Abstract
Recent evidence suggests that neurokinin B (NKB), a member of the neurokinin (tachykinin) peptide family, plays a pivotal role in gonadotropin-releasing hormone (GnRH) pulse generation. Three types of neurokinin receptors (NKRs), NK1R, NK2R and NK3R, are found in the brain. Although NKB preferentially binds to NK3R, other NKRs are possibly also involved in NKB action. The present study examined the effects of intravenous administration of the NKR subtype-selective agonists GR73632 (NK1R), GR64349 (NK2R), and senktide (NK3R) on GnRH pulse generator activity and luteinizing hormone (LH) secretion. Multiple-unit activity (MUA) was monitored in ovariectomized goats (n = 5) implanted with recording electrodes. Characteristic increases in MUA (MUA volleys) were considered GnRH pulse generator activity. Although three NKR agonists dose-dependently induced an MUA volley and an accompanying increase in LH secretion, the efficacy in inducing the volley markedly differed. As little as 10 nmol of senktide induced an MUA volley in all goats, whereas a dose of 1000 nmol was only effective for the NK1R and NK2R agonists in two and four goats, respectively. When the treatment failed to evoke an MUA volley, no apparent change was observed in the MUA or LH secretion. Similar effects of the NK2R and NK3R agonists were observed in the presence of estradiol. The results demonstrated that NK3R plays a predominant role in GnRH pulse generation and suggested that the contributions of NK1R and NK2R to this mechanism may be few, if any, in goats.
Keywords: GnRH pulse generator, Goat, NK1R agonist, NK2R agonist, NK3R agonist
The hypothalamic neural substrate generates rhythmic bursts of firing activity that drive pulsatile gonadotropin-releasing hormone (GnRH) discharges into the hypophyseal portal vessels, thereby enabling pulsatile gonadotropin secretion from the anterior pituitary lobe. This neural substrate is termed the GnRH pulse generator [1, 2]. Because the frequency of pulsatile GnRH secretion is a key determinant of gonadal functions [1, 2], elucidation of the mechanisms underlying the neural activity of the GnRH pulse generator is a prerequisite to advance our understanding of reproduction.
The precise neural identity of the GnRH pulse generator remains to be clarified. However, recent emerging evidence has shed light on a population of neurons that concomitantly contain kisspeptin, neurokinin B (NKB) and dynorphin A, referred to as KNDy neurons [3], as a likely candidate for the intrinsic source of the GnRH pulse generator [3,4,5,6]. In a variety of mammals including mice [4], rats [7], sheep [8], goats [9] and monkeys [10], KNDy neurons exist exclusively in the arcuate nucleus (ARC) of the hypothalamus. The nucleus has been postulated to be the locus of the GnRH pulse generator [1, 11]. Moreover, the multiple-unit activity (MUA) recording method demonstrated that rhythmic increases in MUA (MUA volleys) representing GnRH pulse generator activity are observed in close proximity to KNDy neurons in goats [9, 12].
Of the neuropeptides in KNDy neurons, NKB has being a focus of growing interest as a substance intimately involved in the rhythmic bursting activity of KNDy neurons. NKB, substance P (SP) and neurokinin A (NKA) comprise the neurokinin (tachykinin) peptide family [13]. Three types of neurokinin receptors (NKRs), NK1R, NK2R, and NK3R, are found in the central and peripheral nervous systems and preferentially bind to SP, NKA and NKB, respectively [14]. However, this ligand-receptor interaction is not exclusive, and NKB has the ability to induce responses from both NK1R and NK2R [15].
Anatomical studies have revealed that KNDy neurons are interconnected ipsilaterally and contralaterally with NKB-containing fibers [16, 17], and a majority of KNDy neurons express NK3R [4, 7, 18]. These anatomical features suggest the presence of an NKB-mediated auto-feedback network in the KNDy neurons [6, 19, 20]. On the other hand, electrophysiological studies demonstrated that NKB elicited trains of action potentials in Kiss1- or Tac2 (NKB)-GFP identified neurons in the mouse ARC in vitro [19, 21, 22] and that bolus intracerebroventricular (icv) administration of NKB immediately induced an MUA volley in vivo [9]. These lines of evidence suggest that NKB plays a role in triggering the synchronized firing of KNDy neurons at the time of GnRH pulse generation [6, 19, 20]. Icv administration of a selective NK3R agonist, senktide, increased luteinizing hormone (LH) secretion and induced cFos expression in the KNDy neurons of rats [7]. Prepubertal treatment of rats with senktide advanced puberty onset and tended to increase the LH pulse frequency [23]. Moreover, senktide activated KNDy neurons in vitro [19, 21, 22]. Therefore, it is plausible that NKB-NK3R signaling may play a dominant role in the GnRH pulse generation of KNDy neurons. However, the possibility that the other two types of NKRs are also involved in the action of NKB cannot be ruled out. Noritake et al. [24] reported that icv administration of an antagonist for all 3 receptor subtypes suppressed LH secretion, whereas that of each NKR subtype-selective antagonist was ineffective in rats, suggesting the involvement of all three NKRs in the NKB action of KNDy neurons. In agreement with this in vivo study, the activation of KNDy neurons by NKB was completely abrogated only when all three NKR subtype-selective antagonists were concomitantly applied in an in vitro bath [21].
In the present study, we aimed to clarify the role of NKRs in GnRH pulse generation using the MUA recording technique in goats [9, 12]. Doses of three NKR subtype-selective agonists, GR73632 (NK1R), GR64349 (NK2R) and senktide (NK3R), were intravenously injected into ovariectomized (OVX) and estradiol (E2)-treated OVX goats, and their effects on MUA volleys and LH secretion were examined. The EC50 values of each agonist for NK1R, NK2R and NK3R in rats have been reported to be as follows: NK1R = 4 nM, NK2R = 960 nM, NK3R > 1,000 nM for GR73632; NK1R > 1,000 nM, NK2R = 3.7 nM, NK3R > 1,000 nM for GR64349; and NK1R > 10,000 nM, NK2R > 10,000 nM, NK3R = 18 nM for senktide [25, 26].
Materials and Methods
Animal
Adult (3- to 8-year-old) female Shiba goats (n = 5) weighing 20–30 kg were used. The goats were ovariectomized at least one month prior to the start of the experiment. They were loosely held in individual stanchions in a condition-controlled room (12 h light/dark cycle, 23 C, and 50% relative humidity) and maintained with a standard pellet diet and dry hay. Water and supplemental minerals were always available. All experimental procedures were approved by the Committee on the Care and Use of Experimental Animals at the National Institute of Agrobiological Sciences.
Surgery and MUA recording
The goats were stereotaxically implanted with an array of bilateral recording electrodes aimed at the caudal region of the ARC as described previously [9, 12]. After a recovery period of approximately 4 weeks, the goats were subjected to the experimental procedures. The MUA was measured in conscious animals, and the MUA signal was stored as spikes per 20 sec [9]. A characteristic increase in MUA (MUA volley) was considered to be the electrophysiological manifestation of the GnRH pulse generator.
Steroid treatments
SILASTIC (silicone) tubing (inner diameter, 3 mm; outer diameter, 5 mm; length, 20 mm; Dow Corning, Midland, MI, USA) was filled with crystalline E2 (Sigma-Aldrich, St. Louis, MO, USA). Four of the 5 OVX goats were implanted subcutaneously with an E2 capsule (OVX + E2) to produce E2 levels simulating the luteal phase of the estrous cycle (4–8 pg/ml) [27]. Experiments in the OVX + E2 goats were conducted between 5 and 7 days after implantation of the E2 capsule.
Drug administration and blood sampling
GR73632 (R&D Systems, Minneapolis, MN, USA) and GR64349 (R&D Systems) were dissolved in saline to make working concentrations of 200 or 1000 nmol/2 ml. These doses were employed based on our preliminary experiments. Senktide (synthesized and provided by Drs. Oishi and Fujii, Kyoto University) was dissolved in saline to make working concentrations of 10 or 50 nmol/2 ml. These doses were selected according to a previous study [28]. For comparison of the LH-stimulatory action, human type kisspeptin-10 (Kp-10, Peptide Institute, Osaka, Japan) dissolved in saline (380 nmol/2 ml) [12] was also prepared. The OVX goats received the vehicle (saline) and both doses of GR73632, GR64349, and senktide once each. The OVX + E2 goats received the vehicle and the higher dose of each agonist once. The vehicle and agonists were administered in random order among the goats, and each administration was separated by at least one day.
Prior to the start of the experiment, the goats were fitted with a jugular catheter for drug delivery and blood sampling. On the experimental day, MUA recording was started, and 4 successive MUA volleys were observed in the control period. The mean intervolley interval in the control period (the control interval) was calculated and used to anticipate the occurrence of the next expected MUA volley in each goat. Then, blood sampling was started while monitoring the MUA. After one regularly occurring MUA volley, 2 ml of either vehicle or a drug solution was injected at the midpoint between 2 successive MUA volleys. Blood samples were collected every 3 min for more than 2 h, or every 6 min for more than 4 h in the OVX and OVX + E2 goats, respectively. Blood samples were centrifuged, and plasma was separated and stored at –30 C until assayed for LH.
Assays
LH concentrations in single aliquots of 50 µl plasma samples were measured by a double-antibody radioimmunoassay [29] with rabbit anti-ovine LH serum [30] and expressed in terms of the ovine LH standard (NIDDK-oLH-I-4). The least detectable LH concentration was 0.19 ng/ml for 50 µl plasma samples, and the intra- and inter-assay coefficients of variation were 5.1 and 6.5%, respectively.
MUA data analysis
The mean value and SD of all MUA data (spikes per 20 sec) during the experimental period were calculated in each treatment condition for each individual. When the count at a time point exceeded twice the SD of the mean value, it was designated the start of a volley. For analysis of the MUA data, the time interval between the start of 2 successive MUA volleys (intervolley interval) was determined. The control interval was the mean intervolley interval of 4 successive MUA volleys during the control period. The first posttreatment interval was the intervolley interval between the MUA volleys immediately before and after the injection. The second posttreatment interval was the intervolley interval following the first. When the first posttreatment interval was smaller than the lower 95% confidence limit of the control interval of a given animal, the treatment was considered to have induced the MUA volley.
The mean group value of the first posttreatment interval was statistically compared using one-way ANOVA followed by Tukey’s post hoc test within each treatment group. Comparison of the first mean posttreatment interval between the lower and higher doses of the drug was done using paired t-test. Comparison of the control interval for vehicle treatment between the OVX and OVX + E2 goats was done using the Student’s t-test. An effect was considered to be statistically significant when P < 0.05.
LH data analysis
LH pulses were identified by the PULSAR computer program [31]. The criteria for the identification of LH pulses were as follows: to be considered part of an LH pulse, the difference between a single LH concentration and the baseline concentration had to be 3.0 times greater than the standard deviation (SD) at the level of the LH concentration, the difference between 2 consecutive LH concentrations and the baseline concentrations had to be 1.0 times greater than the SD or the difference between 3 or more consecutive LH concentrations and the baseline concentrations had to be 0.4 times greater than the SD. The SD for each plasma concentration was calculated by the equation y=(1.46 x2 + 2.23 x + 1.99)/100, where x was the LH level and y was the SD for each LH level determined by assaying four series of control plasma samples in 10 replicates. Because relatively short inter-pulse intervals in the OVX animals contained insufficient time points between the peak and nadir values of LH concentrations, computer-aided analysis sometimes failed to identify small increases in LH secretion as an “LH pulse,” even though it occurred immediately after the MUA volley. We described this as a pulse-like increase in LH secretion.
To statistically analyze the effect of the NKR agonists and Kp-10 on LH secretion, the area under the curve (AUC) of the LH response during the 1-h posttreatment period was compared with that during the 1-h pretreatment period in each treatment using paired t-test. Because basal LH secretion markedly varied among individuals (Fig. 1), values of LH concentrations during the 1-h post- and pretreatment periods were divided by the mean LH concentrations during the 2-h sampling period for the vehicle treatment in each goat, and these standardized values were used for the statistical analysis.
Fig. 1.
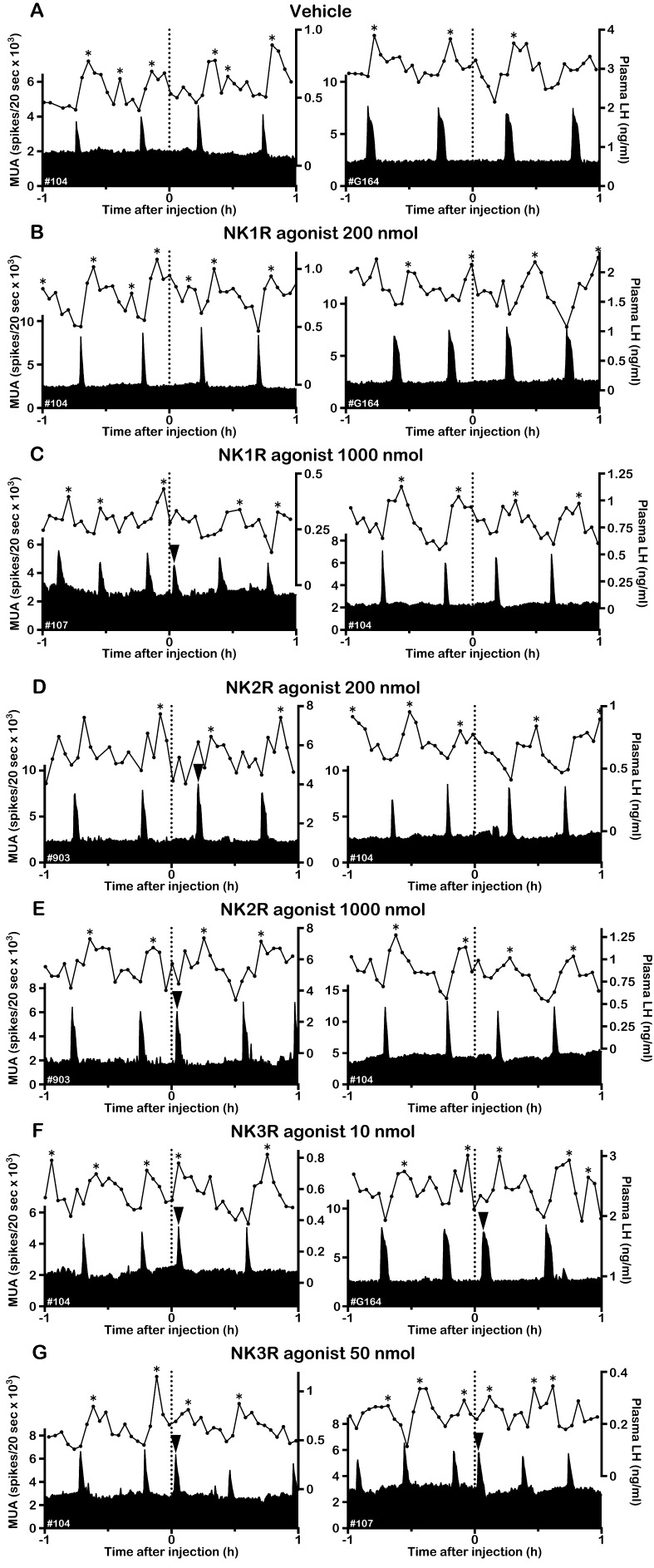
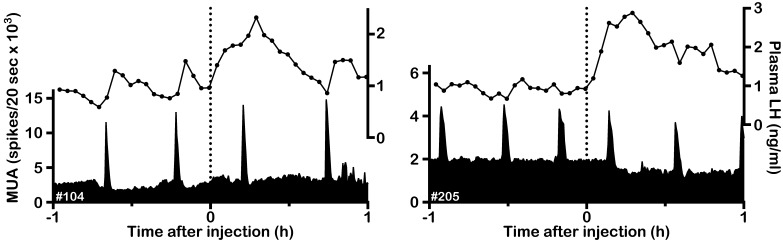
Effects of NKR subtype-selective agonists on the MUA and plasma LH in OVX goats. Representative profiles of the MUA and plasma LH concentrations in two OVX goats that received an intravenous injection of vehicle (A), 200 nmol (B) or 1000 nmol (C) of the NK1R agonist GR73632, 200 nmol (D) or 1000 nmol (E) of the NK2R agonist GR64349 and 10 nmol (F) or 50 nmol (G) of the NK3R agonist senktide are shown. The dotted line indicates the timing of the injection. The arrowhead indicates an MUA volley induced by the treatment. The asterisk represents the LH pulse identified by the PULSAR program.
Results
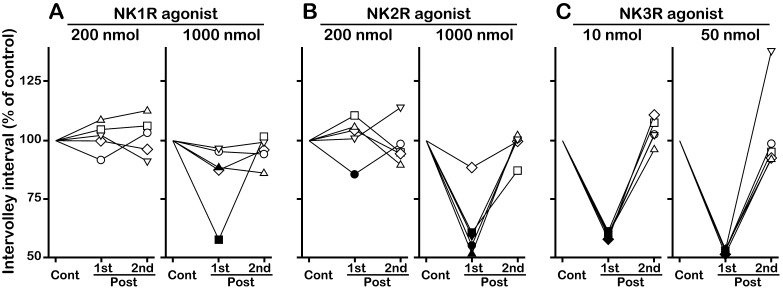
In the OVX goats, spontaneous MUA volleys occurred every 22–33 min. Although the intervolley interval slightly varied among animals, it was relatively constant within the individuals. The MUA volley was always associated with an LH pulse or pulse-like increase in LH secretion. Representative profiles of MUA and LH secretion and individual data concerning the effects of NKR agonists on the first and second posttreatment intervals are shown in Fig. 1 and Fig. 2, respectively. The group MUA data and AUC of the LH response are summarized in Table 1. Intravenous administration of the vehicle had no effect on the MUA (Fig. 1A), and the first posttreatment interval was comparable to the control interval (Table 1). Occurrence of the MUA volley was not affected by 200 nmol of the NK1R agonist GR73632 (Fig. 1B). After 1000 nmol of NK1R agonist administration, the first posttreatment interval was shorter than the lower 95% confidence limit of the control interval in 2 goats (Fig. 1C, left panel, Fig. 2A). Thus, the MUA volley was considered to have been induced by the treatment in those goats. Induction of an MUA volley was not observed in the remaining 3 goats (Fig. 1C, right panel, Fig. 2A). In administration of the NK2R agonist GR64349, an MUA volley occurred after injection of 200 (Fig. 1D, left panel) and 1000 nmol (Fig. 1E, left panel) of the drug in one and 4 goats, respectively (Fig. 2B). Administration of the NK3R agonist senktide at both 10 (Fig. 1F) and 50 (Fig. 1G) nmol doses induced MUA volleys in all goats (Fig. 2C). In all goats, no effect of NKR agonists was observed for the second posttreatment interval (Fig. 2). When either treatment evoked an MUA volley, it was invariably followed by an LH pulse or pulse-like increase in LH secretion. On the other hand, when the treatment failed to evoke an MUA volley, neither basal MUA levels nor LH concentrations showed any apparent changes (Fig. 1).
Fig. 2.
Effects of NKR subtype-selective agonists on the first and second posttreatment intervals in OVX goats. Individual data of the first (1st) and second (2nd) posttreatment intervals for administration of the NK1R agonist GR73632 (A), NK2R agonist GR64349 (B) and NK3R agonist senktide (C) are shown as the percent of the control interval for a given animal. The drug solution was injected at the midpoint between 2 successive MUA volleys (50% of the control interval after the pretreatment MUA volley). The same symbol in A–C represents the value obtained in a single goat. Closed symbols indicate values smaller than the lower 95% confidence limit of the control interval within the individual. The actual value of the control interval in each treatment is shown in Table 1.
Table 1. Effects of neurokinin receptor agonists on multiple-unit activity (MUA) and LH secretion in ovariectomized (OVX) and estradiol-treated (OVX + E2) goats.
| Treatment | Control interval (min) | Induction of an MUA volley (the number of goats) | Posttreatment interval (min) |
LH secretiona |
|||
| 1st | 2nd | Pretreatment | Posttreatment | ||||
| OVX (n=5) | |||||||
| Vehicle | 27.9 ± 2.43 | 0 | 27.8 ± 2.13 | 29.2 ± 3.79 | 58.4 ± 0.93 | 61.8 ± 0.94 | |
| NK1R agonist | 200 nmol | 25.1 ± 1.43 | 0 | 25.5 ± 1.11 | 25.7 ± 1.64 | 60.7 ± 7.83 | 60.5 ± 7.76 |
| 1000 nmol | 26.1 ± 1.57 | 2 | 22.7 ± 2.77 | 24.9 ± 1.19 | 67.0 ± 8.63 | 60.8 ± 7.39 | |
| NK2R agonist | 200 nmol | 26.0 ± 1.74 | 1 | 26.2 ± 1.27 | 25.5 ± 1.67 | 59.8 ± 6.22 | 58.4 ± 3.59 |
| 1000 nmol | 27.3 ± 1.22 | 4 | 17.2 ± 1.70*‡ | 27.0 ± 1.54 | 105 ± 29.5 | 101 ± 30.1 | |
| Senktide | 10 nmol | 27.5 ± 2.43 | 5 | 16.3 ± 1.45* | 28.6 ± 2.43 | 57.9 ± 2.66 | 57.2 ± 2.82 |
| 50 nmol | 27.2 ± 2.25 | 5 | 14.3 ± 1.27*‡ | 27.8 ± 1.95 | 56.3 ± 3.45 | 54.7 ± 4.42 | |
| OVX + E2 (n=4) | |||||||
| Vehicle | 59.4 ± 4.92# | 0 | 60.0 ± 5.52 | 57.3 ± 3.43 | 59.3 ± 1.17 | 60.9 ± 0.67 | |
| NK1R agonist | 1000 nmol | 56.9 ± 5.13 | 0 | 62.7 ± 6.76 | 64.5 ± 7.52 | 62.6 ± 3.33 | 65.0 ± 1.44 |
| NK2R agonist | 1000 nmol | 59.3 ± 3.86 | 4 | 31.0 ± 2.10* | 65.1 ± 8.47 | 72.5 ± 4.99 | 66.5 ± 3.82 |
| Senktide | 50 nmol | 62.0 ± 4.67 | 4 | 31.9 ± 2.25* | 61.8 ± 3.71 | 69.9 ± 2.88 | 71.4 ± 5.50 |
Data are represented as the mean ± SEM. * Significantly smaller than the control interval of the respective treatment (one-way ANOVA, Tukey’s post hoc test, P < 0.05). ‡ Significantly smaller than the lower dose of the same agonist (paired t-test, P < 0.05). # Significantly larger than the control interval of the vehicle treatment in the OVX animals (the Student’s t-test, P<0.05). a Values represent the area under the curve of standardized LH concentrations during the 1-h period.
The overall effect of the NKR agonists in the 5 OVX goats was statistically analyzed by comparing the mean values of the intervolley intervals and the AUCs of the LH response (Table 1). In the NK1R agonist treatment, the first posttreatment interval was comparable to the control interval for both doses. Although the first posttreatment interval was slightly smaller after the higher dose of the NK1R agonist compared with the lower dose, the difference was not statistically significant. In the NK2R agonist treatment, the first posttreatment interval for the higher but not the lower dose was significantly smaller than the control interval of the respective administration (P < 0.05). There was a significant dose effect on the interval in the NK2R agonist treatment (P < 0.05). In senktide administration, both doses of the drug significantly decreased the first posttreatment interval compared with the control interval (P < 0.05). Again, there was a significant dose effect on the interval in the senktide treatment (P < 0.05). The effects of NK2R agonist and senktide on MUA volleys were apparent only for the first posttreatment interval; the second posttreatment interval was comparable to the control interval in both administrations (Table 1). All of the NKR agonist treatments had no effect on overall LH secretion even in the senktide treatment in which an LH pulse or pulse-like increase in LH secretion was induced in all goats (Table 1).
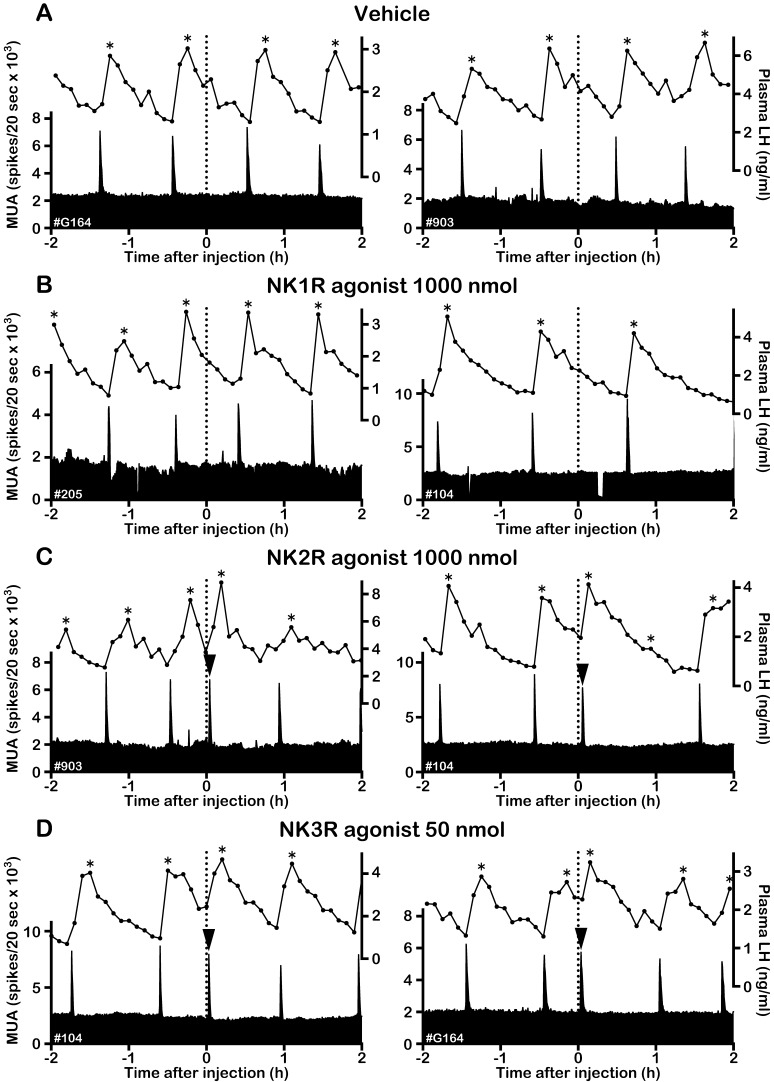
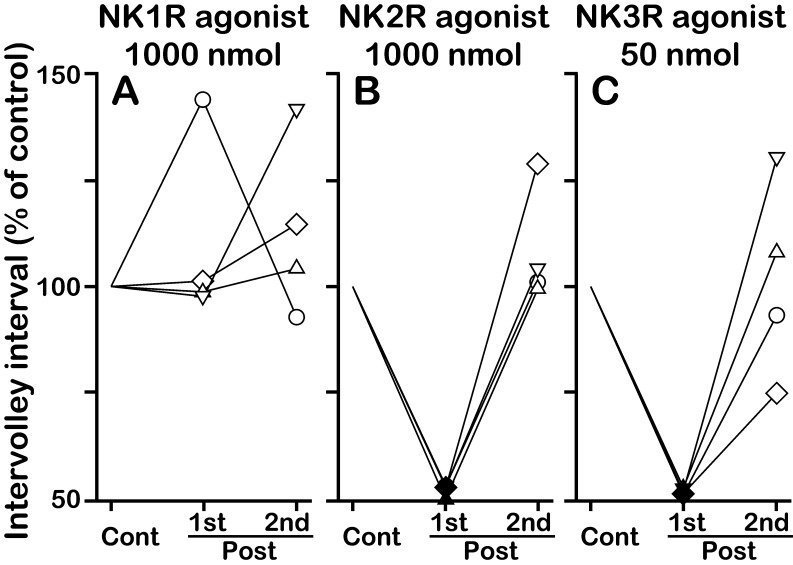
In the OVX + E2 goats, the intervolley interval during the control period of vehicle administration was significantly larger than that in the OVX goats (59.4 ± 4.92 vs. 27.9 ± 2.43 min, P < 0.01), possibly reflecting the negative feedback action of E2 on the GnRH pulse generator [9]. The vehicle had no effect on the MUA (Fig. 3A). In the presence of E2, no MUA volley induction was observed in any goats after administration of 1000 nmol of the NK1R agonist (Fig. 3B, Fig. 4A). In contrast, doses of 1000 nmol of the NK2R agonist (Fig. 3C, Fig. 4B) and 50 nmol of senktide (Fig. 3D, Fig. 4C) induced an MUA volley in all OVX + E2 goats. The first posttreatment intervals of the NK2R agonist and senktide administrations were significantly smaller than the control interval of the respective administration (P < 0.05). The MUA volleys induced by the NKR agonists were also always followed by an LH pulse in the OVX + E2 goats (Fig. 3C, D). The effect of NKR agonists was not observed for the second posttreatment interval (Fig. 4, Table 1). There was no apparent change in LH secretion after administration of 1000 nmol of the NK1R agonist (Fig. 3B). As in the absence of E2, overall LH secretion was not affected by any NKR agonist treatments in the OVX + E2 goats (Table 1).
Fig. 3.
Effects of NKR subtype-selective agonists on the MUA and plasma LH in OVX + E2 goats. Representative profiles of the MUA and plasma LH concentrations in two OVX + E2 goats that received an intravenous injection of vehicle (A), 1000 nmol of the NK1R agonist GR73632 (B), 1000 nmol of the NK2R agonist GR64349 (C) and 50 nmol of NK3R agonist senktide (D) are shown. The dotted line indicates the timing of the injection. The arrowhead indicates an MUA volley induced by the treatment. The asterisk represents the LH pulse identified by the PULSAR program.
Fig. 4.
Effects of NKR subtype-selective agonists on the first and second posttreatment intervals in OVX + E2 goats. Individual data of the first (1st) and second (2nd) posttreatment intervals for administration of the NK1R agonist GR73632 (A), NK2R agonist GR64349 (B) and NK3R agonist senktide (C) are shown as the percent of the control interval for a given animal. The drug solution was injected at the midpoint between 2 successive MUA volleys (50% of the control interval after the pretreatment MUA volley). The same symbol in A–C represents the value obtained in a single goat. Closed symbols indicate values smaller than the lower 95% confidence limit of the control interval within the individual. The actual value of the control interval in each treatment is shown in Table 1.
In the present study, we also examined the effect of kisspeptin on MUA volleys and LH secretion to compare the LH-stimulatory action of kisspeptin and NKR agonists. After administration of Kp-10 in the OVX goats, LH concentrations rapidly increased, reached at a peak around 18 min after injection and then gradually decreased towards the baseline level (Fig. 5). The AUC of the standardized LH concentrations during the 1-h posttreatment period was significantly higher than that during the 1-h pretreatment period (117.2 ± 23.4 vs. 60.6 ± 9.8, P < 0.05). However, the MUA was not affected by Kp-10. The induction of MUA was not observed in any goats, and the first (25.1 ± 1.90 min) and second (28.3 ± 1.80 min) posttreatment intervals were comparable to the control interval.
Fig. 5.
Effects of Kp-10 on the MUA and plasma LH in OVX goats. Representative profiles of the MUA and plasma LH concentrations in two OVX goats that received an intravenous injection of 380 nmol of Kp-10 are shown. The dotted line indicates the timing of the injection.
Discussion
In the present study, we demonstrated that intravenous administration of NKR agonists, GR73632 (NK1R agonist), GR64349 (NK2R agonist), and senktide (NK3R agonist), dose-dependently induced an MUA volley and an accompanying LH pulse or pulse-like increase in LH secretion in OVX goats. The effects of the NK2R and NK3R, but not NK1R, agonists on the MUA and LH secretion were similarly observed in the OVX + E2 goats.
It has been reported in rats that SP and NKA stimulate LH secretion from in vitro cultured anterior pituitary cells [32] and hemi-pituitaries [33], respectively. Moreover, NKRs have been shown to exist in pituitary cells in rats [34] and sheep [35]. These studies suggest that neurokinin peptides affect LH secretion at the pituitary level. In the present study, however, the increase in LH secretion caused by the NKR agonists was observed only when the agonists evoked an MUA volley. When the agonist treatment failed to evoke an MUA volley, there was no apparent change in LH secretion. These results suggest that peripherally administered agonists acted centrally on the GnRH pulse generator and thereby stimulated pulsatile LH secretion. Whether they cross the blood-brain barrier is unknown. However, because the three NKR agonists used in the present study possess a hydrophilic structure and are water-soluble, it is unlikely that they do pass through the blood-brain barrier. Instead, it is possible that the sites of the action of the agonists are the circumventricular organs, regions characterized by an incomplete blood-brain barrier [36] or areas adjacent to such circumventricular organs. The ARC lies immediately dorsal to the median eminence, one of the circumventricular organs, and therefore has been thought to directly sense acute fluctuations of hormones in the peripheral circulation [37]. Previous studies have suggested that the ARC is involved in the control of pulsatile GnRH/LH secretion [1, 11]. Furthermore, it has been shown that NK1R, NK2R, and NK3R are all expressed in this nucleus [7, 18, 38,39,40,41,42,43]. Therefore, the ARC is one of the plausible sites of action of the peripherally administered agonists in inducing MUA volleys.
In the ARC, KNDy neurons have been demonstrated to contain NK3R in mice [4], rats [7, 44] and sheep [18]. Although the presence of NK1R and NK2R in KNDy neurons has not yet been examined, a recent in vitro study in mice showed that a cocktail of all three NKR antagonists was required to completely block the activation of KNDy neurons by NKB [21]. Therefore, it is possible that KNDy neurons co-express the three NKRs and that the peripherally administered agonists act on those receptors. Given that this is the case, it is suggested that the action of NKB in GnRH pulse generation is mediated by the three NKRs. In accordance with this, the present result showed that the three NKR agonists possess the ability to induce MUA volleys. However, there was a marked difference in their efficacies (Table 1). As little as 10 nmol of senktide induced an MUA volley in all the goats. On the other hand, the dose of 200 nmol of the NK1R and NK2R agonists was effective for zero and one goat, respectively, and the dose of 1000 nmol for these same agonists induced a positive MUA response in two and four goats, respectively. Therefore, although pharmacological activation of NK1R and NK2R by the respective agonist resulted in the induction of an MUA volley in the present study, the contributions of NK1R and NK2R signaling in endogenous GnRH pulse generation may be few, or merely supplemental, in goats. Alternatively, it cannot be ruled out that the extremely high doses of the NK1R and NK2R agonists possibly induced MUA volleys via NK3R, even though they are highly specific to their respective NKRs. Collectively, it is plausible to consider that the action of NKB in GnRH pulse generation is predominantly mediated by NK3R in goats. This suggestion is supported by the fact that the induction of an MUA volley and LH pulse by the male pheromone are blocked by the pretreatment of OVX goats with SB222200, an NK3R antagonist [45]. Moreover, an inactivating mutation of TACR3 encoding NK3R produces gonadotropin deficiency and pubertal failure in humans [46].
In contrast, it has been demonstrated in rats that pulsatile LH secretion was suppressed by icv administration of CS-003, an antagonist for all three NKRs, whereas administration of each NKR subtype-selective antagonist alone had no effect [24]. Similarly, in vitro studies showed that the activation of KNDy neurons by NKB was partially reduced by SB222200 in rats [47, 48] or completely abrogated when three NKR subtype-selective antagonists were concomitantly bath applied in mice [21]. These studies suggest that the three NKRs play an indispensable role in the activation of KNDy neurons in rodents. Taken together with the present study, it may be that although the three NKRs are involved in the GnRH pulse generation of KNDy neurons, the ratio of the contribution of each NKR conspicuously varies among species.
It has been reported in rodents that the action of senktide is affected by E2. For example, senktide inhibited LH secretion in OVX mice [4] and rats [7], whereas the peptide increased LH secretion in intact and OVX + E2 rats [7]. To address this issue, we examined the effect of higher doses of the agonists in the OVX + E2 goats. Treatment with the NK2R and NK3R agonists resulted in the induction of an MUA volley in all the OVX + E2 goats. Again, the induced MUA volleys were always followed by an LH pulse, as in the absence of E2. This result suggests that E2 has little, if any, effect on the action of NK2R and NK3R agonists in GnRH pulse generation. In the NK1R agonist treatment, induction of an MUA volley was not observed in the OVX + E2 goats, while it was observed in two OVX goats at the same drug dosage. Because the number of goats that exhibited a positive response was small (n = 2), an evaluation of the effect of E2 on NK1R agonist action could be made in the present study. However, it should be noted that the change in LH secretion induced by the peripherally administered NKR agonists was always stimulatory, and we never observed any inhibitory action of the agonists on LH secretion.
Because the effect of the agonists on MUA was observed only for the volley immediately after the treatment (first), we presume that the administered doses of the drugs were rapidly cleared from and/or broken down in the body. Importantly, the next volley (second) occurred with the regular interval observed in the control period (Table 1). This result indicates that the GnRH pulse generator activity was reset to its initial level after the NKR agonist-induced volley, as after endogenously occurring ones. Moreover, the NKR agonist treatments had no effect on overall LH secretion even in the senktide treatment in which an LH pulse or pulse-like increase in LH secretion was induced in all goats. These results suggest that the action of the NKR agonists on LH secretion is intimately associated with the GnRH pulse-generating mechanism. In addition to NKB, kisspeptin also has been suggested to play a role in GnRH pulse generation. For example, administration of a Kiss1r antagonist into the ARC inhibited the LH pulse frequency in rats [49] and sheep [50]. Moreover, intravenous administration of Kp-10 altered the timing of the occurrence of the LH pulse in men [51]. However, the Kp-10 treatment did not affect the MUA at all, and the profiles of the LH increase in the Kp-10 treatment (Fig. 5) were substantially different from those accompanying the MUA response in the NKR agonist treatments (Fig. 1, 3) in the present study. This result is consistent with previous observations in female rats [52] and castrated male goats [12] and is consistent with recent observations in OVX goats who received icv administration of kisspeptin (Yamamura et al. unpublished data). It is therefore suggested that kisspeptin stimulates LH secretion through mechanisms other than the GnRH pulse generator, unlike the NK3R agonists.
Icv administration of SP [53] or NKA [33], endogenous ligands for NK1R and NK2R, respectively, increased LH secretion, and SP neurons project their fibers to the ARC in rats [54]. Furthermore, colocalization of SP in KNDy neurons has also been suggested to occur in humans [55]. These lines of evidence imply that SP and NKA are also involved in the control of pulsatile GnRH/LH secretion. However, the present results do not support the involvement of NK1R and NK2R in GnRH pulse generation, at least in goats. It is possible that SP and NKA affect GnRH/LH secretion via mechanisms other than the GnRH pulse generator. Further studies are needed to clarify the precise roles of SP and NKA in GnRH/LH secretion and their interaction with NKB signaling.
In summary, the present study demonstrated that NK3R plays a predominant role in GnRH pulse generation. Although the involvement of NK1R and NK2R cannot be ruled out, the results suggest that their contributions to GnRH pulse generation may be few, if any, in goats.
Acknowledgments
We are grateful to Drs S Oishi and N Fujii at Kyoto University for providing senktide. We thank Drs GR Merriam and KW Wachter for the PULSAR computer program. The LH radioimmunoassay and LH pulse analysis were performed at the Nagoya University Radioisotope Center and the Nagoya University Information Technology Center, respectively. This study was supported in part by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Research Program on Innovative Technologies for Animal Breeding, Reproduction and Vaccine Development, REP-2001) and by Grants-in-Aid for Young Scientists (B) 26870842 (TY) and 25871109 (YW) from the Japan Society for the Promotion of Science. The authors wish to thank Ms Y Sakairi for her invaluable technical assistance throughout the study.
References
- 1.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res 1980; 36: 53–88. [DOI] [PubMed] [Google Scholar]
- 2.Karsch FJ. The hypothalamus and anterior pituitary gland. In: Austin CR, Short RV (eds), Reproduction in Mammals, Hormonal Control of Reproduction 2nd ed. Cambridge: Cambridge University Press; 1984: 1–20.
- 3.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010; 151: 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009; 29: 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 2010; 1364: 103–115. [DOI] [PubMed] [Google Scholar]
- 6.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 2010; 1364: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 2011; 300: E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007; 148: 5752–5760. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 2010; 151: 4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkura S, Tsukamura H, Maeda K. Effects of various types of hypothalamic deafferentation on luteinizing hormone pulses in ovariectomized rats. J Neuroendocrinol 1991; 3: 503–508. [DOI] [PubMed] [Google Scholar]
- 12.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009; 21: 813–821. [DOI] [PubMed] [Google Scholar]
- 13.Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 2004; 11: 2045–2081. [DOI] [PubMed] [Google Scholar]
- 14.Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol 1995; 26: 911–944. [DOI] [PubMed] [Google Scholar]
- 15.Reichard GA, Grice CA, Shih NY, Spitler J, Majmundar S, Wang SD, Paliwal S, Anthes JC, Piwinski JJ. Preparation of oxime dual NK(1)/NK(2) antagonists with reduced NK(3) affinity. Bioorg Med Chem Lett 2002; 12: 2355–2358. [DOI] [PubMed] [Google Scholar]
- 16.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 2010; 166: 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among Kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev 2013; 59: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 2010; 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro VM. Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol 2013; 784: 325–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamura H, Yamamura T, Wakabayashi Y. Kisspeptin as a master player in the central control of reproduction in mammals: an overview of kisspeptin research in domestic animals. Anim Sci J 2013; 84: 369–381. [DOI] [PubMed] [Google Scholar]
- 21.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology 2013; 154: 2750–2760. [DOI] [PubMed] [Google Scholar]
- 22.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology 2013; 154: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahara T, Uenoyama Y, Iwase A, Oishi S, Nakamura S, Minabe S, Watanabe Y, Deura C, Noguchi T, Fujii N, Kikkawa F, Maeda K, Tsukamura H. Chronic peripheral administration of kappa-opioid receptor antagonist advances puberty onset associated with acceleration of pulsatile luteinizing hormone secretion in female rats. J Reprod Dev 2013; 59: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev 2011; 57: 409–415. [DOI] [PubMed] [Google Scholar]
- 25.Seabrook GR, Bowery BJ, Hill RG. Pharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slices. Eur J Pharmacol 1995; 273: 113–119. [DOI] [PubMed] [Google Scholar]
- 26.Deal MJ, Hagan RM, Ireland SJ, Jordan CC, McElroy AB, Porter B, Ross BC, Stephens-Smith M, Ward P. Conformationally constrained tachykinin analogues: potent and highly selective neurokinin NK-2 receptor agonists. J Med Chem 1992; 35: 4195–4204. [DOI] [PubMed] [Google Scholar]
- 27.Ichimaru T, Mori Y, Okamura H. A possible role of neuropeptide Y as a mediator of undernutrition to the hypothalamic gonadotropin-releasing hormone pulse generator in goats. Endocrinology 2001; 142: 2489–2498. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi Y, Yamamura T, Ohkura S, Homma T, Sakamoto K, Mori Y, Okamura H. Senktide, a neurokinin B receptor agonist, stimulates pulsatile LH secretion through a mechanism mediated by the GnRH pulse generator in goats. In: Program of the 42nd Society for Neuroscience Annual Meeting; 2012; New Orleans, USA, Abstract 90.07.
- 29.Mori Y, Kano Y. Changes in plasma concentrations of LH, progesterone and oestradiol in relation to the occurrence of luteolysis, oestrus and time of ovulation in the Shiba goat (Capra hircus). J Reprod Fertil 1984; 72: 223–230. [DOI] [PubMed] [Google Scholar]
- 30.Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology 2004; 145: 3239–3246. [DOI] [PubMed] [Google Scholar]
- 31.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 32.Shamgochian MD, Leeman SE. Substance P stimulates luteinizing hormone secretion from anterior pituitary cells in culture. Endocrinology 1992; 131: 871–875. [DOI] [PubMed] [Google Scholar]
- 33.Kalra PS, Sahu A, Bonavera JJ, Kalra SP. Diverse effects of tachykinins on luteinizing hormone release in male rats: mechanism of action. Endocrinology 1992; 131: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 34.Larsen PJ, Saermark T, Mau SE. Binding of an iodinated substance P analogue to cultured anterior pituitary prolactin- and luteinizing hormone-containing cells. J Histochem Cytochem 1992; 40: 487–493. [DOI] [PubMed] [Google Scholar]
- 35.Dupré SM, Miedzinska K, Duval CV, Yu L, Goodman RL, Lincoln GA, Davis JR, McNeilly AS, Burt DD, Loudon AS. Identification of Eya3 and TAC1 as long-day signals in the sheep pituitary. Curr Biol 2010; 20: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol 2010; 518: 943–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belgardt BF, Okamura T, Brüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol 2009; 587: 5305–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buck SH, Helke CJ, Burcher E, Shults CW, O’Donohue TL. Pharmacologic characterization and autoradiographic distribution of binding sites for iodinated tachykinins in the rat central nervous system. Peptides 1986; 7: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 39.Larsen PJ, Jessop DS, Chowdrey HS, Mikkelsen JD, Lightman SL. Osmotic regulation of substance P and neurokinin A peptide content and substance P binding sites in distinct hypothalamic nuclei of the rat. Peptides 1992; 13: 705–712. [DOI] [PubMed] [Google Scholar]
- 40.Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol 1996; 364: 290–310. [DOI] [PubMed] [Google Scholar]
- 41.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol 1996; 372: 395–414. [DOI] [PubMed] [Google Scholar]
- 42.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 2005; 489: 372–386. [DOI] [PubMed] [Google Scholar]
- 43.de Lange RP, Wiegant VM, Stam R. Altered neuropeptide Y and neurokinin messenger RNA expression and receptor binding in stress-sensitised rats. Brain Res 2008; 1212: 35–47. [DOI] [PubMed] [Google Scholar]
- 44.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 2006; 498: 712–726. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto K, Wakabayashi Y, Yamamura T, Tanaka T, Takeuchi Y, Mori Y, Okamura H. A population of kisspeptin/neurokinin B neurons in the arcuate nucleus may be the central target of the male effect phenomenon in goats. PLoS ONE 2013; 8: e81017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 2009; 41: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O’Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology 2012; 153: 4894–4904. [DOI] [PubMed] [Google Scholar]
- 48.Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, García-Galiano D, Hobbs SJ, Manfredi-Lozano M, León S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 2012; 32: 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 2009; 4: e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013; 154: 4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan Y-M, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr, Ren C, Chan KK, Seminara SB. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab 2011; 96: E908–E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinsey-Jones JS, Li XF, Luckman SM, O’Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 2008; 149: 1004–1008. [DOI] [PubMed] [Google Scholar]
- 53.Vijayan E, McCann SM. In vivo and in vitro effects of substance P and neurotensin on gonadotropin and prolactin release. Endocrinology 1979; 105: 64–68. [DOI] [PubMed] [Google Scholar]
- 54.Tsuruo Y, Kawano H, Nishiyama T, Hisano S, Daikoku S. Substance P-like immunoreactive neurons in the tuberoinfundibular area of rat hypothalamus. Light and electron microscopy. Brain Res 1983; 289: 1–9. [DOI] [PubMed] [Google Scholar]
- 55.Hrabovszky E, Borsay BA, Rácz K, Herczeg L, Ciofi P, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Substance p immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS ONE 2013; 8: e72369. [DOI] [PMC free article] [PubMed] [Google Scholar]