Abstract
Melatonin protects luteinized granulosa cells (GCs) from oxidative stress in the follicle during ovulation. However, it is unclear in which cellular components (e.g., nuclei, mitochondria, or plasma membranes) melatonin works as an antioxidant. GCs from immature (3 wks) ICR mice were incubated with hydrogen peroxide (H2O2; 0.01, 0.1, 1, 10 mM) in the presence or absence of melatonin (100 μg/ml) for 2 h. DNA damage was assessed by fluorescence-based immunocytochemistry using specific antibodies for 8-hydroxydeoxyguanosine (8-OHdG), an indicator of oxidative guanine base damage in DNA, and for histone H2AX phosphorylation (γH2AX), a marker of double-strand breaks of DNA. Mitochondrial function was assessed by the fluorescence intensity of MitoTracker Red probes, which diffuse across the membrane and accumulate in mitochondria with active membrane potentials. Lipid peroxidation of plasma membranes was analyzed by measuring hexanoyl-lysine (HEL), a oxidative stress marker for lipid peroxidation. Apoptosis of GCs was assessed by nuclear fragmentation using DAPI staining, and apoptotic activities were evaluated by caspase-3/7 activities. H2O2 treatment significantly increased the fluorescence intensities of 8-OHdG and γH2AX, reduced the intensity of MitoTracker Red in the mitochondria, increased HEL concentrations in GCs, and enhanced the number of apoptotic cells and caspase-3/7 activities. All these changes were significantly decreased by melatonin treatment. Melatonin reduced oxidative stress-induced DNA damage, mitochondrial dysfunction, lipid peroxidation, and apoptosis in GCs, suggesting that melatonin protects GCs by reducing oxidative stress of cellular components including nuclei, mitochondria, and plasma membranes. Melatonin helps to maintain the integrity of GCs as an antioxidant in the preovulatory follicle.
Keywords: Granulosa cells, Melatonin, Oxidative stress, Reactive oxygen species
Granulosa cells are induced to differentiate into luteal cells by a surge of ovulatory luteinizing hormone (LH). The ovulatory LH surge induces the expression of steroidogenic acute regulatory (StAR) protein, a rate-limiting step for progesterone synthesis [1,2,3]. Progesterone produced by granulosa cells undergoing luteinization is necessary for ovulation and subsequent corpus luteum formation. On the other hand, during ovulation after the LH surge, reactive oxygen species (ROS) are locally produced by macrophages, neutrophils and vascular endothelial cells within the follicle [4], suggesting that granulosa cells are exposed to elevated levels of ROS. ROS play a physiological role in ovulation, e.g., in follicle rupture, while an excessive amount of ROS can damage both the ovum and the granulosa cells undergoing luteinization.
ROS damage cellular components including nuclei, mitochondria and plasma membranes, resulting malfunctioning DNA, loss of membrane integrity and mitochondrial dysfunction; the latter in particular is often related to apoptosis [5,6,7,8]. Importantly, antioxidant enzymes including superoxide dismutase, glutathione peroxidase and catalase, and non-enzymatic antioxidants such as melatonin, vitamin E, vitamin C, glutathione, uric acid and albumin are present in the follicles [9,10,11]. The balance between ROS and the antioxidants within the follicle seems to be critical to the integrity and function of granulosa cells undergoing luteinization during ovulation.
Melatonin and its metabolites are powerful free radical scavengers and broad-spectrum antioxidants [12,13,14]. Interestingly, melatonin is present in high concentrations in the preovulatory follicle [15,16,17,18,19], and the concentration of melatonin in follicular fluids increases with increasing follicle size [15, 16]. Because of its small size and highly lipophilic properties [12, 20], melatonin passes through all cell membranes and easily reaches cellular components including nuclei, mitochondria, and plasma membranes, where it seems to accumulate in high concentrations [21,22,23]. Melatonin prevents DNA damage [24, 25] and lipid peroxidation of plasma membranes [26, 27]. In particular, melatonin preserves optimal mitochondrial function and homeostasis by reducing and preventing oxidative stress, thereby curtailing subsequent apoptotic events and cell death [12, 28, 29]. We recently reported that hydrogen peroxide (H2O2) inhibited progesterone production by human luteinized granulosa cells and that melatonin abolished the inhibitory effect of H2O2 [30]. However, it is unclear on which cellular components melatonin works as an antioxidant to protect granulosa cells. Therefore, this study was conducted to investigate whether melatonin reduces oxidative stress in cellular components including in the nuclei, mitochondria, and plasma membranes.
Materials and Methods
Collection and culture of granulosa cells
The experimental protocol was approved by the Committee for Ethics on Animal Experimentation, and performed under the Guidelines for Animal Experiments at Yamaguchi University Graduate School of Medicine in accordance with Law No. 105 and Notification No. 6 of the Japanese Government. Immature (3 wks) ICR mice (Japan SLC, Hamamatsu, Japan) were housed in a controlled room with a 14:10 light:dark photoperiod and free access to standard mouse chow and water. All mice received a subcutaneous injection of 20 units of pregnant mare serum gonadotropin (PMSG, G4877; Sigma-Aldrich, St. Louis, MO, USA) to stimulate the development of multiple follicles. All mice were laparotomized under deep ether anesthesia 48 h after the PMSG injection; the ovaries were quickly removed for the following experiments, and the mice were euthanized by exsanguinations. The ovaries were transferred to alpha Modified Eagle Minimum Essential Medium (αMEM) without phenol red (M4655, Sigma-Aldrich) supplemented with penicillin-streptomycin (15070-063, Invitrogen, Carlsbad, CA, USA). Granulosa cells were collected by puncturing mature preovulatory follicles with a 26-gauge needle under a dissecting microscope. Granulosa cells were isolated, centrifuged at 800 x g, washed in PBS (Wako Pure Chemical Industries, Osaka, Japan) twice, and used for cell culture. The cells were preincubated at a density of 2.5 × 104 cells/well in 100 μl of αMEM for 30 min, and then incubated with H2O2 (8104215, 0.01, 0.1, 1, and 10 mM; Wako Pure Chemical Industries) in the presence or absence of melatonin (M5250, 100 μg/ml; Sigma-Aldrich) for 2 h. After incubation, cells were used for evaluation of DNA damages, mitochondrial function, lipid peroxidation, and apoptosis as described below.
DNA damage
DNA damage was assessed by fluorescence-based immunocytochemistry using specific antibodies for 8-hydroxydeoxyguanosine (8-OHdG), an indicator of oxidative guanine base damage of DNA, and for histone H2AX phosphorylation (γH2AX), a marker of double-strand breaks of DNA. Granulosa cells were fixed with 4% paraformaldehyde (L3N8367, Nacalai Tesque, Kyoto, Japan) for 15 min, washed three times in PBS, and incubated in 0.5% Triton X-100 (T8787, Sigma-Aldrich) in PBS for 15 min. Then, the cells were incubated with primary antibodies against either 8-OHdG (mouse monoclonal IgG, sc-66036, 1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 h, and they were then incubated with a secondary antibody (Alexa Fluor 488-labeled goat anti mouse IgG, A-11001, 1:200 dilution; Invitrogen) at room temperature for 40 min. The cells were also incubated with the antibody against γH2AX (Alexa Fluor 488-labeled rabbit monoclonal IgG, 9719S, 1:100 dilution; Cell Signaling Technology, Danvers, MA, USA) at room temperature for 2 h. To counterstain the DNA material, granulosa cells were mounted onto slides and counterstained with 50 μg/ml 4’,6’-diamidino-2-phenylondole (DAPI, 340-07971; Wako Pure Chemical Industries) in Vectashield mounting medium (H-1200, Vector Laboratories, Burlingame, CA, USA) under a cover slip. Fluorescence of 8-OHdG and γH2AX in the nuclei was detected and imaged under a confocal laser scanning microscope (Zeiss LSM 510 META; Carl Zeiss, Jena, Germany) utilizing a 488 nm excitation. Fluorescence images were captured by ZEN imaging software (ZEN 2008; Carl Zeiss), and the fluorescence intensity from each cell was quantified using the CellProfiler and ImageJ software (National Institutes of Health, Bethesda, MD, USA). The fluorescence intensities of 8-OHdG and γH2AX were measured in at least 200 cells per treatment group, and the mean fluorescence intensity was used as the level of DNA damage.
Mitochondrial function
Mitochondrial function was evaluated by the mitochondrial membrane potential, which was quantified by using mitochondrial-targeted fluorescent probes (MitoTracker Red, M7512, Invitrogen). MitoTracker Red diffuses across cell membranes and accumulates in mitochondria with active membrane potentials. Granulosa cells were incubated with MitoTracker Red (100 nM) at room temperature for 15 min. The cells were washed twice with PBS and fixed with 4% paraformaldehyde for 15 min. Then, the cells were washed three times with PBS and mounted on glass slides. Fluorescence was detected and imaged under a confocal laser scanning microscope (Zeiss LSM 510 META) utilizing 543 nm excitation. Fluorescence images were captured by the ZEN software, and the fluorescence intensity and area of MitoTracker Red staining in at least 200 granulosa cells was quantified by the ImageJ software. A reduction in the intensity and area of MitoTracker Red staining is an indicative of reduced mitochondrial membrane potentials.
Hexanoyl-lysine (HEL) assay
Lipid peroxidation of cell membranes was analyzed by measuring HEL, a stable oxidative stress marker for lipid peroxidation. HEL levels in cells were measured with a competitive enzyme-linked immunosorbent assay (ELISA) kit (KHL-700, Japan Institute for the Control of Aging, Nikken SEIL, Shizuoka, Japan) as reported previously [17]. After incubation, granulosa cells were resuspended in 100 μl αMEM, lysed by sonication and used for the HEL assay. The assay procedures were performed according to the manufacturer’s recommendations. Lipid peroxidation of cell membranes was determined as the overall HEL level per well with 2.5×104 granulosa cells. The minimal detectable concentration of HEL was estimated to be 2 nmol/l.
Apoptosis
Apoptosis of granulosa cells was assessed by morphological changes of nuclei and caspase-3/7 activities. Apoptotic morphological changes of nuclei were evaluated by DAPI staining. Granulosa cells were fixed with 4% paraformaldehyde for 15 min. Then, the cells were washed three times in PBS, mounted on glass slides, and stained with 50 μg/ml DAPI in Vectashield mounting medium under a cover slip. Fluorescence was detected and imaged under a confocal laser scanning microscope (Zeiss LSM 510 META). Apoptotic cells were identified by condensation and fragmentation of the nuclei, and apoptosis was quantified by calculating the percentage of apoptotic nuclei in a total of 200 nuclei in each treatment group.
Caspase-3/7 activities were determined by a Caspase-Glo™ 3/7 Assay (G8091, Promega, Mannheim, Germany) according to the manufacturer’s protocol. Briefly, granulosa cells were cultured on a 96-well plate with 2.5×104 cells in 100 μl medium/well, and 100 μl of Caspase-Glo® reagent was added to each well and incubated for 2 h at room temperature. The luminescence of each well was measured by luminometer (Berthold Micro Lumat LB96P; Berthold Technologies, Bad Wildbad, Germany) with an excitation wavelength of 499 nm and an emission wavelength of 521 nm. Apoptotic activities were quantified as the level of fluorescence emitted from Caspase-Glo® reagent bound to caspase-3/7. Caspase-3/7 activities were determined as the overall activities per well with 2.5×104 granulosa cells and shown as relative fluorescence units (RFU).
Statistical analysis
All experiments were performed with three independent incubations. Statistical analysis was carried out using the computer program SPSS for Windows13.0. The Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction analysis were used. A value of P < 0.05 was considered significant.
Results
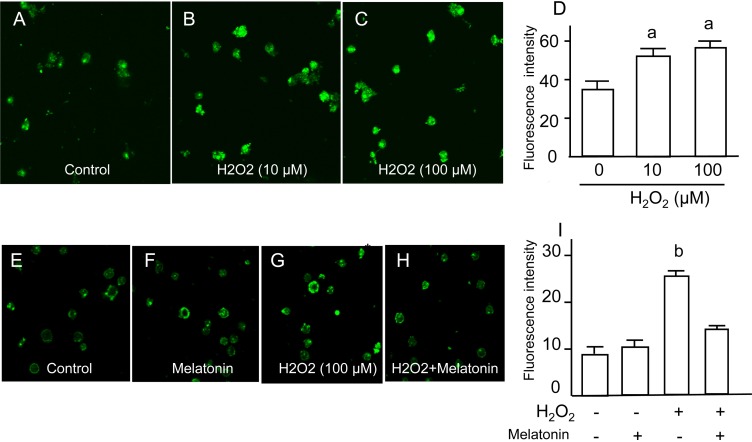
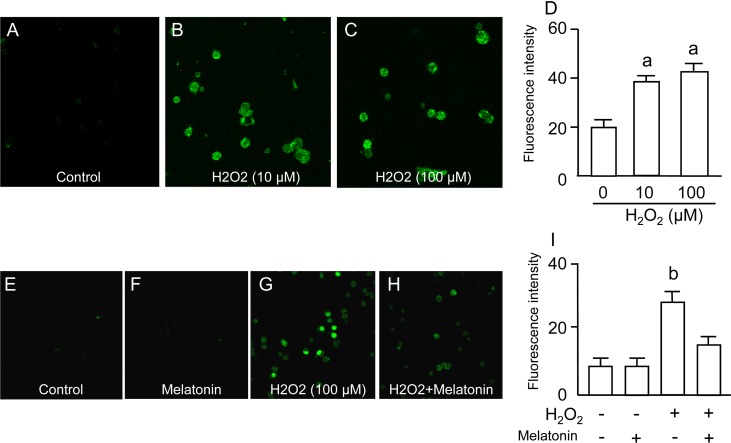
DNA damage in granulosa cells was assessed by fluorescence-based immunocytochemistry using specific antibodies for 8-OHdG (an indicator of oxidative guanine base damage of DNA) and γH2AX (a marker of double-strand breaks of DNA). The fluorescence intensity of 8-OHdG was dose-dependently increased by H2O2 treatment (Fig. 1A–D), and the increase in 8-OHdG intensities induced by H2O2 was completely blocked by melatonin treatment (Fig. 1E–I). The fluorescence intensity of γH2AX was also dose-dependently increased by H2O2 treatment (Fig. 2A–D), and the increase in γH2AX intensities induced by H2O2 was completely blocked by melatonin treatment (Fig. 2E–I).
Fig. 1.
Effects of H2O2 and/or melatonin on 8-Hydroxydeoxyguanosine (8-OHdG) in granulosa cells. Granulosa cells were incubated with H2O2 (0, 10, 100 mM) for 2 h. The oxidative guanine base damage of DNA was assessed by fluorescence-based immunocytochemistry using specific antibodies for 8-OHdG. (A) Control. (B) H2O2 (10 μM). (C) H2O2 (100 μM). (D) The fluorescence intensity of each group was analyzed using the CellProfiler software. Granulosa cells were also incubated with H2O2 (100 μM) in the presence or absence of melatonin (100 μg/ml) for 2 h. (E) Control. (F) Melatonin (100 μg/ml). (G) H2O2 (100 μM). (H) H2O2 (100 μM) + melatonin (100 μg/ml). (I) The fluorescence intensity of each group was analyzed as described above. Data are shown as the mean ± SEM of three independent incubations. a, P < 0.05 vs. control, and b, P < 0.05 vs. the other groups (Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction).
Fig. 2.
Effects of H2O2 and/or melatonin on histone H2AX phosphorylation (gH2AX) in granulosa cells. Granulosa cells were incubated with H2O2 (0, 10, 100 μM) for 2 h. The double-strand breaks of DNA were assessed by fluorescence-based immunocytochemistry using specific antibodies for gH2AX. (A) Control. (B) H2O2 (10 μM). (C) H2O2 (100 μM). (D) The fluorescence intensity of each group was analyzed using the CellProfiler software. Granulosa cells were also incubated with H2O2 (100 μM) in the presence or absence of melatonin (100 μg/ml) for 2 h. (E) Control. (F) Melatonin (100 μg/ml). (G) H2O2 (100 μM). (H) H2O2 (100 μM) + melatonin (100 μg/ml). (I) The fluorescence intensity of each group was analyzed as described above. Data are shown as the mean ± SEM of three independent incubations. a, P < 0.05 vs. control, and b, P < 0.05 vs. the other groups (Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction).
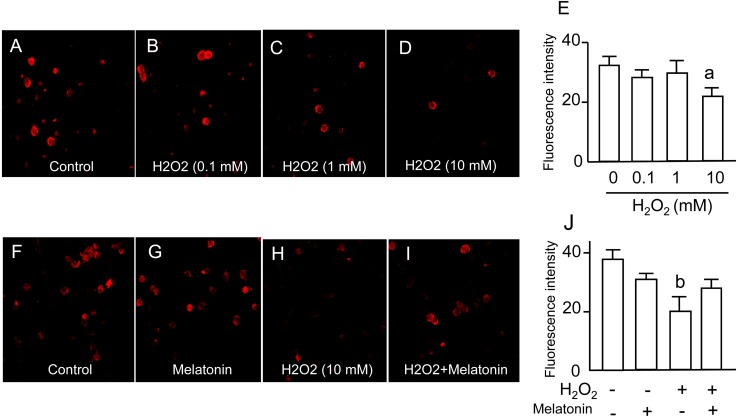
Mitochondrial function of granulosa cells was assessed by the fluorescence intensities of MitoTracker Red probes, which diffuse across the membrane and accumulate in mitochondria with active membrane potentials. The intensity of MitoTracker Red was significantly decreased by 10 mM of H2O2 (Fig. 3A–E). The decrease in MitoTracker Red intensities caused by H2O2 was significantly reversed by melatonin treatment (Fig. 3F–J).
Fig. 3.
Effects of H2O2 and/or melatonin on mitochondrial function. Granulosa cells were incubated with H2O2 (0.1, 1, 10 mM) for 2 h, and then the cells were loaded with a mitochondrial-targeted fluorescent probe, MitoTracker Red, at a concentration of 100 nM for 15 min. The fluorescence images were obtained using a confocal laser scanning microscope. (A) Control. (B) H2O2 (0.1 μM). (C) H2O2 (1 mM). (D) H2O2 (10 mM). (E) The fluorescence intensity of each group was analyzed using the ImageJ software. Granulosa cells were also incubated with H2O2 (10 mM) in the presence or absence of melatonin (100 μg/ml) for 2 h. (F) Control. (G) Melatonin (100 μg/ml). (H) H2O2 (10 mM). (I) H2O2 (10 mM) + melatonin (100 μg/ml). (J) The fluorescence intensity of each group was analyzed as described above. Data are shown as the mean ± SEM of three independent incubations. a, P < 0.05 vs. control, and b, P < 0.05 vs. the other groups (Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction).
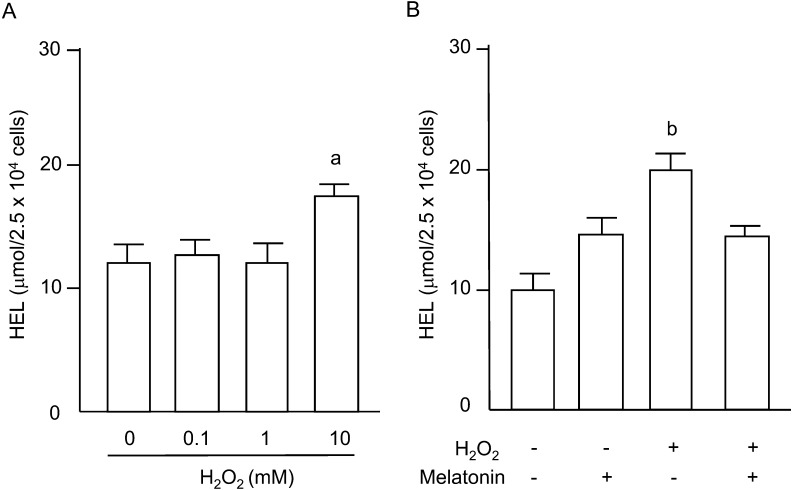
Lipid peroxidation of plasma membranes in granulosa cells was evaluated by measuring HEL, a stable oxidative stress marker for lipid peroxidation. The HEL concentrations were significantly increased by 10 mM of H2O2 (Fig. 4A), and the increase was significantly blocked by melatonin treatment (Fig. 4B).
Fig. 4.
Effects of H2O2 and/or melatonin on lipid peroxidation. (A) Granulosa cells were incubated with H2O2 (0.1, 1, 10 mM) for 2 h. (B) Granulosa cells were incubated with H2O2 (10 mM) in the presence or absence of melatonin (100 μg/ml) for 2 h. Lipid peroxidation of cell membranes was analyzed by measuring hexanoyl-lysine (HEL). Data are shown as the mean ± SEM of three independent incubations. a, P < 0.05 vs. control, and b, P < 0.05 vs. the other groups (Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction).
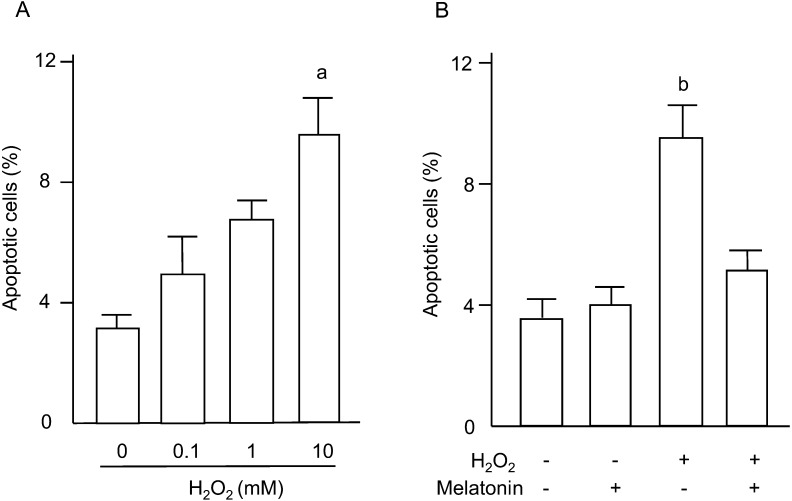
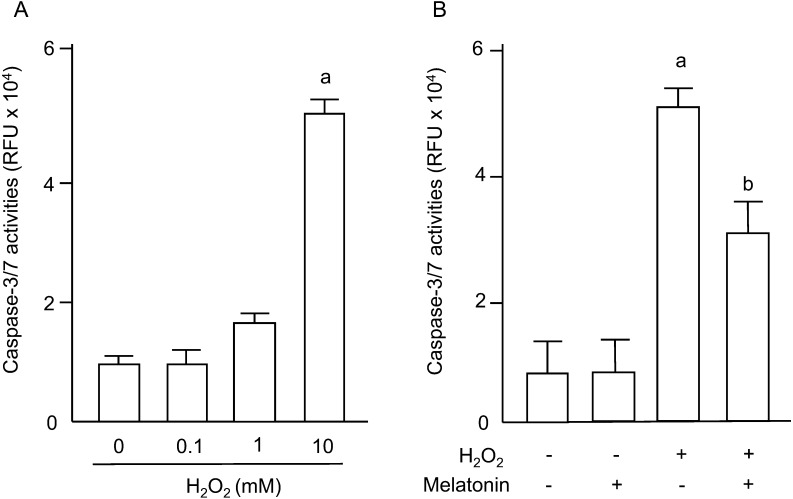
Apoptosis of granulosa cells was assessed by nuclear fragmentation using DAPI staining and by the caspase-3/7 activities. The percentage of apoptotic cells was dose-dependently increased by H2O2 (Fig. 5A), and the increase in numbers of apoptotic cells induced by H2O2 was completely blocked by melatonin treatment (Fig. 5B). Caspase-3/7 activities in granulosa cells were significantly increased by 10 mM of H2O2 (Fig. 6A), and the increase was significantly decreased by melatonin treatment (Fig. 6B).
Fig. 5.
Effects of H2O2 and/or melatonin on apoptosis of granulosa cells. (A) Granulosa cells were incubated with H2O2 (0.1, 1, 10 mM) for 2 h. (B) Granulosa cells were incubated with H2O2 (10 mM) in the presence or absence of melatonin (100 μg/ml) for 2 h. Apoptosis of granulosa cells was assessed by nuclear fragmentation using DAPI staining. Apoptotic cells were identified by condensation and fragmentation of the nuclei. Apoptosis was quantified by calculating the percentage of apoptotic nuclei in a total of 200 nuclei in each group. Data are shown as the mean ± SEM of three independent incubations. a, P < 0.05 vs. control, and b, P < 0.05 vs. the other groups (Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction).
Fig. 6.
Effects of H2O2 and/or melatonin on caspase-3/7 activities of granulosa cells. (A) Granulosa cells were incubated with H2O2 (0.1, 1, 10 mM) for 2 h. (B) Granulosa cells were incubated with H2O2 (10 mM) in the presence or absence of melatonin (100 μg/ml) for 2 h. Caspase-3/7 activities were determined as overall activities in 2.5 × 104 granulosa cells and shown as relative fluorescence units (RFU). Data are shown as the mean ± SEM of three independent incubations. a, P < 0.01 vs. control, and b, P < 0.05 vs. H2O2 (Kruskal-Wallis H-test and Mann-Whitney U-test with Bonferroni correction).
Discussion
The present study showed that oxidative stress damages nuclei, mitochondria and plasma membranes in granulosa cells, resulting in DNA damage, mitochondrial dysfunction, and lipid peroxidation of plasma membranes, which likely cause disruption of cellular integrity and apoptosis. Furthermore, our results clearly showed that melatonin protects the integrity of granulosa cells by reducing oxidative stress in each of these cellular components.
Various indicators of DNA damage have been reported; these include, oxidative base damage, telomere shortening, chromosome fragmentation, single-strand breaks and double-strand breaks. The double-strand breaks are the most lethal forms of DNA damages because they cause cellular senescence and apoptosis [31,32,33]. In the present study, DNA damage by oxidative stress was evaluated by γH2AX (a sensitive marker of double-strand breaks) [33] in addition to 8-OHdG (an indicator of oxidative guanine base damage). The present study showed that melatonin blocks both oxidative stress-induced guanine base damages and double-strand breaks in granulosa cells. This is consistent with previous reports that melatonin reduces the DNA damage as assessed by γH2AX in rat germ cells [34] and the rat brain [35].
The mitochondrial membranes are important sites for steroidogenesis in granulosa cells. Cholesterol, a substrate of steroid hormones, is transferred from the outer to the inner mitochondrial membrane by StAR protein [36, 37], which is in turn metabolized to pregnenolone by the cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc). Damage to mitochondrial membranes by oxidative stress impairs steroidogenesis in granulosa cells. In fact, oxidative stress has been reported to inhibit steroidogenic enzymes and a mitochondrial carrier protein (StAR protein) involved in cholesterol transport into mitochondria of luteal cells [38]. The present study showed that oxidative stress damages mitochondrial function of granulosa cells and that melatonin reduces the oxidative stress in the mitochondria, suggesting that melatonin protects granulosa cells by reducing the mitochondrial membrane damage caused by oxidative stress. These findings support the previous reports that melatonin preserves mitochondrial function by reducing electron leakage and protecting the mitochondrial membrane [39, 40] and that melatonin increases respiratory chain complex I and IV activities and ATP synthesis [41].
Oxidative stress-induced damage to plasma membranes was evaluated by lipid peroxidation. A number of studies have reported that oxidative stress inhibits progesterone production by luteal cells through lipid peroxidation of the plasma membrane [38, 42]. Our previous study also showed that H2O2 inhibits progesterone production by human luteinized granulosa cells and that melatonin abolished the inhibitory effect of H2O2 on progesterone production [30]. Furthermore, it has been reported that lipid peroxidation of the plasma membranes of luteal cells is involved in corpus luteum regression through the disruption of cellular integrity [43, 44]. Taken together, the present results document that melatonin prevents oxidative stress-induced lipid peroxidation of plasma membranes in granulosa cells, as in other cells.
The primary function of mitochondria is to generate ATP through the five-complex electron transport chain in the mitochondrial membrane. Mitochondrial damage by oxidative stress is commonly related to cell death. Oxidative stress induces the mitochondrial membrane to release cytochrome c, which activates caspase-9 activity and triggers the downstream caspase cascade including the activation of caspase-3 [45]. Caspases are central mediators of apoptosis [46]; in particular, caspase-3 activation is a major contributor to apoptotic processes [47]. The present study showed that oxidative stress increased the caspase-3/7 activities and the percentage of apoptotic granulosa cells; moreover, the results showed that the apoptotic effects of oxidative stress were blocked by melatonin treatment. Thus, melatonin likely prevented apoptosis of granulosa cells by reducing oxidative stress. These data are consistent with recent reports that melatonin prevents apoptosis by regulating caspase-3 and Bax/BCL-2 in the gastric mucosa [48], leukocytes [49], and bone marrow mesenchymal stem cells [50].
Although melatonin directly scavenges free radicals, recent reports showed that melatonin receptors are present in granulosa cells [51, 52]. However, the role of receptor-mediated actions of melatonin in ovarian function is unclear, and little information is available concerning how melatonin receptors change during estrous cycle. It has been reported that melatonin increases the expression of other antioxidant enzymes such as SOD and glutathione peroxidase through melatonin receptors [53, 54]. In this study, the antioxidant effect was found 2 h after melatonin treatment, suggesting the direct antioxidant action of melatonin, but not the receptor-mediated action. However, it would be interesting to investigate whether melatonin works as an antioxidant through its receptors, for example, by upregulating the expression of antioxidant enzymes, in a future study.
H2O2 caused significant DNA damage at the concentration of 10 μM, while concentrations of H2O2 higher than 10 mM were necessary to cause mitochondrial damages, lipid peroxidation and apoptosis with increased caspase 3/7 activities. The difference in the effective concentrations of H2O2 may be due to the difference in sensitivities to ROS among cellular components of granulosa cells. DNA has high sensitivity to ROS, but it is rapidly repaired. In contrast, mitochondria is relatively resistant to ROS because it is always exposed to ROS that it produces. Therefore, DNA is damaged by relatively low H2O2 concentrations, and higher concentrations of H2O2 are necessary to cause mitochondrial damages and the subsequent apoptotic events.
The melatonin concentration used in this study was high compared with the physiological concentrations in the follicle [15]. After melatonin reacts with ROS, the melatonin metabolites are produced and accumulated in vivo. Interestingly, the melatonin metabolites also work as antioxidants, resulting in melatonin and melatonin metabolites working together as powerful antioxidants in vivo. Therefore, a high concentration of melatonin was used in this study to well reflect the in vivo condition.
The present study showed that melatonin reduces the oxidative stress-induced DNA damage, mitochondrial dysfunction, lipid peroxidation, and apoptosis of granulosa cells, showing that melatonin protects these cells by reducing free radical damage of cellular components including nuclei, mitochondria, and plasma membranes. We previously reported that melatonin is present in high concentrations in the preovulatory follicle [15, 16]. Collectively, these results suggest that melatonin helps to maintain the integrity of granulosa cells in the follicle as an antioxidant. In addition, we recently found that melatonin protects oocytes from oxidative stress in the follicle during ovulation [15,16,17,18,19, 55]. Thus, melatonin, acting as an antioxidant, contributes to not only oocyte maturation but also luteinization of granulosa cells in the follicle during ovulation.
Acknowledgments
This work was supported in part by JSPS KAKENHI Grants 24592471, 24791704, 24791705, 25293343, 25462559, 25462560, 25861495, 26670726, 26861328, 26861329, 26861330, and 26462492 for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Hickey GJ, Chen SA, Besman MJ, Shively JE, Hall PF, Gaddy-Kurten D, Richards JS. Hormonal regulation, tissue distribution, and content of aromatase cytochrome P450 messenger ribonucleic acid and enzyme in rat ovarian follicles and corpora lutea: relationship to estradiol biosynthesis. Endocrinology 1988; 122: 1426–1436. [DOI] [PubMed] [Google Scholar]
- 2.Lee L, Asada H, Kizuka F, Tamura I, Maekawa R, Taketani T, Sato S, Yamagata Y, Tamura H, Sugino N. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology 2013; 154: 458–470. [DOI] [PubMed] [Google Scholar]
- 3.Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 1998; 139: 303–315. [DOI] [PubMed] [Google Scholar]
- 4.Brännström M, Norman RJ. Involvement of leukocytes and cytokines in the ovulatory process and corpus luteum function. Hum Reprod 1993; 8: 1762–1775. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 2001; 18: 685–716. [DOI] [PubMed] [Google Scholar]
- 6.Mishra DP, Dhali A. Endotoxin induces luteal cell apoptosis through the mitochondrial pathway. Prostaglandins Other Lipid Mediat 2007; 83: 75–88. [DOI] [PubMed] [Google Scholar]
- 7.Nagy IZ. On the true role of oxygen free radicals in the living state, aging, and degenerative disorders. Ann N Y Acad Sci 2001; 928: 187–199. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Jing L, Yuan H, Peng SQ. T-2 toxin induces apoptosis in ovarian granulosa cells of rats through reactive oxygen species-mediated mitochondrial pathway. Toxicol Lett 2011; 202: 168–177. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Gubory KH, Bolifraud P, Germain G, Nicole A, Ceballos-Picot I. Antioxidant enzymatic defence systems in sheep corpus luteum throughout pregnancy. Reproduction 2004; 128: 767–774. [DOI] [PubMed] [Google Scholar]
- 11.Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med 2008; 44: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res 2010; 48: 297–310. [DOI] [PubMed] [Google Scholar]
- 13.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 2007; 42: 28–42. [DOI] [PubMed] [Google Scholar]
- 14.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res 2013; 54: 245–257. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril 2003; 80: 1012–1016. [DOI] [PubMed] [Google Scholar]
- 16.Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril 2009; 92: 328–343. [DOI] [PubMed] [Google Scholar]
- 17.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008; 44: 280–287. [DOI] [PubMed] [Google Scholar]
- 18.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y, Sugino N. The role of melatonin as an antioxidant in the follicle. J Ovarian Res 2012; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J 2013; 60: 1–13. [DOI] [PubMed] [Google Scholar]
- 20.Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol 2009; 44: 175–200. [DOI] [PubMed] [Google Scholar]
- 21.León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res 2005; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- 22.Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 2000; 14: 1677–1679. [DOI] [PubMed] [Google Scholar]
- 23.Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 2012; 52: 217–227. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto HA, Mohanan PV. Melatonin attenuates brain mitochondria DNA damage induced by potassium cyanide in vivo and in vitro. Toxicology 2002; 179: 29–36. [DOI] [PubMed] [Google Scholar]
- 25.Erenberk U, Dundaroz R, Gok O, Uysal O, Agus S, Yuksel A, Yilmaz B, Kilic U. Melatonin attenuates phenytoin sodium-induced DNA damage. Drug Chem Toxicol 2014; 37: 233–239. [DOI] [PubMed] [Google Scholar]
- 26.Akbulut KG, Gonul B, Akbulut H. The role of melatonin on gastric mucosal cell proliferation and telomerase activity in ageing. J Pineal Res 2009; 47: 308–312. [DOI] [PubMed] [Google Scholar]
- 27.Carretero M, Escames G, López LC, Venegas C, Dayoub JC, García L, Acuña-Castroviejo D. Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res 2009; 47: 192–200. [DOI] [PubMed] [Google Scholar]
- 28.Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, Chen JY, Wu HY, Chen CC, Hsu LF. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res 2007; 43: 389–403. [DOI] [PubMed] [Google Scholar]
- 29.Milczarek R, Hallmann A, Sokołowska E, Kaletha K, Klimek J. Melatonin enhances antioxidant action of alpha-tocopherol and ascorbate against NADPH- and iron-dependent lipid peroxidation in human placental mitochondria. J Pineal Res 2010; 49: 149–155. [DOI] [PubMed] [Google Scholar]
- 30.Taketani T, Tamura H, Takasaki A, Lee L, Kizuka F, Tamura I, Taniguchi K, Maekawa R, Asada H, Shimamura K, Reiter RJ, Sugino N. Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res 2011; 51: 207–213. [DOI] [PubMed] [Google Scholar]
- 31.Bekker-Jensen S, Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst) 2010; 9: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 32.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 2010; 40: 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mah LJ, El-Osta A, Karagiannis TC. GammaH2AX as a molecular marker of aging and disease. Epigenetics 2010; 5: 129–136. [DOI] [PubMed] [Google Scholar]
- 34.Wu HJ, Liu C, Duan WX, Xu SC, He MD, Chen CH, Wang Y, Zhou Z, Yu ZP, Zhang L, Chen Y. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat Res 2013; 752: 57–67. [DOI] [PubMed] [Google Scholar]
- 35.Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, Gu J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J Pineal Res 2002; 33: 48–56. [DOI] [PubMed] [Google Scholar]
- 36.Arakane F, Kallen CB, Watari H, Foster JA, Sepuri NB, Pain D, Stayrook SE, Lewis M, Gerton GL, Strauss JF., 3rdThe mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem 1998; 273: 16339–16345. [DOI] [PubMed] [Google Scholar]
- 37.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 1994; 269: 28314–28322. [PubMed] [Google Scholar]
- 38.Behrman HR, Aten RF. Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology 1991; 128: 2958–2966. [DOI] [PubMed] [Google Scholar]
- 39.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005; 27: 119–130. [DOI] [PubMed] [Google Scholar]
- 40.Reiter RJ, Paredes SD, Korkmaz A, Jou MJ, Tan DX. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip Toxicol 2008; 1: 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López A, García JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 2009; 46: 188–198. [DOI] [PubMed] [Google Scholar]
- 42.Kodaman PH, Aten RF, Behrman HR. Lipid hydroperoxides evoke antigonadotropic and antisteroidogenic activity in rat luteal cells. Endocrinology 1994; 135: 2723–2730. [DOI] [PubMed] [Google Scholar]
- 43.Sawada M, Carlson JC. Rapid plasma membrane changes in superoxide radical formation, fluidity, and phospholipase A2 activity in the corpus luteum of the rat during induction of luteolysis. Endocrinology 1991; 128: 2992–2998. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Yao K, Carlson JC. Plasma membrane changes in the rat corpus luteum induced by oxygen radical generation. Endocrinology 1993; 133: 491–495. [DOI] [PubMed] [Google Scholar]
- 45.Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res 2001; 306: 347–361. [DOI] [PubMed] [Google Scholar]
- 46.Denault JB, Salvesen GS. Apoptotic caspase activation and activity. Methods Mol Biol 2008; 414: 191–220. [DOI] [PubMed] [Google Scholar]
- 47.Tang XL, Yang XY, Jung HJ, Kim SY, Jung SY, Choi DY, Park WC, Park H. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol Pharm Bull 2009; 32: 1399–1405. [DOI] [PubMed] [Google Scholar]
- 48.Akbulut KG, Akbulut H, Akgun N, Gonul B. Melatonin decreases apoptosis in gastric mucosa during aging. Aging Clin Exp Res 2012; 24: 15–20. [DOI] [PubMed] [Google Scholar]
- 49.Espino J, Bejarano I, Redondo PC, Rosado JA, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB. Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J Membr Biol 2010; 233: 105–118. [DOI] [PubMed] [Google Scholar]
- 50.Wang FW, Wang Z, Zhang YM, Du ZX, Zhang XL, Liu Q, Guo YJ, Li XG, Hao AJ. Protective effect of melatonin on bone marrow mesenchymal stem cells against hydrogen peroxide-induced apoptosis in vitro. J Cell Biochem 2013; 114: 2346–2355. [DOI] [PubMed] [Google Scholar]
- 51.Niles LP, Wang J, Shen L, Lobb DK, Younglai EV. Melatonin receptor mRNA expression in human granulosa cells. Mol Cell Endocrinol 1999; 156: 107–110. [DOI] [PubMed] [Google Scholar]
- 52.Soares JM, Jr, Masana MI, Erşahin C, Dubocovich ML. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther 2003; 306: 694–702. [DOI] [PubMed] [Google Scholar]
- 53.Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 2002; 59: 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004; 36: 1–9. [DOI] [PubMed] [Google Scholar]
- 55.Tamura H, Takasaki A, Taketani T, Tanabe M, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y, Sugino N. Melatonin and female reproduction. J Obstet Gynaecol Res 2014; 40: 1–11. [DOI] [PubMed] [Google Scholar]