–15 μl which was comparable to volume (20 μl) used in earlier studies [7, 11, 12]. Only notable difference in protocols was in the volume of culture drop used for embryo culture. We used 15 μl culture drop (group culture containing 3–4 embryos) where as the previous investigators have used a 50 μl drop for similar number of embryos [7, 11, 12]. Since we used lower volume of culture drop for identical number of embryos, the concentration of embryotrophic factors would have been higher compared to those in previous trials [7, 11, 12] hence an unlikely cause for the lower implantation n live birth rates obtained in our study.
One of the plausible reasons for a lower implantation rate in the SEET group could be the role of endometrial injury by the additional procedure (introduction of catheter and injection of supernatant fluid). The recent Cochrane review evaluating the role of endometrial injury prior to ART cycle concluded improvement in live birth rate following intervention compared to control group [13]. However this improvement was seen only in patients who had endometrial injury in the cycle prior to ART. In the subgroup analysis, a significantly lower ongoing pregnancy rates was obtained in patients who had endometrial injury on the day of oocyte retrieval followed by transfer in the same cycle [14]. Though introduction of the catheter and injection of supernatant fluid is unlikely to be traumatic, the possibility of a deleterious effect on the endometrium affecting embryo implantation cannot be ruled out.
One of the limitations of our study was the lack of blinding. Possibility of bias cannot be ruled out since both the clinicians and the embryologist were aware of group allotment, since one arm had the SEET procedure done prior to embryo transfer. However there was no difference in either the mean number or quality of blastocyst transferred in both the groups. Embryo transfer was done by a limited group of clinicians, hence any operator dependent effect is unlikely. Another limitation is the small sample size which is based on the clinical pregnancy rate: hence not adequately powered to detect differences in live birth rate. Our findings need further validation in larger trials in a similar vitrified warmed blastocyst cycle.
Our randomized trial is the first study in which the transfer of supernatant culture medium prior to vitrified warmed blastocyst transfer was found to have significant negative impact on implantation and live birth rate compared to direct embryo transfer. There is a need to conduct larger well designed trials (with possible blinding) in similar patients planned for vitrified warmed blastocyst transfer to arrive at firmer conclusions.
Acknowledgments
We thank Ms. M.S. Gowri, Department of Biostatistic, Christian Medical College, Vellore for providing the statistical support.
Study funding
Research Grant, Christian Medical College, Vellore.
Conflict of interest
None to declare.
Authorship role
KG and MSK conceived and designed the study. MM and MSK analyzed and interpreted the data. MM, MSK and KG wrote the manuscript. KB, NNV, AJ, MM, MK and MSK contributed to data collection and/or performed procedures. All the authors contributed to write the manuscript. All the authors approved the final version of the manuscript.
Footnotes
Capsule
In women undergoing vitrified warmed blastocyst transfer, the clinical pregnancy rate following prior transfer of supernatant embryo culture fluid (SEET) did not improve compared to direct transfer.
TRIAL REGISTRATION NUMBER: CTRI/2013/01/003280.
References
- 1.Emiliani S, Delbaere A, Devreker F, Englert Y. Embryo-maternal interactive factors regulating the implantation process: implications in assisted reproductive. Reprod Biomed Online. 2005;10(4):527–40. doi: 10.1016/S1472-6483(10)60831-0. [DOI] [PubMed] [Google Scholar]
- 2.Lopata A. Blastocyst-endometrial interaction: an appraisal of some old and new ideas. Mol Hum Reprod. 1996;2(7):519–25. doi: 10.1093/molehr/2.7.519. [DOI] [PubMed] [Google Scholar]
- 3.Barañao RI, Piazza A, Rumi LS, Polak de Fried E. Determination of IL-1 and IL-6 levels in human embryo culture-conditioned media. Am J Reprod Immunol N Y N 1989. 1997 Feb;37(2):191–4. [DOI] [PubMed]
- 4.Sheth KV, Roca GL, Al-Sedairy ST, Parhar RS, Hamilton CJ, Al-Abdul Jabbar F. Prediction of successful embryo implantation by measuring interleukin-1-alpha and immunosuppressive factor(s) in preimplantation embryo culture fluid. Fertil Steril. 1991;55(5):952–7. doi: 10.1016/s0015-0282(16)54305-2. [DOI] [PubMed] [Google Scholar]
- 5.Diedrich K, Fauser BCJM, Devroey P, Griesinger G. Evian annual reproduction (EVAR) workshop group. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13(4):365–77. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- 6.Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD002118.pub4. [DOI] [PubMed] [Google Scholar]
- 7.Goto S, Kadowaki T, Hashimoto H, Kokeguchi S, Shiotani M. Stimulation of endometrium embryo transfer (SEET): injection of embryo culture supernatant into the uterine cavity before blastocyst transfer can improve implantation and pregnancy rates. Fertil Steril. 2007;88(5):1339–43. doi: 10.1016/j.fertnstert.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Li X, Fu Z, Tang Z, Chen X, Zhou Y, et al. Injection of day 2 embryo culture supernatant into the uterine cavity did not improve the pregnancy rate of day 3 embryo transfer in patients who underwent in vitro fertilization-embryo transfer: a randomized clinical trial. Fertil Steril. 2010;93(7):2216–21. doi: 10.1016/j.fertnstert.2009.01.098. [DOI] [PubMed] [Google Scholar]
- 9.Balaban B, Yakin K, Urman B. Randomized comparison of two different blastocyst grading systems. Fertil Steril. 2006;85(3):559–63. doi: 10.1016/j.fertnstert.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Costigan S, Henman M, Stojanov T. P-206. Fertil Steril. 2006 Sep 1; 86(3):S208.
- 11.Goto S, Kadowaki T, Hashimoto H, Kokeguchi S, Shiotani M. Stimulation of endometrium embryo transfer can improve implantation and pregnancy rates for patients undergoing assisted reproductive technology for the first time with a high-grade blastocyst. Fertil Steril. 2009;92(4):1264–8. doi: 10.1016/j.fertnstert.2008.08.076. [DOI] [PubMed] [Google Scholar]
- 12.Tehraninejad ES, Tanha FD, Ghajarzadeh M, Zandieh Z, Aziminekoo E, Zanjani HR. Stimulation of the endometrium with high-grade blastocyst culture supernatant (SEHB) can improve pregnancy outcome for couples undergoing intracytoplasmic sperm injection (ICSI): a randomized clinical trial. Arch Gynecol Obstet. 2012;285(4):1167–71. doi: 10.1007/s00404-011-2143-z. [DOI] [PubMed] [Google Scholar]
- 13.Nastri CO, Gibreel A, Raine-Fenning N, Maheshwari A, Ferriani RA, Bhattacharya S, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD009517.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Karimzade MA, Oskouian H, Ahmadi S, Oskouian L. Local injury to the endometrium on the day of oocyte retrieval has a negative impact on implantation in assisted reproductive cycles: a randomized controlled trial. Arch Gynecol Obstet. 2010;281(3):499–503. doi: 10.1007/s00404-009-1166-1. [DOI] [PubMed] [Google Scholar]