Abstract
Background and aims
Genetic factors are important in the pathogenesis of Premature ovarian failure (POF). Notably, estrogen receptor-a (ESR1) has been suggested as a possible candidate gene for POF; however, published studies of ESR1 gene polymorphisms have been hampered by small sample sizes and inconclusive or ambiguous results. The aim of this meta analysis is to investigate the associations between two novel common ESR1 polymorphisms (intron 1 polymorphisms PvuII-rs2234693: T.C and XbaI-rs9340799: A.G) and POF.
Methods
A comprehensive search was conducted to identify all studies on the association of ESR1 gene polymorphisms with POF up to August 2014. Pooled odds ratio (OR) and corresponding 95 % confidence interval (CI) were calculated using fixed-or random-effects model in the meta-analysis.
Results
Three studies covering 1396 subjects were identified. Pooled data showed significant association between ESR1 gene PvuII polymorphism and risk of POF: [allele model: Cvs. T, OR = 0.735, 95%CI: 0.624 ~ 0.865, p = 0.001; co-dominant models: CCvs.TT, OR = 0.540, 95%CI: 0.382 ~ 0.764, p = 0.001, CTvs.TT, OR = 0.735, 95%CI: 0.555 ~ 0.972, p = 0.031; dominant model: CT + CCvs.TT, OR = 0.618, 95%CI: 0.396 ~ 0.966, p = 0.035; recessive model: CCvs.TT + CT, OR = 0.659, 95%CI: 0.502 ~ 0.864, p = 0.003]. Subgroup analyses showed a significant association in all models in Asian population, but no significant association in any model in European population. For the XbaI polymorphism, overall, no significant association was observed under any genetic models. However, under dominant model, ESR1 gene XbaI polymorphism is significantly association with risk of POF in Asian population.
Conclusion
The present meta-analysis suggests that ESR1gene PvuII polymorphism is significantly associated with an increased risk of POF. And ESR1gene XbaI polymorphism is not association with risk of POF overall. However, under dominant model, ESR1gene XbaI polymorphism is significantly association with risk of POF in Asian population. Further large and well-designed studies are needed to confirm the association.
Keywords: Estrogen receptor alpha gene, Polymorphism, Premature ovarian failure, Meta-analysis
Introduction
POF is defined as a cessation of ovarian function with low estrogen level and elevated gonadotrophins before the age of 40 [1], and results in amenorrhea, infertility, and other systemic consequences (such as Cardiovascular disease, osteoporosis, and so on) because of sex-steroid deficiency [2]. Which affects approximately 1 % of women of reproductive age [3]. It is characterized by the absence of menstruation for a period longer than 6 months (secondary amenorrhea), but it can occur before menarche, leading to primary amenorrhea [4–7].
Although many etiologies are suggested as the cause of POF including genetic, autoimmune and metabolic causes, amongst others [8–10], the etiology remains unknown in a large proportion of cases. It is well known that follicular growth and maturation occurs by the synergic influence of the hormones estrogen, FSH and LH on the ovary [11]. Considering that initial follicular pool size and the rate of follicular depletion are associated with the age of menopause, genetic variants in sex hormone receptor genes could affect the risk of POF. Studies on female α-ER knockout mice showed anovulation and completed infertility suggesting the importance of ER-α in reproduction [12].
Two ER subtypes exist in humans, including estrogen receptor-α (ER-α) and estrogen receptor-β (ER-β), encoded by ESR1 and ESR2 gene [13], respectively. Estrogen acts through ER-α at the hypothalamus-hypophysis-ovarian (HPO) axis to stimulate the release of gonadotrophins to regulate folliculogenesis, and through ER-β in the ovary to enhance follicular development [14]. Intron 1 of the ESR1 gene contains two common single-nucleotide polymorphisms (SNPs) at Pvu II (−397 T/C, rs2234693, NM_000125.3:c.453-397 T > C) and Xba I (−351 A/G, rs9340799, NM_000125.3:c.453-351A > G) restriction enzyme sites. Recently, several studies on the association between ESR1 Pvu II (−397 T/C, rs2334693) and Xba I (−351 A/G, rs9340799) polymorphisms and POF risk among different populations including Chinese Han population. However, with relatively small sample sizes, and in Chinese Han population these former studies provided limited information and could not draw a convincing conclusion. Therefore, in this study, a meta-analysis was performed on previous reports to assess the association between the ESR1 gene Pvu II (−397 T/C, rs2334693) and Xba I (−351 A/G, rs9340799) polymorphisms and the risk of POF .
Materials and methods
Patient and control recruitment
The diagnostic criteria for POF following the definition include at least 4 months of amenorrhea before the age of 40 years, with high serum FSH levels (40 CIU/l). All the patients were assessed clinically for complete medical and gynecological history, including the menstrual history, menopausal age, serum FSH levels (two times at 1-month interval), LH levels, TSH levels and for any history of autoimmune disease and mental retardation. Patients with endocrinopathies, autoimmune disorders or chromosomal abnormalities (determined by G-banded karyotype analysis) or with a past history of hysterectomy, pelvic surgery, and chemotherapy were excluded from the study.
Search strategy
A comprehensive electronic search of Pubmed、Ovid、Chinese National Knowledge Infrastructure (CNKI), and Chinese Biomedical Literature Database (CBM) and WanFang Database published up to August 2014. Literature searches were performed by an expert using the following key words and MeSH terms: “Premature ovarian failure” or “POF”, “estradiol receptor alpha” or “ER alpha” or “ESR1” or “estrogen receptor 1” and “genetic polymorphism” or “genetic variants” or “single nucleotide polymorphisms”.
Inclusion and exclusion criteria
Studies included in our meta-analysis had to meet the following criteria: (a) population-based case–control or cohort study focusing on associations of ESR1 PvuII and/or XbaI polymorphisms with POF; (b) the papers offered the size of the samples, source of controls, distribution of alleles, genotypes, or other information that could help us calculation of odds ratio (OR) with 95 % confidence interval (CI); (c) the following criteria for diagnosing POF were used [15]: >4 months of amenorrhea and two serum FSH levels of >40 mIU/ml in a women aged <40 years. (d) published in the English or Chinese language. While the major reasons for exclusion of studies were as follows: (a) insufficient or error data; (b) lack of control-group or genotype distribution is deviation from the test of Hardy–Weinberg equilibrium (HWE) in controls; (c) studies were meta-analyses, letters, reviews or editorial articles; (d) when multiple publications reported on the same or overlapping data, we used the most recent or largest population as recommended by Little et al. [16].
Data extraction and quality assessment
All data from included studies were extracted independently by two investigators (He and Shu) using a piloted data standardized form (any disagreement were resolved through discussion and, when necessary, adjudicated by a third reviewer). The following data elements were extracted from the studies: first author, year of publication, origin of country, ethnicity of subjects, deviation fromHWE in controls, source of controls, genotyping method, distribution of alleles and genotypes in case and control groups.
Statistical analysis
The association of ESR1 gene PvuII/XbaI polymorphism with the POF susceptibility were estimated by calculating odds ratios (ORs) with the corresponding 95 % confidence intervals (CIs) under all genetic models. Data analysis was performed using STATA Software (version 12.0, Stata Corp.). P <0.05 was considered statistically significant. Five comparison models for the PvuII/XbaI polymorphism were evaluated: an allele model (T vs. C/ A vs. G), a co-dominant model (TT vs. CC and CT vs. CC / AA vs. GG and AA vs. AG), a recessive model (TT + CT vs. CC / AA + AG vs. GG), and a dominant model (TT vs. CT + CC/AA vs. GG + GA).
The between-study heterogeneity was examined by Q statistic test. P value <0.1 was considered statistically significant [17]. When P value >0.10 and I2 < 50 %, the between-study heterogeneity was not significant, we used the fixed-effects (Mantel–Haenszel) model, otherwise, the random-effects (DerSimonian–Laird) model was used to calculate the data. In the subgroup analysis, we evaluated the effect of the PvuII/XbaI polymorphism on the susceptibility to POF in the different populations stratified by geographic location (Asian and European).
Sensitivity analysis was performed by sequentially removing an individual study each time to check whether any single study could bias the overall estimate [18]. The potential publication bias was investigated using Begg’ funnel plot and Egger’s regression test [19]. P < 0.05 was regarded as statistically significant. In our meta-analysis, the P value for the control population in HWE was calculated by a Chi-square test again. The HWE was considered statistically significant, when the P value was less than 0.05 [20].
Results
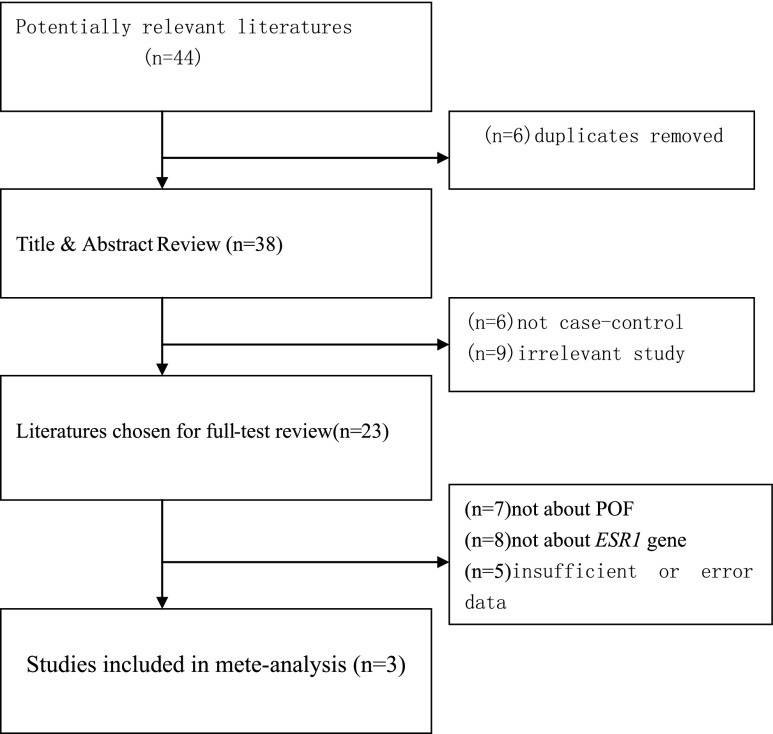
Three studies that met the inclusion criteria were included in the meta-analysis [21–23]. The detailed selection process is illustrated in Fig. 1. The characteristics of the extracted information from each article are summarized in Table 1. Among the eligible studies, a total of 1396 subjects (478 POF cases, 918 healthy controls) were included for this meta-analysis, two were performed in Asia, one was performed in Europe, respectively. All the genotype frequencies in the control populations were in agreement with HWE.
Fig. 1.
Flow chart of the study selection process
Table 1.
Main characteristics of individual studies in the meta-analysis of the ESR1 gene PvuII/XbaI polymorphism and POF
| Author | Country | Geographic location | age of case | age of control | Sample size | HWE | |
|---|---|---|---|---|---|---|---|
| Case | Control | ||||||
| Li | Serbia | European | 34.2 ± 8.6 | 33.6 ± 11.1 | 197 | 547 | 0.586 |
| Lipeng | China | Asian | 30.05 ± 4.10 | 29.61 ± 3.74 | 155 | 150 | 0.476 |
| Yoon | Korea | Asian | 27.5 ± 9.0 | 30.14 ± 3.38 | 126 | 221 | 0.357 |
Quantitative synthesis of data
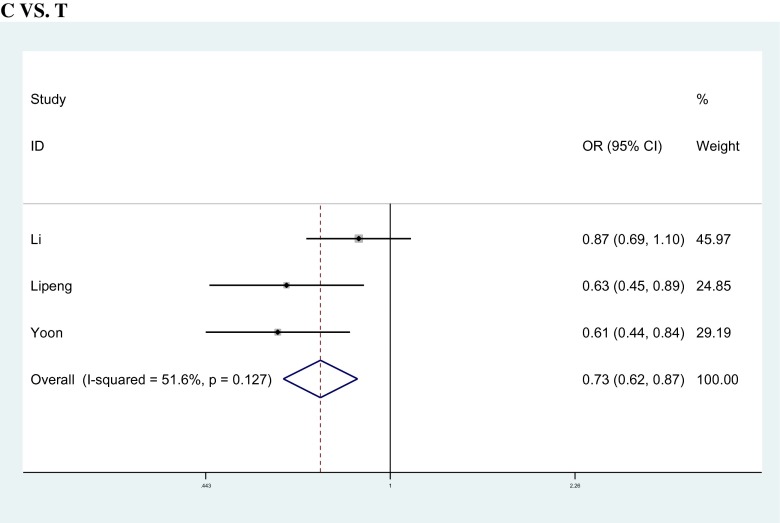
The relationship between ESR1 gene PvuII/XbaI polymorphism and the risk of POF were explored through 3 case–control studies including 1396 subjects (478 cases, 918 controls). For the PvuII polymorphism, overall, there were significant association observed under all genetic models [allele model:Cvs.T, OR = 0.735, 95%CI: 0.624 ~ 0.865, p = 0.001; co-dominant models: CCvs.TT, OR = 0.540, 95%CI: 0.382 ~ 0.764, p = 0.001, CTvs.TT, OR = 0.735, 95%CI: 0.555 ~ 0.972, p = 0.031; dominant model: CT + CCvs.TT, OR = 0.618, 95%CI: 0.396 ~ 0.966, p = 0.035; recessive model: CCvs.TT + CT, OR = 0.659, 95%CI: 0.502 ~ 0.864, p = 0.003]. (Figure 2). When the subgroup analysis was categorized into Asian and European populations, significant association were observed between ESR1 gene PvuII polymorphism and the risk of POF under all genetic models in the Asian (Table. 2). However, there were no significant association in any model in the European populations (Table. 2).
Fig. 2.
Meta-analysis of the association between ESR1 PvuII (T/C) polymorphisms and the risk of POF under allele model (C vs. T)
Table. 2.
Results of the relationship between the meta-analysis of the ESR1 gene PvuII polymorphism and POF
| Comparison | Population | N | Sample Size | Test of association | M | test of heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ORa | 95%CIb | POR | x 2 | Pvalue c | I2(%) | |||||
| C vs. T | overall | 3 | 956 | 1836 | 0.735 | 0.624 | 0.865 | 0.001 | F | 4.13 | 0.127 | 51.6 |
| asian | 2 | 562 | 742 | 0.620 | 0.491 | 0.782 | 0.001 | F | 0.03 | 0.871 | 0.0 | |
| european | 1 | 394 | 1094 | 0.870 | 0.690 | 1.096 | – | – | – | – | – | |
| CC vs. TT | overall | 3 | 246 | 370 | 0.540 | 0.382 | 0.764 | 0.001 | F | 4.21 | 0.122 | 52.54 |
| asian | 2 | 148 | 190 | 0.359 | 0.212 | 0.610 | 0.001 | F | 0.00 | 0.956 | 0.0 | |
| european | 1 | 98 | 280 | 0.751 | 0.471 | 1.198 | 0.001 | – | – | – | – | |
| CT vs. TT | overall | 3 | 364 | 562 | 0.735 | 0.555 | 0.972 | 0.031 | F | 3.30 | 0.192 | 39.5 |
| asian | 2 | 207 | 249 | 0.552 | 0.363 | 0.893 | 0.005 | F | 0.02 | 0.889 | 0.0 | |
| european | 1 | 157 | 413 | 0.933 | 0.637 | 1.367 | 0.031 | – | – | – | – | |
| CT + CC vs. TT | overall | 3 | 478 | 818 | 0.618 | 0.396 | 0.966 | 0.035 | R | 4.67 | 0.097 | 57.2 |
| asian | 2 | 281 | 371 | 0.458 | 0.325 | 0.722 | 0.001 | F | 0.12 | 0.732 | 0.0 | |
| european | 1 | 197 | 547 | 0.873 | 0.609 | 1.251 | 0.035 | – | – | – | – | |
| CCVS.TT + CT | overall | 3 | 478 | 818 | 0.659 | 0.502 | 0.864 | 0.003 | F | 1.51 | 0.469 | 0.0 |
| asian | 2 | 281 | 371 | 0.566 | 0.392 | 0.819 | 0.002 | F | 0.15 | 0.701 | 0.0 | |
| european | 1 | 197 | 547 | 0.785 | 0.527 | 1.169 | 0.003 | – | – | – | – | |
a, OR odds ratio
b, 95 % confidence interval
c, Pvalue for heterogeneity based on Q test
M model of meta-analysis, F fixed-effects model, R random-effects model
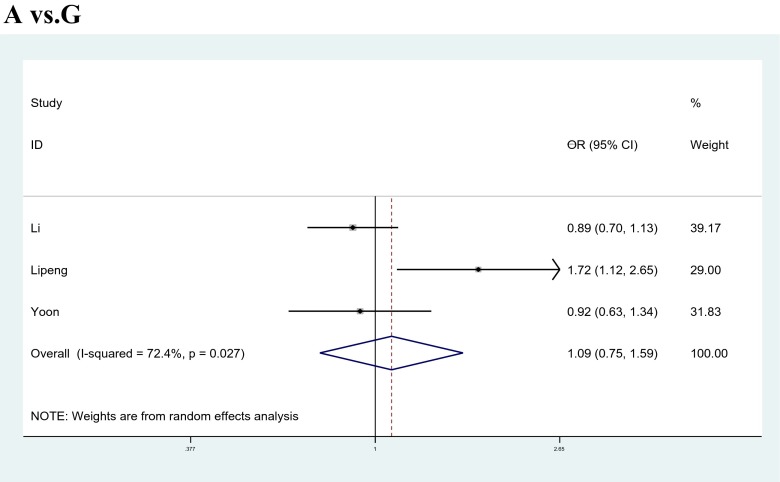
For the XbaI polymorphism, overall, no significant association was observed under any genetic models [allele model:Gvs.A, OR = 1.090, 95%CI: 0.746 ~ 1.591, p = 0.656; co-dominant models: GGvs.AA, OR = 0.790, 95%CI: 0.510 ~ 1.225, p = 0.293, GAvs.AA, OR = 1.257, 95%CI: 0.721 ~ 2.191, p = 0.420; dominant model: GG + GAvs. AA, OR = 1.186, 95%CI: 0.706 ~ 1.993, p = 0.519; recessive model: GGvs.AA + GA, OR = 0.789, 95%CI: 0.520 ~ 1.196, p = 0.264] (Fig. 3). In the subgroup analysis by ethnicity (Asian and European population), a significant association was observed between ESR1 gene Xbal polymorphism and the risk of POF under dominant model (GG + GAvs.AA, OR = 0.458, 95%CI: 0.325 ~ 0.722, p = 0.001) in Asian populations. However, there were no significant association in any model in the European populations. The overall and subgroup results are displayed in Table 3.
Fig. 3.
Meta-analysis of the association between ESR1 XbaI (A/G) polymorphisms and the risk of POF under allele model (A vs. G)
Table 3.
Results of the relationship between the meta-analysis of the ESR1 gene XbaI polymorphism and POF
| Comparison | Population | N | Sample Size | Test of association | M | test of heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ORa | 95%CIb | POR | x 2 | PvalueC | I2(%) | |||||
| G vs. A | Overall | 3 | 956 | 1836 | 1.090 | 0.746 | 1.591 | 0.656 | R | 7.26 | 0.027 | 72.4 |
| Asian | 2 | 562 | 742 | 1.250 | 0.677 | 2.306 | 0.475 | R | 4.59 | 0.032 | 78.2 | |
| European | 1 | 394 | 1094 | 0.888 | 0.699 | 1.128 | – | – | – | – | – | |
| GG vs. AA | Overall | 3 | 288 | 558 | 0.790 | 0.510 | 1.225 | 0.293 | F | 0.19 | 0.909 | 0.0 |
| Asian | 2 | 183 | 266 | 0.873 | 0.388 | 1.963 | 0.743 | F | 0.11 | 0.744 | 0.0 | |
| European | 1 | 105 | 292 | 0.935 | 0.659 | 1.327 | – | – | – | – | – | |
| GA vs. AA | Overall | 3 | 444 | 819 | 1.257 | 0.721 | 2.191 | 0.420 | R | 9.37 | 0.009 | 78.6 |
| Asian | 2 | 171 | 354 | 1.498 | 0.606 | 3.703 | 0.382 | R | 6.58 | 0.01 | 84.8 | |
| European | 1 | 173 | 465 | 0.935 | 0.659 | 1.327 | – | – | – | – | – | |
| GG + GA vs. AA | Overall | 3 | 478 | 918 | 1.186 | 0.706 | 1.993 | 0.519 | R | 8.99 | 0.011 | 77.8 |
| Asian | 2 | 281 | 371 | 0.458 | 0.325 | 0.722 | 0.001 | F | 0.12 | 0.732 | 0.0 | |
| European | 1 | 197 | 547 | 0.892 | 0.640 | 1.244 | – | – | – | – | – | |
| GGVS.AA + GA | Overall | 3 | 478 | 918 | 0.789 | 0.520 | 1.196 | 0.264 | F | 0.00 | 1.000 | 0.0 |
| Asian | 2 | 281 | 371 | 0.794 | 0.355 | 1.776 | 0.574 | F | 0.00 | 0.986 | 0.0 | |
| European | 1 | 197 | 547 | 0.787 | 0.483 | 1.281 | – | – | – | – | – | |
a, OR odds ratio
b, 95 % confidence interval
c, P value for heterogeneity based on Q test
M model of meta-analysis, F fixed-effects model, R random-effects model
Sensitivity analysis
Sensitivity analysis, after removing one study at a time, was performed to evaluate the stability of the results. For the ESR1 gene PvuII /XbaI polymorphisms, when successively excluded one study (data not shown), the estimated pooled odd ration remains unchangeable. Sensitivity analysis indicated that our results are reliable and stable.
Publication bias diagnostics
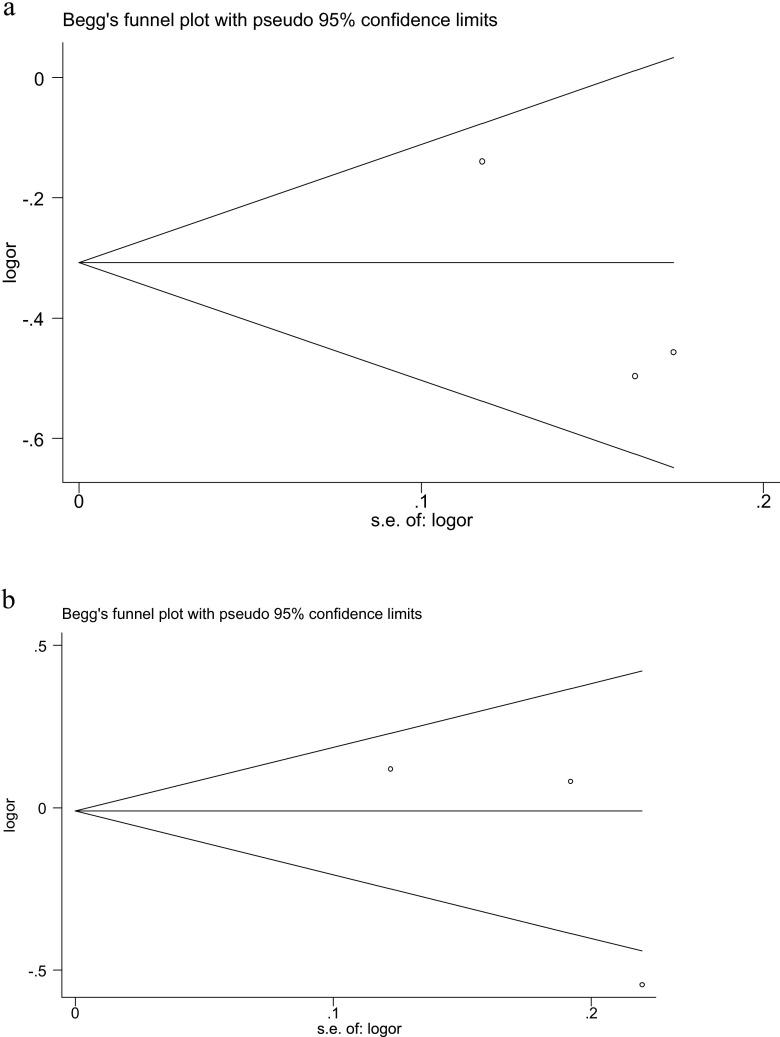
We conducted a Begg’s funnel plot and Egger’s regression test to strengthen further the confidence level in the results. The funnel plot did not reveal evidence asymmetry, which suggested there was no obvious publication bias and Egger’s test also showed that there was no statistical significance for the evaluation of publication bias under the allele model T vs. C /A vs. G (Fig. 4).
Fig. 4.
Begg’s funnel plot of publication bias on the association between ESR1 PvuII (T/C) (a) and XbaI (A/G) (b) polymorphisms and the risk of POF under allele model in the overall populations
Discussion
The role of sex steroid hormones in reproductive function, especially estrogen, has for long been studied and positive correlations have been found by diverse groups and gynecological diseases [24–27]. Indeed, studies with ESR1 knockout mice showed important evidences of reproductive impairment as anovulation and complete infertility in the absence of this gene [12].
Human ESR1 gene is located on chromosome 6q25.1, wild-type ESR1 gene length of 140 kb, and consists of eight exons separated by seven introns. Study indicated that the ESR1 is a major mediator of the atheroprotective effect of estrogen on animal and human [28]. When ERs bind to estrogen, a conformational change ensues that enables the homodimerization of the complex, allowing for binding to estrogen response elements and subsequently altering the expression of relevant target genes, and the result that thereby regulating the growth, reproduction, differentiation and function of many target organs, including the breast tissue, cardiovascular system, nervous system, bone tissue, liver, and so on. Recently, several studies have focused on the ESR1 rs2234693 and rs9340799 polymorphisms with risk of POF. In fact, the results were inconclusive. Such as study by Liu [21] the ESR1 rs2234693 and rs9340799 polymorphisms were significantly association with POF, but study by LI.J [22], no significantly association with POF in both the ESR1 rs2234693 and rs9340799 polymorphisms. However, study by Yoon SH [23], their results have demonstrated that the genetic variation in ESR1 gene (PvuII polymorphism) is associated to POF risk. Therefore, we decided to perform a meta-analysis of all eligible case–control studies on POF risk in order to reveal a more accurate relationship between the PvuII and XbaI polymorphisms of the ESR1 gene and risk of POF.
To our knowledge, this is the first meta-analysis which comprehensively assessed the associations between the ESR1 rs2234693 and rs9340799 polymorphisms and risk of POF in different populations. Three studies covering 1396 subjects were identified. Pooled data showed significant association between ESR1 gene PvuII polymorphism and risk of POF: [allele model:Cvs.T, OR = 0.735, 95%CI: 0.624 ~ 0.865, p = 0.001; co-dominant models: CCvs.TT, OR = 0.540, 95%CI: 0.382 ~ 0.764, p = 0.001, CTvs.TT, OR = 0.735, 95%CI: 0.555 ~ 0.972, p = 0.031; dominant model: CT + CCvs.TT, OR = 0.618, 95%CI: 0.396 ~ 0.966,p = 0.035; recessive model: CCvs.TT + CT, OR = 0.659, 95%CI: 0.502 ~ 0.864, p = 0.003]. Subgroup analyses showed a significant association in all models in Asian population, but no significant association in any model in European population. For the XbaI polymorphism, overall, no significant association was observed under any genetic models. However, under dominant model, ESR1gene XbaI polymorphism is significantly association with risk of POF in Asian population.
Few studies have focused on ESR1 XbaI polymorphisms associated with age at menopause or POF. However, the X allele of XbaI was reported to be related to increased bone mineral density, reduced risk of osteoporosis, and cardiovascular diseases, which suggest higher levels of estrogen [29–33]. So, we hypothesize a series of mechanisms for POF and ER genetic polymorphisms: (1) Estrogen binds to ERs in reproductive tissues, such as the ovaries, uterus, and vagina [34]. The low activity gene encoding protein leads to the low of activity of ERs, and then estrogenic action in the tissue may be weak; (2) Continuous weak estrogenic effect in the reproductive tissue, especially the ovaries, may have a negative feedback on the pituitary gland, especially follicle stimulating hormone (FSH) secretion; (3) FSH, in turn, can accelerate the rapid depletion of the ovarian follicles, leading to the development of POF because of ovarian dysfunction. To clarify the exact mechanisms between ER genes and POF.
We used a fixed-effects or a random-effects model in our analysis of the studies based on heterogeneity testing (Table 2). For the XbaI polymorphism, all the comparison models revealed large heterogeneity between the studies for the overall populations and the Asian subgroup. Differences in the studied populations with different genetic backgrounds and variations in sample selection and environmental exposures may result in these heterogeneities. Our meta-regression analysis also showed that the ethnicity in case groups and control groups significantly contributed to the heterogeneity.
Begg’s funnel plot and Egger’s test did not show any evidence of significant publication bias in all the comparison models. Sensitivity analysis indicated that none of the studies study influenced the results of this meta-analysis. So, the results of our study can be interpreted with a high confidence level.
Limitation
We acknowledged that there were some limitations in our study. First, sample size in our study was comparatively small and had insufficient statistical power to detect the association. Second, the effect of gene-gene and gene-environment interactions was not considered in this meta-analysis. Third, in the current meta-analysis study, two were performed in Asia, one was performed in Europe, but not from Africa and North America et al. Fourth, this meta-analysis was based on unadjusted estimates, whereas a more precise analysis could be obtained if all individual raw data were available. Thus, we hope that these issues will be considered in future by the related researchers.
Conclusions
In conclusion, although these limits, the results of our meta-analysis strongly suggests that ESR1 gene PvuII polymorphism was significant associated with an increased risk of POF. And ESR1 gene XbaI polymorphism is not association with risk of POF overall. But, ESR1gene XbaI polymorphism was significantly association with risk of POF in dominant model in Asian population. In the future, well-designed studies are performed to re-evaluate the potential associations between ESR1gene polymorphisms with other candidate gene polymorphisms and POF risk..
Acknowledgments
This work was not supported by any funds.
Conflict of Interest
The authors declare that they have no competing interests with respect to the authorship and/or publication of this article.
Footnotes
Meirong He and Jingcheng Shu contributed equally to this study and should be considered as co-first authors.
Capsule
In a case-control study comprising 478 women with POF and 918 women without the disease. For the PvuII polymorphism, we were able to demonstrate that there were significant association observed under all genetic models, overall and in Asian population. However,for the XbaI polymorphism,overall, no significant association was observed under any genetic models. But ESR1 gene XbaI polymorphism is significantly association with risk of POF under dominant model in Asian population.
Contributor Information
Xing Huang, Phone: +8618277177579, Email: 18376646056@163.com.
Hui Tang, Email: 175917736@qq.com.
References
- 1.Burton KA, Van Ee CC, Purcell K, Winship I, Shelling AN. Autosomal translocation associated with premature ovarian failure. J Med Genet. 2000;37(5):E2. doi: 10.1136/jmg.37.5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalantaridou SN, Nelson LM. Premature ovarian failure is not premature menopause. Ann N Y Acad Sci. 2000;900:393–402. doi: 10.1111/j.1749-6632.2000.tb06251.x. [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–6. [PubMed] [Google Scholar]
- 4.Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris) 2003;64(2):87–92. [PubMed] [Google Scholar]
- 5.Timmreck LS, Reindollar RH. Contemporary issues in primary amenorrhea. Obstet Gynecol Clin N Am. 2003;30(2):287–302. doi: 10.1016/S0889-8545(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 6.Jeong HJ, Cho SW, Kim HA, Lee SH, Cho JH, Choi DH, et al. G769A variation of inhibin alpha-gene in korean women with premature ovarian failure. Yonsei Med J. 2004;45(3):479–82. doi: 10.3349/ymj.2004.45.3.479. [DOI] [PubMed] [Google Scholar]
- 7.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 8.Rebar RW. Hypergonadotropic amenorrhea and premature ovarian failure: a review. J Reprod Med. 1982;27(4):179–86. [PubMed] [Google Scholar]
- 9.Coulam CB, Stringfellow S, Hoefnagel D. Evidence for a genetic factor in the etiology of premature ovarian failure. Fertil Steril. 1983;40(5):693–5. doi: 10.1016/s0015-0282(16)47433-9. [DOI] [PubMed] [Google Scholar]
- 10.Conway GS. Premature ovarian failure. Curr Opin Obstet Gynecol. 1997;9(3):202–6. doi: 10.1097/00001703-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Cordts EB, Christofolini DM, Dos Santos AA, Bianco B, Barbosa CP. Genetic aspects of premature ovarian failure: a literature review. Arch Gynecol Obstet. 2011;283(3):635–43. doi: 10.1007/s00404-010-1815-4. [DOI] [PubMed] [Google Scholar]
- 12.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3(5):281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 13.Molvarec A, Vér A, Fekete A, Rosta K, Derzbach L, Derzsy Z, et al. Association between estrogen receptor alpha (ESR1) gene polymorphisms and severe preeclampsia. Hypertens Res. 2007;30(3):205–11. doi: 10.1291/hypres.30.205. [DOI] [PubMed] [Google Scholar]
- 14.Kolibianakis EM, Papanikolaou EG, Fatemi HM, Devroey P. Estrogen and folliculogenesis: is one necessary for the other? Curr Opin Obstet Gynecol. 2005;17(3):249–53. doi: 10.1097/01.gco.0000169101.83342.96. [DOI] [PubMed] [Google Scholar]
- 15.Anasti JN. Premature ovarian failure: an update. Fertil Steril. 1998;70(1):1–15. doi: 10.1016/S0015-0282(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 16.Little J, Bradley L, Bray MS, Clyne M, Dorman J, Ellsworth DL, et al. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am J Epidemiol. 2002;156(4):300–10. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Teng Z, Cai S, Wang D, Zhao X, Yu K. The association between the PPARγ2 Pro12Ala polymorphism and nephropathy susceptibility in type 2 diabetes: a meta-analysis based on 9,176 subjects. Diagn Pathol. 2013;8:118. doi: 10.1186/1746-1596-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong T, Yang M, Qi L, Shen M, Du Y. Association of MCP-1–2518A/G and -362G/C variants and tuberculosis susceptibility: a meta-analysis. Infect Genet Evol. 2013;20:1–7. doi: 10.1016/j.meegid.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, Wang W, Zhai S, Dang S, Sun M. IL6 gene polymorphisms and susceptibility to colorectal cancer: a meta-analysis and review. Mol Biol Rep. 2012;39(8):8457–63. doi: 10.1007/s11033-012-1699-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Tan R, Cui Y, Liu J, Wu J. Estrogen receptor α gene (ESR1) polymorphisms associated with idiopathic premature ovarian failure in Chinese women. Gynecol Endocrinol. 2013;29(2):182–5. doi: 10.3109/09513590.2012.731113. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Vujovic S, Dalgleish R, Thompson J, Dragojevic-Dikic S, Al-Azzawi F. Lack of association between ESR1 gene polymorphisms and premature ovarian failure in Serbian women. Climacteric. 2014;17(3):247–51. doi: 10.3109/13697137.2013.819330. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Choi YM, Hong MA, Lee GH, Kim JJ, Im HJ, et al. Estrogen receptor {alpha} gene polymorphisms in patients with idiopathic premature ovarian failure. Hum Reprod. 2010;25(1):283–7. doi: 10.1093/humrep/dep375. [DOI] [PubMed] [Google Scholar]
- 24.Christofolini DM, Vilarino FL, Mafra FA, André GM, Bianco B, Barbosa CP. Combination of polymorphisms in luteinizing hormone β, estrogen receptor β and progesterone receptor and susceptibility to infertility and endometriosis. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):260–4. doi: 10.1016/j.ejogrb.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Du JW, Xu KY, Fang LY, Qi XL. Association between mutations of the luteinizing hormone β subunit and female infertility. Mol Med Rep. 2012;5(2):473–6. doi: 10.3892/mmr.2011.683. [DOI] [PubMed] [Google Scholar]
- 26.Romerius P, Giwercman A, Moëll C, Relander T, Cavallin-Ståhl E, Wiebe T, et al. Estrogen receptor α single nucleotide polymorphism modifies the risk of azoospermia in childhood cancer survivors. Pharmacogenet Genomics. 2011;21(5):263–9. doi: 10.1097/FPC.0b013e328343a132. [DOI] [PubMed] [Google Scholar]
- 27.Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107(3):333–40. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, et al. GENOMOS Study. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004;292(17):2105–14. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 29.Lorentzon M, Lorentzon R, Bäckström T, Nordström P. Estrogen receptor gene polymorphism, but not estradiol levels, is related to bone density in healthy adolescent boys: a cross-sectional and longitudinal study. J Clin Endocrinol Metab. 1999;84(12):4597–601. doi: 10.1210/jcem.84.12.6238. [DOI] [PubMed] [Google Scholar]
- 30.Weiderpass E, Persson I, Melhus H, Wedrén S, Kindmark A, Baron JA. Estrogen receptor alpha gene polymorphisms and endometrial cancer risk. Carcinogenesis. 2000;21(4):623–7. doi: 10.1093/carcin/21.4.623. [DOI] [PubMed] [Google Scholar]
- 31.van Meurs JB, Schuit SC, Weel AE, van der Klift M, Bergink AP, Arp PP, et al. Association of 5′ estrogen receptor alpha gene polymorphisms with bone mineral density, vertebral bone area and fracture risk. Hum Mol Genet. 2003;12(14):1745–54. doi: 10.1093/hmg/ddg176. [DOI] [PubMed] [Google Scholar]
- 32.Schuit SC, Oei HH, Witteman JC, van Kessel Geurts CH, van Meurs JB, Nijhuis RL, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291(24):2969–77. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 33.Lorentzon M, Lorentzon R, Bäckström T, Nordström P. Estrogen receptor gene polymorphism, but not estradiol levels, is related to bone density in healthy adolescent boys: a cross-sectional and longitudinal study. J Clin Endocrinol Metab. 1999;84(12):4597–601. doi: 10.1210/jcem.84.12.6238. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85(12):4835–40. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]