Few studies have tested whether mutualisms may affect species distributions by altering the niches of partner species. We show that a fungal endophyte is associated with a shift in the soil moisture niche of its host plant relative to a co-occurring, endophyte-free congener. The endophyte appeared to initially restrict its host's distribution to wetter microsites before positively affecting its growth, suggesting the value of considering symbiont effects at different partner life stages. Our study identifies a symbiotic relationship as a potential mechanism facilitating the coexistence of two species, suggesting that symbiont effects on host niche may have community-level consequences.
Keywords: Epichloë, fungal endophyte, mutualism, Poa leptocoma, Poa reflexa, symbiosis
Abstract
Mutualisms can play important roles in influencing species coexistence and determining community composition. However, few studies have tested whether such interactions can affect species distributions by altering the niches of partner species. In subalpine meadows of the Rocky Mountains, USA, we explored whether the presence of a fungal endophyte (genus Epichloë) may shift the niche of its partner plant, marsh bluegrass (Poa leptocoma) relative to a closely related but endophyte-free grass species, nodding bluegrass (Poa reflexa). Using observations and a 3-year field experiment, we tested two questions: (i) Do P. leptocoma and P. reflexa occupy different ecological niches? and (ii) Does endophyte presence affect the relative fitness of P. leptocoma versus P. reflexa in the putative niches of these grass species? The two species were less likely to co-occur than expected by chance. Specifically, P. leptocoma grew closer to water sources and in wetter soils than P. reflexa, and also had higher root colonization by mycorrhizal fungi. Endophyte-symbiotic P. leptocoma seeds germinated with greater frequency in P. leptocoma niches relative to P. reflexa niches, whereas neither endophyte-free (experimentally removed) P. leptocoma seeds nor P. reflexa seeds showed differential germination between the two niche types. Thus, endophyte presence constrained the germination and early survival of host plants to microsites occupied by P. leptocoma. However, endophyte-symbiotic P. leptocoma ultimately showed greater growth than endophyte-free plants across all microsites, indicating a net benefit of the symbiosis at this life history stage. Differential effects of endophyte symbiosis on different host life history stages may thus contribute to niche partitioning between the two congeneric plant species. Our study therefore identifies a symbiotic relationship as a potential mechanism facilitating the coexistence of two species, suggesting that symbiont effects on host niche may have community-level consequences.
Introduction
Mutualisms can play important roles in influencing species coexistence and determining community composition (Palmer et al. 2003; Umbanhowar and McCann 2005; Jones et al. 2012). Such positive interactions can alter species' range sizes by expanding or shifting the niches of individual partners (Bruno et al. 2003; Poisot et al. 2011). In addition, mutualists may increase the competitive dominance of host species, thereby decreasing species coexistence and the species richness of competitors in the community (Hartnett and Wilson 1999; Rudgers et al. 2007). In other systems, mutualisms appear to increase species diversity if they benefit multiple community members or preferentially benefit rare species (Müller and Godfray 1999; Hart et al. 2003).
A small number of studies have tested whether mutualisms have the potential to influence species coexistence by altering the niches of partner organisms. Considerable niche overlap among species is predicted to result in competitive exclusion; mutualisms may bring about niche shifts or changes in competitive hierarchies that allow for species coexistence (Bruno et al. 2003). For instance, mutualistic ants were found to shift the niche of Lycaeides melissa caterpillars, increasing their survival on a novel, sub-optimal host plant by providing protection from predators (Forister et al. 2011). In some plants, arbuscular mycorrhizal (AM) fungi may increase species coexistence by reducing the ability of one plant species to repress the other via competition for shared soil resources (Wagg et al. 2011). An endosymbiont was found to affect the long-distance airborne dispersal of the Erigone atra spider, decreasing the spider's dispersal range and thereby limiting the spider's niche (Goodacre et al. 2009). In contrast, ant-mediated dispersal of Hexastylis arifolia seeds was not found to result in plant niche expansion via enhanced resource utilization or decreased density dependence (Warren et al. 2010). Although not all mutualisms bring about niche alteration, these positive interactions may affect the niche dimensions of partners in some systems.
This study focused on the potential role of endophytic fungi in influencing host plant niche dimensions. Endophytic fungi and bacteria are ubiquitous symbionts of plant tissues (Bacon and White 2000) and can range from mutualists to pathogens. Certain endophytes are responsible for the production of secondary metabolite compounds previously attributed to plants (Strobel 2002) and can buffer their hosts against damage by pathogens and herbivores (Clay and Schardl 2002) as well as abiotic stress (Rodriguez et al. 2008). Systemic, vertically transmitted fungal endophytes, which commonly form symbioses with cool-season grasses, often act as mutualists and are known to improve host tolerance to drought, increase plant nitrogen and phosphorus content, and produce alkaloids that deter herbivores (Malinowski and Belesky 2000; Clay and Schardl 2002; Cheplick and Faeth 2009; Schardl 2010). Such effects on host plants can in turn have community- and ecosystem-level consequences, influencing processes such as plant succession (Rudgers et al. 2007) and decomposition (Lemons et al. 2005; Omacini et al. 2012). However, whether an endophyte can shift the niche of its host and thereby alter host interactions with closely related competitor plants remains undocumented.
We evaluated whether the presence of a fungal endophyte may alter the niche of Poa leptocoma (marsh bluegrass) and affect its co-occurrence with a similar but naturally endophyte-free grass species, Poa reflexa (nodding bluegrass) in subalpine meadows of the Rocky Mountains, USA. Although it has been suggested that P. leptocoma prefers wetter habitats (Barkworth et al. 2007), no data exist that document niche characteristics of the two species. For our study, we conceptualized the ‘niche’ of a species as the distinctive set of abiotic and biotic microsite conditions associated with a species' current distribution (Levine and HilleRisLambers 2009). We surveyed P. leptocoma and P. reflexa populations to document endophyte symbiosis and carried out endophyte species characterization via DNA sequencing. We then addressed two questions to explore the effect of endophyte symbiosis on multiple stages of host life history: (i) Do P. leptocoma and P. reflexa occupy different ecological niches? and (ii) Does endophyte presence affect the relative fitness of P. leptocoma versus P. reflexa in their putative niches? We hypothesized that (i) the two species occupy different ecological niches in the habitats examined, and that (ii) endophyte symbiosis alters the relative fitness of P. leptocoma versus P. reflexa in the two species' putative niches. We predicted that endophyte-symbiotic P. leptocoma seeds would perform best in P. leptocoma microsites (niches), and that P. reflexa seeds would perform best in P. reflexa microsites. If the endophyte were to shift the niche dimensions of its host, then endophyte-free P. leptocoma individuals would have niche requirements more similar to those of P. reflexa; thus, we predicted that P. leptocoma endophyte-free seeds would perform better in P. reflexa microsites than in P. leptocoma microsites.
Methods
Study organisms
Both P. leptocoma (sect. Oreinos) and P. reflexa (sect. Homalopoa) grow in western North America in subalpine meadow and forest understory environments near streams (Barkworth et al. 2007). Poa leptocoma has scabrous panicle branches, whereas P. reflexa has smooth branches (Shaw 2008), and it is difficult to differentiate between the two species prior to flowering. Thus, naturally occurring plants used in the study were identified while they were flowering.
We examined the symbiosis between P. leptocoma and a systemic, vertically transmitted fungal endophyte in the genus Epichloë. Epichloë spp. are plant-symbiotic fungi in the family Clavicipitaceae and occur in ∼20–30 % of all grass species (Leuchtmann 1992; Rodriguez et al. 2009). Fungal endophytes can be transmitted to a host plant horizontally via spores and/or vertically from plant to seed (Clay and Schardl 2002); the genus Epichloë includes non-hybrid sexual species (horizontal and some vertical transmission) and hybrid and non-hybrid asexual species, formerly called Neotyphodium spp. (vertical transmission) (Leuchtmann et al. 2014).
Endophyte frequency and species characterization
To evaluate endophyte symbiosis in the two Poa species, we collected leaf samples from P. leptocoma and P. reflexa populations in the Rocky Mountains of Colorado, USA, from the Rocky Mountain National Park and surrounding areas (Larimer, Grand and Boulder counties) south to Gunnison County [see Supporting Information]. Thin sections from the inner leaf sheaths of collected samples were stained with aniline blue lactic acid dye following the methods in Bacon and White (1994). Stained sections were checked for endophyte presence using a microscope at ×200 magnification.
We determined Epichloë species diversity present in P. leptocoma using plant material collected from six sites in Gunnison County, CO [see Supporting Information]. Total DNA was extracted from P. leptocoma plant material using a QIAGEN MagAttract 96 DNA Plant Core Kit (QIAGEN Inc., Valencia, CA, USA). Multiplex PCR was carried out using 19 established genus-specific primers representing housekeeping, mating type and key alkaloid biosynthesis genes (Charlton et al. 2012; Takach et al. 2012) to determine endophyte presence and alkaloid production potential.
We identified species by direct sequencing the housekeeping genes tefA and tubB, using primer sets tef1-exon1d-1 and tef1-exon6u-1 for tefA and tubB F1 (5′ CTCTGTTTGTCTTGGGGACC 3′) and T1.2 for tubB (Craven et al. 2001; Young et al. 2005; Charlton et al. 2012). Sequences representing Epichloë species diversity were analysed using phylogeny.fr with default settings (Dereeper et al. 2008, 2010). Sequences were aligned using MUSCLE (version 3.7) (Edgar 2004), phylogenetic trees were inferred by maximum likelihood using PHYML (version 3.0) (Guindon and Gascuel 2003) with an approximate likelihood ratio test (Anisimova and Gascuel 2006), and the trees were rendered with TREEDYN (version 198.3) (Chevenet et al. 2006). We annotated clades in accordance with Leuchtmann et al. (2014).
Do P. leptocoma and P. reflexa occupy different ecological niches?
Surveys
To document the current distributions of the bluegrass species, we marked naturally occurring plants at four sites. First, during August 2008, we marked 77 P. leptocoma plants and 40 P. reflexa plants near a stream system in Virginia Basin, Gunnison County, CO (38°58.314N, 106°58.942W, elevation 3229 m). The nearest individual of each species was marked approximately every 2 m along transects placed in three regions of the watershed: directly in and along a shady streambed (where only P. leptocoma was present), next to a sunny streambed (both species) and in a wet meadow between the two streams (both species). Then, during August 2011, we used the same transect method to mark a minimum of 10 individuals per species at each of two sites near Schofield Pass (Schofield and Maroon, 39°01.329N, 107°02.938W, elevation 3218 m) and at one site on Snodgrass Mountain (38°55.083N, 106°59.175W, elevation 3357 m) [see Supporting Information for sample sizes].
Co-occurrence
To examine natural patterns of co-occurrence, we compared P. leptocoma and P. reflexa densities in randomly placed quadrats versus quadrats placed around target plants of each species (24–25 July 2012). For the random samples, we placed 30 × 30 cm quadrats at 2 m intervals along eight transects in the Virginia Basin study area and in nearby Copper Creek (38°58.01N, 106°58.17W). We counted the number of flowering P. leptocoma and P. reflexa individuals per quadrat to determine an expected density per square metre for reproductive adults of each species, evaluating a total of 58 quadrats. At each transect interval, we also located the nearest bluegrass individual (P. leptocoma or P. reflexa), placed the quadrat around the centre of the identified target plant and counted the number of flowering individuals of each species in the quadrat to quantify plant density. We evaluated a total of 24 P. leptocoma targeted quadrats and 25 P. reflexa targeted quadrats. We used a one-tailed, unequal variance t-test to compare the density of each species in the randomly located quadrats against their densities in the targeted quadrats. These analyses tested (i) whether targeted quadrats had higher densities of conspecifics than expected by chance, indicative of a clumped species distribution and (ii) whether the two species were less likely to co-occur than expected by chance, suggestive of different niches or competitive exclusion.
Niche dimensions
As anecdotal data suggested that P. leptocoma prefers wetter environments relative to P. reflexa (Barkworth et al. 2007), we examined water availability at four survey sites to describe the abiotic microhabitats of the two bluegrasses. During 8–10 August 2011, we measured the distance of each marked plant (to the nearest 0.01 m) to the nearest source of water (a stream or snow bank, measuring to the closer of the two). We also measured soil volumetric water content (VWC) at each plant using a soil moisture metre (HydroSense meter, Campbell Scientific, Inc., Logan, UT, USA) with 12 cm probes, consistent with rooting depth. At the Virginia Basin site, we additionally measured soil moisture at marked plants approximately weekly during the 2011 growing season following snowmelt on 12 July 2011, excluding eight plants in locations too rocky to obtain accurate soil moisture data [see Supporting Information for sample sizes].
To evaluate one biotic niche dimension, we examined P. leptocoma and P. reflexa roots for colonization by fungal symbionts other than Epichloë spp., which do not occupy roots. Root fungi were of particular interest because Epichloë presence has been shown to reduce mycorrhizal colonization in grass hosts (Mack and Rudgers 2008). During 8–12 August 2012, we collected roots from marked plants at the four survey sites. Roots were stored in 70 % ethanol, cleared with 10 % KOH and stained with trypan blue (Phillips and Hayman 1970). We mounted stained roots onto glass slides and assessed fungal colonization using the magnified intersection method of McGonigle et al. (1990). At ×400 magnification, we noted the presence of mycorrhizal hyphae, vesicles and arbuscules, as well as dark septate endophyte hyphae and microsclerotia, at each root intersection.
Statistical analysis: niche characteristics
We analysed niche characteristics with ANOVA, including the factor of plant species identity, the random effect of site and the site × species interaction (SAS version 9.2, SAS Institute, Inc., Cary, NC, USA). A significant species × site interaction would indicate inconsistency in species niche characteristics across sites. When such interactions were significant, we used a priori orthogonal contrasts to compare species within each site. We log-transformed the responses of distance to water and VWC to improve normality of residuals and homogeneity of variances.
Does endophyte presence affect the relative fitness of P. leptocoma versus P. reflexa in their putative niches?
Experimental approach
We used the marked plants at the Virginia Basin site as replicates of each species' specific microhabitat, i.e. its putative niche. This approach eliminated the need to replicate the precise abiotic and biotic conditions characteristic of each species' microhabitat. To test whether plant performance differed between species and/or with endophyte symbiosis, we planted seeds into natural P. leptocoma and P. reflexa microhabitats and compared germination, seedling survival and plant growth.
Seed types and sources
Surrounding each marked, naturally occurring plant, we planted a total of nine seeds: three naturally endophyte-free (E−) P. reflexa seeds collected from field plants; two naturally endophyte-symbiotic (E+) P. leptocoma seeds collected from field plants (‘E+ natural’); three endophyte-free (E−) P. leptocoma seeds collected from greenhouse-grown plants from which the endophyte was experimentally removed; and one P. leptocoma endophyte-symbiotic (E+) ‘control’ seed collected from a greenhouse-grown plant. This approach controlled for maternal effects by decoupling endophyte presence from effects associated with plant genotype or maternal environment.
For the P. leptocoma E+ natural and P. reflexa seed treatments, we collected seeds from the Virginia Basin site during late August 2009 and stored them at 4 °C until planting. Poa leptocoma E− seeds and E+ control seeds came from plants grown in a common greenhouse environment from the Virginia Basin seed stock. To create endophyte-free (E−) P. leptocoma seeds, we experimentally removed the endophyte by germinating seeds for 5 weeks on wet filter paper in standard plastic Petri plates containing the fungicide benomyl (2 g/L). Seeds for the P. leptocoma E+ control treatment were germinated in Petri plates that contained water only. Following germination, P. leptocoma E+ control and E− seedlings were transplanted into 2.5 × 4 cm plastic pots and grown for 2–6 weeks on a light bench. Once seedlings had established a root system, they were transplanted into 10 cm2 pots with a 1 : 1 mixture of Pro-mix BX (Premier Horticulture, Quakertown, PA, USA) and sterile sand and grown until reproductive. Endophyte status of each plant was confirmed microscopically following the methods in Bacon and White (1994). Seeds for the experiment were collected in the greenhouse during July 2010.
Experimental methods
Prior to planting, we lightly glued seeds to plastic toothpicks (Wright et al. 2006) with water-soluble glue (Elmer's Washable School Glue, Elmer's Products, Inc., Columbus, OH, USA). We used a different toothpick colour for each type of seed, and planted the toothpicks in the ground to position the seeds just at the soil surface. The seeds (N = 920) were planted on 9 July 2011 in randomized order, forming a circle with a radius of ∼5 cm around each original, naturally occurring plant [see Supporting Information]. This spacing mimicked realistic field seedling densities.
In 2011, we scored seed germination weekly for 7 weeks. After allowing the plants to overwinter, we scored germination, measured the height of the tallest leaf (cm) and counted the number of leaves and tillers on each plant twice in 2012 (14 June and 20 August) and twice in 2013 (20 June and 2 September). On each of these dates, for seeds that had previously germinated, we also recorded plant survival. On 2 September 2013, we harvested all remaining plants and counted the total number of leaves and the number of live leaves on each plant. We rinsed the roots of harvested plants to remove soil, divided each plant at the soil interface into aboveground and belowground biomass and dried the samples at 60 °C. Following drying, we weighed samples using a microbalance (Sartorius Cubis MSE 3.6P, Data Weighing Systems, Inc., Elk Grove, IL, USA).
Statistical analysis
Analyses were designed to test our predictions that P. leptocoma E+ seeds would perform best in P. leptocoma microsites, P. reflexa seeds would perform best in P. reflexa microsites and endophyte removal from P. leptocoma would shift the species' niche toward P. reflexa microsites. All models included the fixed effects of seed identity, microsite (P. leptocoma or P. reflexa niche) and their interaction, as well as the random effect of plant identity (nested within microsite) to account for the non-independence of seeds that were placed around the same naturally occurring plant. To test a priori hypotheses, we constructed contrasts to compare performance between the P. leptocoma and P. reflexa niches within each seed type. We also specifically compared the responses of greenhouse-grown seeds (P. leptocoma E+ control versus P. leptocoma E−) within each microsite.
We analysed germination and survival data with a log-linear model, including the binomial response (0/1) with a logit link function (Proc GLIMMIX, SAS version 9.2, SAS Institute, Inc.). For the subset of seeds that germinated, we analysed the time to germinate (in weeks), and for the subset of seedlings that died, we analysed survival time (in weeks) using generalized linear models with a Gaussian distribution. Growth responses (number of live leaves at the time of harvest, aboveground biomass and belowground biomass) were analysed using MANOVA followed by univariate analysis. Aboveground and belowground biomass data were log-transformed, and live leaf data were square-root-transformed. All models included the fixed effects of seed identity, microsite (P. leptocoma or P. reflexa niche) and their interaction, as well as the random effect of plant identity (nested within microsite) to account for the non-independence of seeds that were placed around the same naturally occurring plant. To test a priori hypotheses, we constructed contrasts to compare performance between the P. leptocoma and P. reflexa niches within each seed type. We also specifically compared the responses of greenhouse-grown seeds (P. leptocoma E+ control versus P. leptocoma E−) within each microsite. For growth responses, P. leptocoma E+ control and P. leptocoma E+ natural data were combined because few individuals were alive at the time of harvest.
Results
Endophyte frequency and species characterization
In our field surveys, Epichloë species approached 100 % frequency in P. leptocoma [see Supporting Information]. No systemic endophyte was found in P. reflexa. In greenhouse-grown seedlings, the endophyte was vertically transmitted to 95 % of established seedlings (J. A. Rudgers, unpubl. data).
Examined samples contained single copies of endophyte tubB and tefA genes, indicating the presence of a non-hybrid Epichloë species in P. leptocoma. Phylogenies revealed that all tubB and tefA sequence data grouped in the Epichloë typhina subspecies poae subclade [see Supporting Information]. Sexual Epichloë spp. are heterothallic, requiring individuals of two mating types for successful reproduction; all examined samples were positive for mtBA markers, suggesting that the examined populations of P. leptocoma endophytes comprise a single mating type, MTB. While it is possible that the examined P. leptocoma endophytes are capable of sexual reproduction, we saw no evidence of both mating types within the populations we evaluated. In addition, stromata were not observed in field or greenhouse-grown plants, further suggesting that the examined endophytes likely represent asexual, non-hybrid populations of E. typhina subsp. poae.
We also found genes linked to alkaloid production within the E. typhina subsp. poae populations. All samples were positive for perA (PER locus required for peramine production) markers, indicating the potential to produce peramine, a known insect feeding deterrent. All samples from the Washington Gulch population and half of samples from the Slate River population also contained ergot alkaloid biosynthesis genes (EAS locus), indicating the potential to produce the ergot alkaloid intermediate chanoclavine. All samples examined from the Virginia Basin site contained identical endophyte genotypes and were positive for PER markers but negative for EAS markers.
Do P. leptocoma and P. reflexa occupy different ecological niches?
Co-occurrence
Our species distribution surveys indicated low local co-occurrence of the two Poa species. Poa leptocoma individuals were absent from quadrats placed around target P. reflexa individuals, and in the randomly placed quadrats were significantly less likely to co-occur with P. reflexa than with a conspecific (Fig. 1, t(23) = −2.77, P = 0.005) or than expected by chance (Fig. 1, t(23) = −2.34, P = 0.014). Poa reflexa was significantly clumped relative to the randomly sampled quadrats (Fig. 1, t(24) = −1.76, P = 0.045), and significantly more likely to co-occur with a conspecific target than with P. leptocoma (Fig. 1, t(24) = −1.92, P = 0.034). However, P. reflexa was equally abundant in quadrats with a target P. leptocoma relative to the random expectation (Fig. 1, t(24) = 1.05, P = 0.306).
Figure 1.
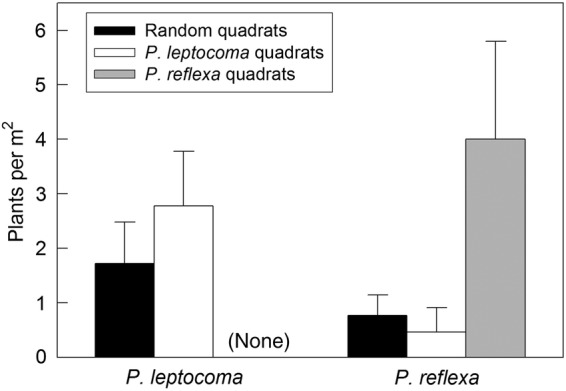
Natural densities of P. leptocoma and P. reflexa individuals in Gunnison Co., CO, USA, sampled in three quadrat types: randomly placed quadrats along transects, quadrats centred on a focal P. leptocoma individual, and quadrats centred on a focal P. reflexa individual. Bars show the mean number of individuals per square metre (excluding the focal plant) with s.e.
Niche dimensions
Poa leptocoma plants typically occupied wetter microenvironments than P. reflexa. For instance, at the Virginia Basin site, P. leptocoma grew in environments an average of 8.3 m from streams, while P. reflexa grew in environments an average of 22.0 m from streams (Fig. 2A). Correspondingly, P. leptocoma sites were 92 % wetter than P. reflexa sites (Fig. 2B). The trend for higher soil moisture at P. leptocoma microsites was consistent throughout the 2011 growing season at Virginia Basin (Fig. 3).
Figure 2.
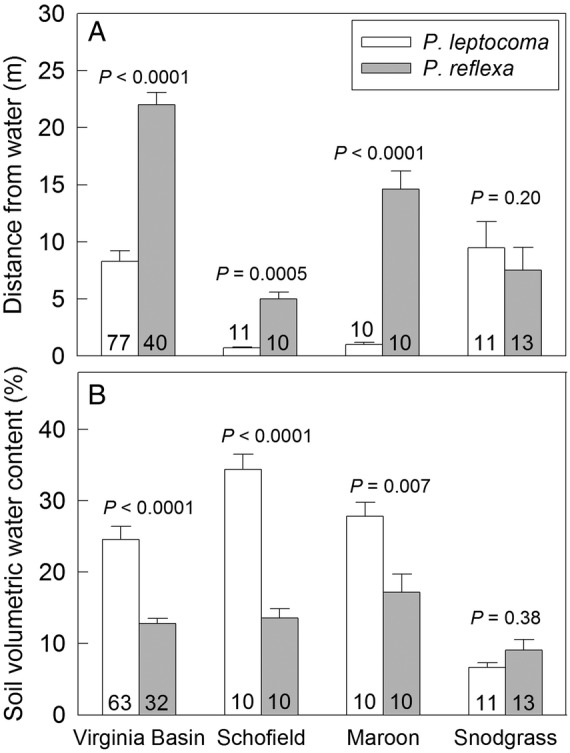
Abiotic niche characteristics of naturally occurring P. leptocoma and P. reflexa plants at each of four sites in Gunnison Co., CO, USA. (A) Mean distance from the nearest water source (± s.e.). (B) Mean volumetric water content of the soil (%) (±s.e.) measured during August 2011. Sample sizes are given on each bar, and P-values are shown for contrasts between pairs of means within each site.
Figure 3.
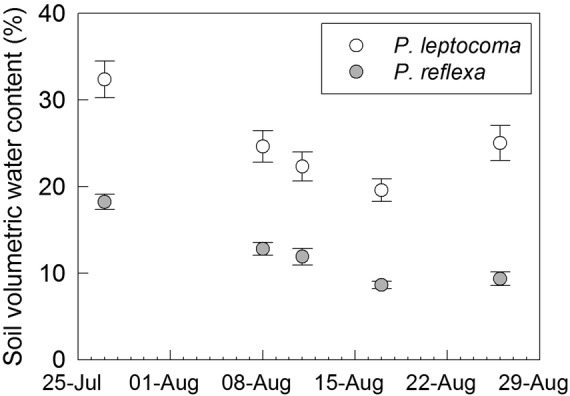
Seasonal variation in mean volumetric water content (%) (± s.e.) of the soil at naturally occurring P. leptocoma and P. reflexa plants at the Virginia Basin site in Gunnison Co., CO, USA in 2011.
Similar differences were observed at two of the three additional locations we surveyed (Fig. 2). At Schofield and Maroon, P. leptocoma grew in environments 600–1300 % closer to streams with 60–150 % higher soil moisture levels relative to P. reflexa environments. However, at Snodgrass Mountain, there were no significant differences between the two plant species in mean distance from a water source or mean VWC (Fig. 2). Soil moisture was lower overall at Snodgrass Mountain than at the other sites.
Colonization of roots by AM fungi was ∼15 % greater for P. leptocoma than for P. reflexa: on average, 54 % of P. leptocoma root intersections (±2 % s.e.) contained AM hyphae, compared with 47 % of P. reflexa root intersections (±2 % s.e.) (species F1,57 = 6.36, P = 0.015). Colonization by other types of root fungi did not differ significantly between the two Poa species or among sites (model F5,57 = 1.27, P = 0.288).
Does endophyte presence affect the relative fitness of P. leptocoma versus P. reflexa in their putative niches?
Germination
Generally, each species germinated best in its own microsite, and endophyte absence/removal decreased the magnitude of this effect. In accordance with our predictions, a significantly greater percentage of endophyte-symbiotic P. leptocoma seeds germinated in P. leptocoma habitats relative to P. reflexa habitats (Fig. 4A, Table 1). This was the case for both greenhouse-grown (control) and field-collected (natural) E+ P. leptocoma seeds, which showed 100 % and 75 % higher germination rates in the P. leptocoma niche relative to the P. reflexa niche, respectively. The endophyte-free P. leptocoma seeds showed a much weaker difference in germination between microsites. Poa leptocoma endophyte-free (E−) seeds had 85–187 % higher germination in P. reflexa microsites than did P. leptocoma E+ control seeds (P = 0.13) or E+ natural seeds (P = 0.0005), respectively. For the P. reflexa seeds, there was no significant difference in percentage germination between P. leptocoma and P. reflexa niches, contrary to our original prediction (Fig. 4A).
Figure 4.
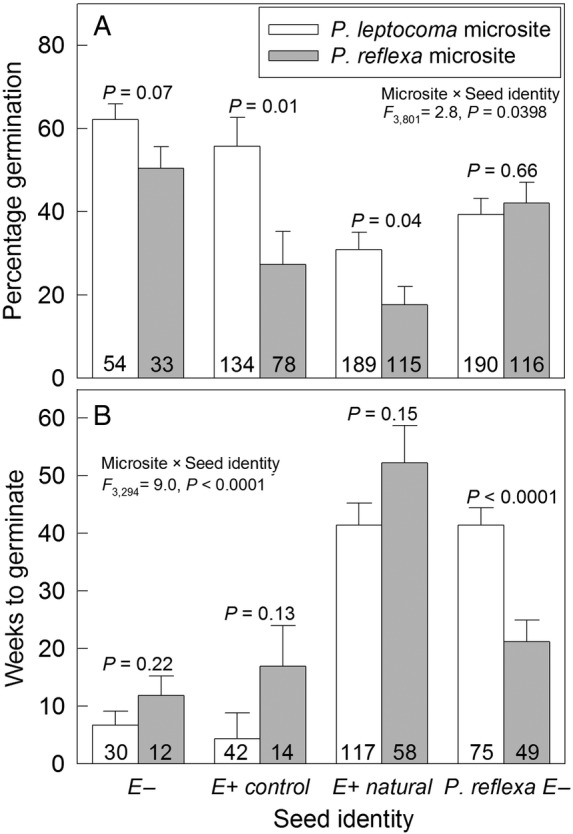
Germination responses of P. leptocoma and P. reflexa seeds in P. leptocoma and P. reflexa microsites. (A) Mean (±s.e.) percentage of seeds that germinated. (B) Mean (±s.e.) number of weeks from date of planting to date of germination. E+ natural signifies endophyte-symbiotic P. leptocoma seeds collected from field plants, E+ control signifies endophyte-symbiotic P. leptocoma seeds collected from greenhouse-grown plants, E− signifies greenhouse-grown, endophyte-free P. leptocoma seeds resulting from experimental disinfection, and P. reflexa E− seeds were collected from field plants and were always endophyte-free. Sample sizes are given on each bar, and P-values are shown for contrasts within each seed identity type.
Table 1.
Results of statistical models testing the effects of seed identity (P. leptocoma E+ control, P. leptocoma E+ natural, P. leptocoma E− or P. reflexa E−), microsite type (P. leptocoma or P. reflexa) and their interaction on (a) whether or not a seed germinated, (b) the number of weeks to germination for seeds that germinated, (c) whether or not a germinated seedling survived, (d) number of weeks surviving for seedlings that died and (e) combined growth responses (number of live leaves at the time of harvest, aboveground biomass and belowground biomass) using MANOVA.
| Numerator df | Denominator df | F-ratio | P | |
|---|---|---|---|---|
| (a) Effect on germination (0/1) | ||||
| Seed identity | 3 | 801 | 15.5 | <0.0001 |
| Microsite | 1 | 100 | 8.4 | 0.0045 |
| Microsite × seed identity | 3 | 801 | 2.8 | 0.0398 |
| (b) Effect on weeks to germinate | ||||
| Seed identity | 3 | 294 | 38.4 | <0.0001 |
| Microsite | 1 | 95 | 0.3 | 0.5643 |
| Microsite × seed identity | 3 | 294 | 9.0 | <0.0001 |
| (c) Effect on seedling survival (0/1) | ||||
| Seed identity | 3 | 296 | 6.5 | 0.0003 |
| Microsite | 1 | 95 | 1.9 | 0.1741 |
| Microsite × seed identity | 3 | 296 | 1.7 | 0.1700 |
| (d) Effect on weeks surviving for seedlings that died | ||||
| Seed identity | 3 | 234 | 5.7 | 0.0009 |
| Microsite | 1 | 92 | 3.7 | 0.0575 |
| Microsite × seed identity | 3 | 234 | 2.5 | 0.0599 |
| (e) Effect on plant growth (MANOVA) | ||||
| Seed identity | 6 | 118 | 2.40 | 0.0315 |
| Microsite | 3 | 58 | 0.73 | 0.5379 |
| Microsite × seed identity | 6 | 118 | 0.35 | 0.9113 |
In contrast, amongst seeds that germinated, P. reflexa seeds germinated 20 weeks earlier, on average, if planted into conspecific microsites relative to P. leptocoma microsites (Fig. 4B, Table 1). Poa leptocoma seeds with a similar maternal history (E+ natural) took an additional 31 weeks, on average, to germinate compared with P. reflexa seeds (Fig. 4B, P < 0.0001). The length of time to germinate was not affected by microsite for any of the P. leptocoma seed types (Fig. 4B, Table 1). In general, greenhouse-grown seeds germinated in fewer weeks than did seeds harvested from field plants (Fig. 4B).
Survival
Generally, P. leptocoma microsites were better for seedling survival than P. reflexa microsites. In P. leptocoma microsites, P. reflexa seedlings showed a higher percentage survival, and P. leptocoma seedlings survived for a longer amount of time. Endophyte presence altered the plant survival response to microsite: in P. reflexa microsites, endophyte-free P. leptocoma seedlings survived 50 % longer than control E+ seeds (Fig. 5A, P = 0.058, with low power due to low germination rates in these microsites, n = 12). For P. reflexa, the effects of microsite on survival were opposite to the effects of microsite on time to germination, with 133 % higher survival of P. reflexa seedlings in the P. leptocoma microsite relative to the P. reflexa microsite (Fig. 5B, Table 1). Greenhouse-grown P. leptocoma seedlings (E− and E+ control) generally survived longer in P. leptocoma microsites than in P. reflexa microsites (Fig. 5A), while no difference was observed in length of survival between microsites for E+ natural P. leptocoma plants.
Figure 5.
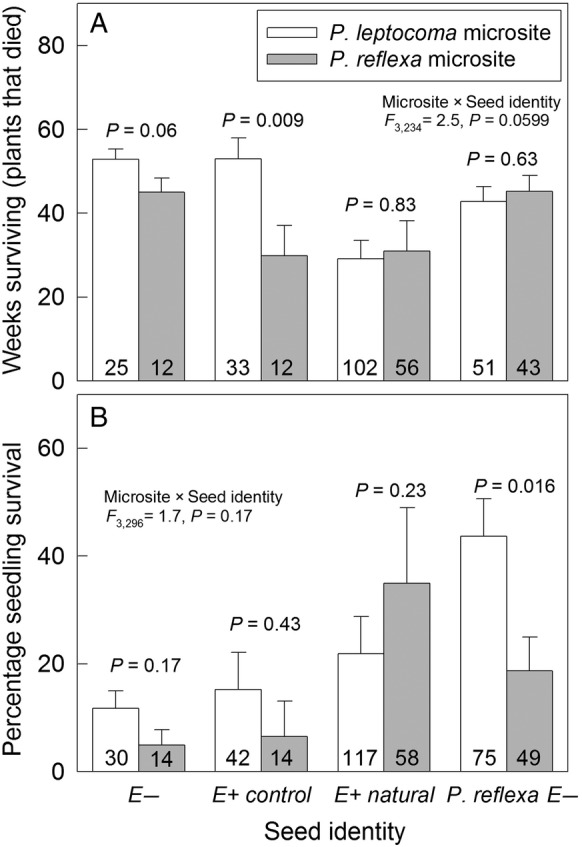
Survival responses of P. leptocoma and P. reflexa seeds in P. leptocoma and P. reflexa microsites. (A) Mean (±s.e.) number of weeks that seedlings survived (for seedlings that died). (B) Mean (±s.e.) percentage of seedlings that survived. E+ natural signifies endophyte-symbiotic P. leptocoma seeds collected from field plants, E+ control signifies endophyte-symbiotic P. leptocoma seeds collected from greenhouse-grown plants, E− signifies greenhouse-grown, endophyte-free P. leptocoma seeds resulting from experimental disinfection, and P. reflexa E− seeds were collected from field plants and were always endophyte-free. Sample sizes are given on each bar, and P-values are shown for contrasts within each seed identity type.
Growth
Plant growth was affected only by seed identity and not by microsite (Table 1, microsite × seed identity, P > 0.9). Mean aboveground biomass was significantly greater for P. leptocoma E+ individuals relative to P. leptocoma E− individuals, with E+ plants having ∼600 % more biomass than E− plants (Fig. 6A). Mean aboveground biomass did not differ significantly between P. leptocoma E+ and P. reflexa plants (Fig. 6A). Poa leptocoma E+ individuals had a significantly greater number of live leaves, on average, relative to P. reflexa individuals, and a marginally greater number of live leaves relative to P. leptocoma E− individuals (Fig. 6B). There was no significant effect of seed identity on belowground biomass (P = 0.1483).
Figure 6.
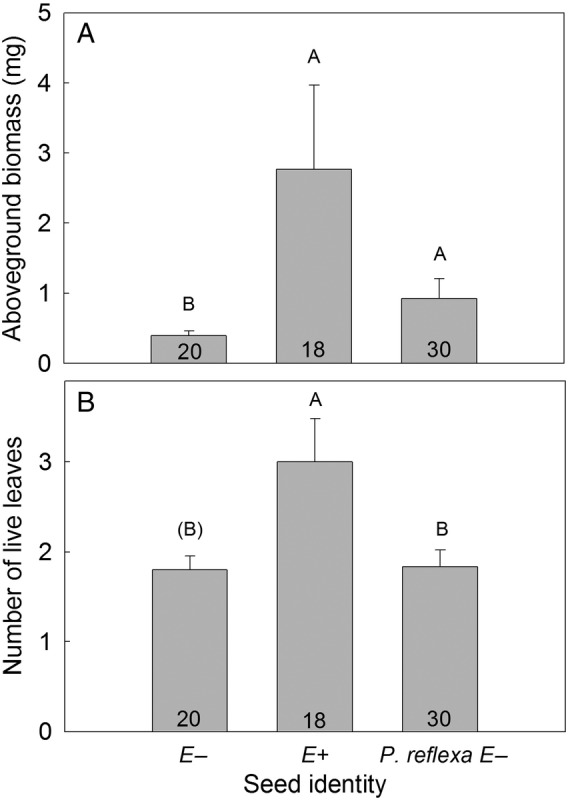
Growth responses of endophyte-free (E−) P. leptocoma seeds, endophyte-symbiotic (E+) P. leptocoma seeds and endophyte-free P. reflexa (P. reflexa E−) seeds. (A) Mean (±s.e.) aboveground biomass of plants harvested in September 2013. (B) Mean (±s.e.) number of live leaves on plants harvested in September 2013. For each growth response, means annotated with different letters significantly differed. Parentheses around a letter indicate a marginally significant difference between that mean and a mean annotated with a different letter.
Discussion
Our observational data indicate that P. leptocoma and P. reflexa typically occupy distinct ecological niches, with lower levels of co-occurrence than expected by chance. Poa leptocoma was more common in wet microsites and supported higher rates of root colonization by mycorrhizal fungi than P. reflexa. With regards to seed germination and seedling survival, endophyte presence appeared to constrain the distribution of P. leptocoma to wetter microsites, where its congener was largely absent. However, for those plants that survived their first 3 years, endophyte symbiosis yielded net benefits to plant growth, regardless of the microhabitat. Differential effects of endophyte symbiosis on different host life history stages may contribute to niche partitioning between the two congeneric plant species. Given the knowledge that endophyte effects can vary among host life history stages (Rudgers et al. 2012), it is premature to designate the endophyte–P. leptocoma interaction as a net mutualism. Here, however, we for the first time document beneficial effects of endophytes at the seedling to 3-year-old age range.
Water can be a key resource for plants in subalpine habitats (Kuramoto and Bliss 1970; Harte and Shaw 1995), but may also cause oxidative stress due to flooding, as could be the case for P. leptocoma individuals observed growing in running water. Our results are consistent with anecdotal observations that P. leptocoma inhabits wetter environments than P. reflexa (Barkworth et al. 2007). It is surprising that P. leptocoma, the endophyte host species, is associated with wetter areas, given that endophytes are known to promote drought tolerance in other grass species (Cheplick and Faeth 2009). However, in other systems, fungal endophyte symbiosis has been shown to enhance host tolerance to stress via pathways involving the interplay of reactive oxygen species and antioxidants (Hamilton et al. 2012). For instance, endophyte symbiosis appeared to convey tolerance to heat and salt stress in three plant species by lowering accumulation of reactive oxygen species in host tissues (Rodriguez et al. 2008). The fungal endophyte of P. leptocoma may confer greater tolerance of oxidative stress to its host via a similar mechanism, if indeed flooding is stressful to plants in this system.
Observations of edaphic factors, such as soil nutrient levels and pH, could yield further insight into variables that affect the distributions of P. leptocoma and P. reflexa. For instance, interspecific differences in chemical form, soil depth and timing of nitrogen uptake have been documented within plant communities, and such resource partitioning can in turn influence species coexistence and community composition (McKane et al. 2002; Miller and Bowman 2002; Pornon et al. 2007), including amongst grass species (Weigelt et al. 2005). Endophyte symbiosis has been found to affect the accumulation of inorganic and organic nitrogen in host grass tissues (Lyons et al. 1990); uptake of different nitrogen forms could be an endophyte-mediated axis of niche differentiation between the two bluegrasses examined here. Further study could also consider morphological characteristics, such as rooting depth, that may contribute to niche partitioning between the two species by allowing differential access to nitrogen and other soil resources (McKane et al. 2002).
Susceptibility to herbivory may be an additional biotic niche dimension that differentiates the two Poa species. All examined Epichloë populations symbiotic with P. leptocoma contained the perA gene required for the production of peramine, a documented insect deterrent (Tanaka et al. 2005; Schardl et al. 2013b). If the encoded perA gene is functional, then endophyte-symbiotic P. leptocoma individuals may be less susceptible to herbivory by insects relative to P. reflexa. Similarly, in some P. leptocoma populations, we found Epichloë species with genes for the production of the ergot alkaloid intermediate chanoclavine. While the toxicity of chanoclavine has not been well studied, many ergot alkaloids are known to be active against mammals (Schardl et al. 2013a). Poa leptocoma populations symbiotic with endophytes that possess genes for chanoclavine production may therefore be less affected by mammalian herbivory relative to P. reflexa, if the encoded genes are functional and expressed. In particular, pocket gophers (Thomomys talpoides), which consume both roots and shoots of grasses (Ward 1960), have been observed in the study area and may be a significant source of mortality in the system. Additional work could examine expression of the identified defensive genes, consider rates of herbivory on the two Poa species and manipulate herbivory levels to investigate this potential axis of niche differentiation.
While our data provide evidence of niche differences between P. leptocoma and P. reflexa, our results also indicate a certain degree of niche overlap between the two species. At one of four sites (Snodgrass Mountain), significant differences were not observed between P. leptocoma and P. reflexa in mean distance from the closest water source or in soil water content. At Snodgrass Mountain, soil moisture was overall much lower than at the other sites, and differences between P. leptocoma and P. reflexa habitats were smaller, perhaps accounting for the results obtained. Overall densities of both species were also low at this site relative to the other surveyed locations, possibly indicative of marginal habitat for both species. Other, less closely related Poa species are common in the general region that we sampled, and future studies might incorporate their distributions in a community phylogenetics context (Cavender-Bares et al. 2009).
Our results contribute to growing evidence that fungal endophyte presence can have opposing effects on different host life history stages. In accordance with our expectations, endophyte presence appeared to shift the niche of P. leptocoma relative to that of P. reflexa, affecting plant performance in microsites typical of each species. However, our results indicate that more complex dynamics are at play than we initially predicted. Endophyte symbiosis appeared to negatively affect host germination and early survival, constraining the niche of P. leptocoma; however, endophyte presence positively influenced the growth of plants that successfully germinated and survived, regardless of their location. Similar stage-dependent effects were observed in an experiment that removed a fungal endophyte from its host grass and showed that endophyte presence reduced host survival but increased reproduction; model predictions indicated that the beneficial effects of the endophyte on reproduction overwhelmed its negative impacts on survival (Rudgers et al. 2012). Our findings are also consistent with a growing number of studies that document the context-dependency of symbioses (Johnson et al. 1997). For instance, abiotic and biotic factors have been shown to influence the relative costs and benefits to a host plant of associations with fungal endophytes (Davitt et al. 2011) and AM fungi (Jones and Smith 2004; Hoeksema et al. 2010). Our results suggest that the outcome of a symbiosis can depend on host life history stage as well.
It is worthwhile to note that our study examined only the early life history stages of P. leptocoma and P. reflexa when testing the effects of fungal endophyte presence or absence. It is possible that the consideration of adult grasses and sexual reproduction would yield still different results regarding how the fungal endophyte contributes to niche partitioning between the two Poa species. An ongoing experiment is examining adult grasses and their growth and survival in P. leptocoma and P. reflexa niches. Furthermore, a competition experiment could allow for more targeted examination of how endophyte symbiosis may affect species coexistence in this system.
Further work might also consider the broader geographic distributions of the two Poa species. While species' range limits are expected to reflect their niche limits across continuous environments, evidence suggests that range and niche limits may or may not coincide, depending on the relative importance of factors such as dispersal, biotic interactions and temporal fluctuations (Hargreaves et al. 2014). In North America, the ranges of P. leptocoma and P. reflexa overlap, but P. reflexa has not been reported in California or Canada, whereas P. leptocoma has a broader distribution to both the north and west (USDA, NRCS 2014). Investigations into the respective geographic ranges of the two Poa species could identify whether local microsite preferences are consistent with factors that constrain range-wide species distribution limits. Transplants could be conducted outside of the range limits of each species (Hargreaves et al. 2014), with endophyte status manipulated in P. leptocoma. Recent work has shown that endophytes in the genus Epichloë can affect species distributions at a range-wide scale (Afkhami et al. 2014).
In addition, considering symbioses using phylogenetic approaches can yield insight into symbiont effects on host niche within the broader context of host evolutionary history (Poisot et al. 2011). For instance, examining the symbiosis between gall-inducing insects and fungi in a phylogenetic context, Joy (2013) found evidence supporting the hypothesis that symbiosis can lead to host niche expansion and diversification. Similarly, using phylogenetic approaches on an obligate pollination mutualism, Kawakita et al. (2010) found data suggesting that participation in a mutualism favours higher levels of specialization. However, Elias et al. (2008) found the opposite pattern, suggesting that mutualisms increase niche convergence. Similarly examining symbiont effects across the Poa clade could be informative of possible phylogenetic constraints on grass species' microsite preferences, and could shed light on whether trends documented in this study are present in other endophyte-symbiotic Poa species.
Conclusions
Our study provides evidence that P. leptocoma and P. reflexa occupy different ecological niches in the habitats examined. The presence of an endophyte in P. leptocoma appears to play a role in partitioning the niches of the two species, perhaps by initially restricting its host's distribution to wetter microsites before positively affecting its growth. While mutualisms are known to cause shifts in partner niche dimensions, our results suggest the value of considering such effects at different partner life stages. In a broader context, our study identifies a symbiotic relationship as a potential mechanism facilitating the coexistence of two species, suggesting that symbiont effects on host niche may have community-level consequences.
Sources of Funding
Our work was funded by the National Science Foundation (USA) (grants DEB-1354972 and DEB-1145588), the Rocky Mountain Biological Laboratory (National Science Foundation grants DBI-0753774 and OIA-0963529) and the University of New Mexico Department of Biology.
Contributions by the Authors
M.R.K. contributed to data collection and analysis and wrote the manuscript. C.L.D. and J.A.R. designed the experiment, carried out data collection and contributed to writing the manuscript. L.R. and Y.A.C. contributed to experimental design and collected data. W.Q.H. collected data and, with N.D.C. and C.A.Y., completed DNA sequencing and phylogenetic analysis. T.H.P. completed root fungal colonization data collection and analysis. All authors edited the manuscript.
Conflicts of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article –
Table S1. Locality information of sites at which P. leptocoma and P. reflexa plants were collected and/or marked, along with date of sampling, sample size and percentage of plants that were symbiotic with the fungal endophyte E. typhina subsp. poae.
Image S1. Photograph showing placement of plastic toothpicks around a focal, naturally occurring Poa individual.
Figure S1. Gene phylogenies placing the fungal endophyte of P. leptocoma within the E. typhina subsp. poae subclade.
Acknowledgement
We thank Liz Siefert and Bethany Haley for their assistance with greenhouse and laboratory work.
Literature Cited
- Afkhami ME, McIntyre PJ, Strauss SY. Mutualist-mediated effects on species’ range limits across large geographic scales. Ecology Letters. 2014;17:1265–1273. doi: 10.1111/ele.12332. [DOI] [PubMed] [Google Scholar]
- Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- Bacon CW, White JF. Stains, media, and procedures for analyzing endophytes. In: Bacon CW, White JF, editors. Biotechnology of endophytic fungi of grasses. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Bacon CW, White JF. Microbial endophytes. New York: M. Dekker; 2000. [Google Scholar]
- Barkworth ME, Capels KM, Long S, Anderton LK, Piep MB. Flora of North America Volume 24: North of Mexico: Magnoliophyta: Commelinidae (in part): Poaceae, part 1. New York: Oxford University Press; 2007. [Google Scholar]
- Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends in Ecology and Evolution. 2003;18:119–125. doi: 10.1016/S0169-5347(02)00045-9. [DOI] [Google Scholar]
- Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. The merging of community ecology and phylogenetic biology. Ecology Letters. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- Charlton ND, Craven KD, Mittal S, Hopkins AA, Young CA. Epichloë canadensis, a new interspecific epichloid hybrid symbiotic with Canada wildrye (Elymus canadensis) Mycologia. 2012;104:1187–1199. doi: 10.3852/11-403. [DOI] [PubMed] [Google Scholar]
- Cheplick GP, Faeth SH. Ecology and evolution of the grass-endophyte symbiosis. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- Chevenet F, Brun C, Bañuls AL, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. The American Naturalist. 2002;160:S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- Craven KD, Hsiau PTW, Leuchtmann A, Hollin W, Schardl CL. Multigene phylogeny of Epichloe species, fungal symbionts of grasses. Annals of the Missouri Botanical Garden. 2001;88:14–34. doi: 10.2307/2666129. [DOI] [Google Scholar]
- Davitt AJ, Chen C, Rudgers JA. Understanding context-dependency in plant–microbe symbiosis: the influence of abiotic and biotic contexts on host fitness and the rate of symbiont transmission. Environmental and Experimental Botany. 2011;71:137–145. doi: 10.1016/j.envexpbot.2010.11.004. [DOI] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Audic S, Claverie JM, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evolutionary Biology. 2010;10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M, Gompert Z, Jiggins C, Willmott K. Mutualistic interactions drive ecological niche convergence in a diverse butterfly community. PLoS Biology. 2008;6:2642–2649. doi: 10.1371/journal.pbio.0060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forister ML, Gompert Z, Nice CC, Forister GW, Fordyce JA. Ant association facilitates the evolution of diet breadth in a lycaenid butterfly. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1539–1547. doi: 10.1098/rspb.2010.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre SL, Martin OY, Bonte D, Hutchings L, Woolley C, Ibrahim K, Thomas CFG, Hewitt GM. Microbial modification of host long-distance dispersal capacity. BMC Biology. 2009;7:32. doi: 10.1186/1741-7007-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hamilton CE, Gundel PE, Helander M, Saikkonen K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal Diversity. 2012;54:1–10. doi: 10.1007/s13225-012-0158-9. [DOI] [Google Scholar]
- Hargreaves AL, Samis KE, Eckert CG. Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. The American Naturalist. 2014;183:157–173. doi: 10.1086/674525. [DOI] [PubMed] [Google Scholar]
- Hart MM, Reader RJ, Klironomos JN. Plant coexistence mediated by arbuscular mycorrhizal fungi. Trends in Ecology and Evolution. 2003;18:418–423. doi: 10.1016/S0169-5347(03)00127-7. [DOI] [Google Scholar]
- Harte J, Shaw R. Shifting dominance within a montane vegetation community: results of a climate-warming experiment. Science. 1995;267:876–880. doi: 10.1126/science.267.5199.876. [DOI] [PubMed] [Google Scholar]
- Hartnett DC, Wilson GWT. Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology. 1999;80:1187–1195. doi: 10.1890/0012-9658(1999)080[1187:MIPCSA]2.0.CO;2. [DOI] [Google Scholar]
- Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecology Letters. 2010;13:394–407. doi: 10.1111/j.1461-0248.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Graham JH, Smith FA. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytologist. 1997;135:575–586. doi: 10.1046/j.1469-8137.1997.00729.x. [DOI] [Google Scholar]
- Jones EI, Bronstein JL, Ferrière R. The fundamental role of competition in the ecology and evolution of mutualisms. Annals of the New York Academy of Sciences. 2012;1256:66–88. doi: 10.1111/j.1749-6632.2012.06552.x. [DOI] [PubMed] [Google Scholar]
- Jones MD, Smith SE. Exploring functional definitions of mycorrhizas: are mycorrhizas always mutualisms? Canadian Journal of Botany. 2004;82:1089–1109. [Google Scholar]
- Joy JB. Symbiosis catalyses niche expansion and diversification. Proceedings of the Royal Society B: Biological Sciences. 2013;280 doi: 10.1098/rspb.2012.2820. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita A, Okamoto T, Goto R, Kato M. Mutualism favours higher host specificity than does antagonism in plant–herbivore interaction. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2765–2774. doi: 10.1098/rspb.2010.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto RT, Bliss LC. Ecology of subalpine meadows in the Olympic Mountains, Washington. Ecological Monographs. 1970;40:317–347. doi: 10.2307/1942286. [DOI] [Google Scholar]
- Lemons A, Clay K, Rudgers JA. Connecting plant–microbial interactions above and belowground: a fungal endophyte affects decomposition. Oecologia. 2005;145:595–604. doi: 10.1007/s00442-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Leuchtmann A. Systematics, distribution, and host specificity of grass endophytes. Natural Toxins. 1992;1:150–162. doi: 10.1002/nt.2620010303. [DOI] [PubMed] [Google Scholar]
- Leuchtmann A, Bacon CW, Schardl CL, White JF, Tadych M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia. 2014;106:202–215. doi: 10.3852/13-251. [DOI] [PubMed] [Google Scholar]
- Levine JM, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- Lyons PC, Evans JJ, Bacon CW. Effects of the fungal endophyte Acremonium coenophialum on nitrogen accumulation and metabolism in tall fescue. Plant Physiology. 1990;92:726–732. doi: 10.1104/pp.92.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack KML, Rudgers JA. Balancing multiple mutualists: asymmetric interactions among plants, arbuscular mycorrhizal fungi, and fungal endophytes. Oikos. 2008;117:310–320. doi: 10.1111/j.2007.0030-1299.15973.x. [DOI] [Google Scholar]
- Malinowski DP, Belesky DP. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science. 2000;40:923–940. doi: 10.2135/cropsci2000.404923x. [DOI] [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytologist. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- Miller AE, Bowman WD. Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species: do species partition by nitrogen form? Oecologia. 2002;130:609–616. doi: 10.1007/s00442-002-0927-3. [DOI] [PubMed] [Google Scholar]
- Müller CB, Godfray HCJ. Predators and mutualists influence the exclusion of aphid species from natural communities. Oecologia. 1999;119:120–125. doi: 10.1007/s004420050767. [DOI] [PubMed] [Google Scholar]
- Omacini M, Semmartin M, Pérez LI, Gundel PE. Grass–endophyte symbiosis: a neglected aboveground interaction with multiple belowground consequences. Applied Soil Ecology. 2012;61:273–279. doi: 10.1016/j.apsoil.2011.10.012. [DOI] [Google Scholar]
- Palmer TM, Stanton ML, Young TP. Competition and coexistence: exploring mechanisms that restrict and maintain diversity within mutualist guilds. The American Naturalist. 2003;162:S63–S79. doi: 10.1086/378682. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970;55:158–161. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- Poisot T, Bever JD, Nemri A, Thrall PH, Hochberg ME. A conceptual framework for the evolution of ecological specialisation. Ecology Letters. 2011;14:841–851. doi: 10.1111/j.1461-0248.2011.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornon A, Escaravage N, Lamaze T. Complementarity in mineral nitrogen use among dominant plant species in a subalpine community. American Journal of Botany. 2007;94:1778–1785. doi: 10.3732/ajb.94.11.1778. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS. Stress tolerance in plants via habitat-adapted symbiosis. The ISME Journal. 2008;2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytologist. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Holah J, Orr SP, Clay K. Forest succession suppressed by an introduced plant–fungal symbiosis. Ecology. 2007;88:18–25. doi: 10.1890/0012-9658(2007)88[18:FSSBAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Miller TEX, Ziegler SM, Craven KD. There are many ways to be a mutualist: endophytic fungus reduces plant survival but increases population growth. Ecology. 2012;93:565–574. doi: 10.1890/11-0689.1. [DOI] [PubMed] [Google Scholar]
- Schardl CL. The epichloae, symbionts of the grass subfamily Poöideae. Annals of the Missouri Botanical Garden. 2010;97:646–665. doi: 10.3417/2009144. [DOI] [Google Scholar]
- Schardl CL, Florea S, Pan J, Nagabhyru P, Bec S, Calie PJ. The epichloae: alkaloid diversity and roles in symbiosis with grasses. Current Opinion in Plant Biology. 2013a;16:480–488. doi: 10.1016/j.pbi.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG, Schweri KK, Voisey CR, Farman ML, Jaromczyk JW, Roe BA, O'Sullivan DM, Scott B, Tudzynski P, An ZQ, Arnaoudova EG, Bullock CT, Charlton ND, Chen L, Cox M, Dinkins RD, Florea S, Glenn AE, Gordon A, Guldener U, Harris DR, Hollin W, Jaromczyk J, Johnson RD, Khan AK, Leistner E, Leuchtmann A, Li CJ, Liu JG, Liu JZ, Liu M, Mace W, Machado C, Nagabhyru P, Pan J, Schmid J, Sugawara K, Steiner U, Takach JE, Tanaka E, Webb JS, Wilson EV, Wiseman JL, Yoshida R, Zeng Z. Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genetics. 2013b;9:e1003323. doi: 10.1371/journal.pgen.1003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RB. Grasses of Colorado. Boulder, CO: University Press of Colorado; 2008. [Google Scholar]
- Strobel GA. Rainforest endophytes and bioactive products. Critical Reviews in Biotechnology. 2002;22:315–333. doi: 10.1080/07388550290789531. [DOI] [PubMed] [Google Scholar]
- Takach JE, Mittal S, Swoboda GA, Bright SK, Trammell MA, Hopkins AA, Young CA. Genotypic and chemotypic diversity of Neotyphodium endophytes in tall fescue from Greece. Applied and Environmental Microbiology. 2012;78:5501–5510. doi: 10.1128/AEM.01084-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Tapper BA, Popay A, Parker EJ, Scott B. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Molecular Microbiology. 2005;57:1036–1050. doi: 10.1111/j.1365-2958.2005.04747.x. [DOI] [PubMed] [Google Scholar]
- Umbanhowar J, McCann K. Simple rules for the coexistence and competitive dominance of plants mediated by mycorrhizal fungi. Ecology Letters. 2005;8:247–252. doi: 10.1111/j.1461-0248.2004.00714.x. [DOI] [Google Scholar]
- USDA, NRCS. The PLANTS database. Greensboro, NC: National Plant Data Team; 2014. http://plants.usda.gov. (4 November 2014) [Google Scholar]
- Wagg C, Jansa J, Stadler M, Schmid B, van der Heijden MGA. Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology. 2011;92:1303–1313. doi: 10.1890/10-1915.1. [DOI] [PubMed] [Google Scholar]
- Ward AL. Mountain pocket gopher food habits in Colorado. The Journal of Wildlife Management. 1960;24:89–92. doi: 10.2307/3797361. [DOI] [Google Scholar]
- Warren RJ, Giladi I, Bradford MA. Ant-mediated seed dispersal does not facilitate niche expansion. Journal of Ecology. 2010;98:1178–1185. doi: 10.1111/j.1365-2745.2010.01694.x. [DOI] [Google Scholar]
- Weigelt A, Bol R, Bardgett RD. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia. 2005;142:627–635. doi: 10.1007/s00442-004-1765-2. [DOI] [PubMed] [Google Scholar]
- Wright JW, Davies KF, Lau JA, McCall AC, McKay JK. Experimental verification of ecological niche modeling in a heterogeneous environment. Ecology. 2006;87:2433–2439. doi: 10.1890/0012-9658(2006)87[2433:EVOENM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Young CA, Bryant MK, Christensen MJ, Tapper BA, Bryan GT, Scott B. Molecular cloning and genetic analysis of a symbiosis-expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Molecular Genetics and Genomics. 2005;274:13–29. doi: 10.1007/s00438-005-1130-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


