Abstract
In humans, effortful cognitive processing frequently takes place during social interaction, with eye contact being an important component. This study shows that the effect of eye contact on memory for nonsocial information is different in children with typical development than in children with autism, a disorder of social communication. Direct gaze facilitated memory performance in children with typical development (n = 25, 6 years old), but no such facilitation was seen in the clinical group (n = 10, 6 years old). Eye tracking conducted during the cognitive test revealed strikingly similar patterns of eye movements, indicating that the results cannot be explained by differences in overt attention. Collectively, these findings have theoretical significance and practical implications for testing practices in children.
Being looked at is a strong signal, indicating that the other person is attending to you and processing information about you. In many nonhuman species, direct gaze functions as an aversive stimulus, likely because of the threat value associated with eye contact (Emery, 2000). In humans, collaborative communication and problem solving is frequently embedded in face-to-face interaction (Kaplan, Hooper, & Gurven, 2009).
Several different theoretical models specify how eye contact could exercise influence on cognition. According to the natural pedagogy theory, direct gaze, by virtue of being a cue of communicative intent, causes the receiver to search for referents and expecting to be taught something that is generalizable across contexts (Csibra & Gergely, 2009). Supporting this model, one study showed that 6-month-old infants followed another person's gaze shift only if that gaze shift was preceded by direct gaze or other cues of communicative intent (Senju & Csibra, 2008). Another study showed that infants encode kind relevant, generalizable knowledge (object identity) better when addressed with direct gaze and child-directed speech than when cues of communicative intent are absent (Yoon, Johnson, & Csibra, 2008). According to another model, eye contact activates fast and automatic subcortical face processing, which in turns modulates activity in the social brain on short timescales and the development of the social brain on longer timescales (Johnson, 2005). This model is supported by data indicating that the amygdala encodes face-related information early after the onset of the stimulus (e.g., Krolak-Salmon, Henaff, Vighetto, Bertrand, & Mauguiere, 2004). Furthermore, infants' sensitivity to low-spatial-frequency information from faces indicates that this circuit could be early emerging. A final set of theories assumes that direct gaze affects arousal or motivation (Dalton et al., 2005; Kylliainen et al., 2012). Because arousal influences information processing in the whole brain (Sara & Bouret, 2012), altered arousal patterns in response to eye contact could have far-reaching behavioral and cognitive consequences. For related reviews and recent data, see Elsabbagh et al. (2013) and Senju and Johnson (2009a, 2009b).
Autism spectrum disorder (ASD) is a highly heritable neurodevelopmental disorder defined by sociocommunicative impairments and repetitive and stereotyped behaviors, affecting around 1% of the population (Bölte, 2010). According to one influential view, ASD is best conceived as quantitative traits at the extreme end of a phenotypic and etiological continuum (Lundstrom et al., 2012). Altered reaction to eye contact is likely to be one important part of the ASD phenotype. Recent data show that already in infancy and childhood, individuals with ASD process direct gaze differently compared to typically developing (TD) individuals (Elsabbagh et al., 2012; Grice et al., 2005; Kylliainen, Braeutigam, Hietanen, Swithenby, & Bailey, 2006; Kylliainen & Hietanen, 2006; Senju, Tojo, Yaguchi, & Hasegawa, 2005). For example, Senju, Yaguchi, Tojo, and Hasegawa (2003) showed that while TD children were better at detecting direct gaze than averted gaze, no such difference was found in children with ASD. Another study showed that direct gaze (compared to averted gaze) enhanced gender discrimination in typical children, but not in children with ASD (Pellicano & Macrae, 2009). Thus, there may be important downstream consequences of altered eye gaze processing in ASD, at least in the social domain. The design of the current study mimicked the Pellicano and Macrae (2009) study, with the important difference that we tested the children's performance on a nonsocial cognitive task.
Pellicano and Macrae (2009) noted that the differences they observed in gender discrimination between the ASD and the typical group could potentially be explained by different patterns of overt attention (eye movements) in the two groups. Modern eye-tracking technology provides an effective and nonintrusive way of studying eye movements in children. Many studies have found altered eye movements in ASD during observation of social scenes and faces (for a review, see Falck-Ytter, Bölte, & Gredebäck, 2013). In the present study, we recorded the eye movements of the participants during the task, allowing group comparisons of both cognitive performance and overt attention.
Most available eye-tracking studies of children use stimuli that are presented on a computer screen. An important aspect of the current study was that the eye tracking was done live, during a face-to-face interaction between the child and the experimenter. Studying sociocognitive processes live is important in order to increase the ecological validity of the findings (Freeth, Foulsham, & Kingstone, 2013; Gredebäck, Fikke, & Melinder, 2010; Laidlaw, Foulsham, Kuhn, & Kingstone, 2011; Risko, Laidlaw, Freeth, Foulsham, & Kingstone, 2012). In the present study, we address directly the generalizability of some of our findings across live and computer contexts.
There is evidence that a live context may be necessary in order to study certain phenomena related to eye contact. One study found that when the stimuli were live faces, direct gaze caused left-sided frontal EEG alpha-band activation (indicative of a tendency to approach) in TD individuals, whereas an averted gaze caused a right-sided activation (indicative of avoidance; Hietanen, Leppanen, Peltola, Linna-Aho, & Ruuhiala, 2008). When the stimuli were shown as pictures, these effects were not present. Similarly, galvanic skin responses were only different between conditions in the live setting.
We administered a modified version of the digit-span task to 4- to 10-year-old participants with either typical development or ASD. In this task, which measures short-term memory, the child is verbally presented with a series of random digits and is prompted to repeat them directly afterward in the same order. The child and experimenter are sitting down facing each other during the whole task. The digit-span task is highly correlated with general intelligence and typically embedded in gold-standard IQ tests, including the Wechsler scales (Wechsler, 1967/2002, 2003).
In our study, the manipulation of primary interest was the gaze direction of the experimenter administering the test: On half of the digit-span trials he looked the child in the eyes; on remaining trials he looked at the test protocol (hereafter, “averted gaze”). There is some evidence that direct gaze facilitates cognitive performance in TD children (Otteson & Otteson, 1980) and adults (Fry & Smith, 1976; Fullwood & Doherty-Sneddon, 2006; Kelley & Gorham, 1988; but see also Nemeth, Turcsik, Farkas, & Janacsek, 2013). Kelley and Gorham (1988) suggested that eye contact and physical intimacy produced similar cognitive gains, and argued that the results were likely to reflect improved attention via modulation of arousal. Against this background, we predicted that children with typical development would perform better when being looked at than when the experimenter looked down. We hypothesized, based on the literature demonstrating diminished sensitivity to eye contact in ASD, that eye contact would influence performance less in this group than in the TD group (Pellicano & Macrae, 2009; Senju & Johnson, 2009a, 2009b).
On the basis of the existing literature, we also predicted that in terms of eye movements, both groups would look less at the face when thinking and answering than when listening (Doherty-Sneddon, Bruce, Bonner, Longbotham, & Doyle, 2002; Doherty-Sneddon & Phelps, 2005; Doherty-Sneddon, Riby, & Whittle, 2012). We expected children to look more at the experimenter in direct gaze trials than in indirect gaze trials (Freeth et al., 2013).
Method
Participants
Ten high-functioning individuals with ASD (1 girl and 9 boys, age range = 4.9–10.4 years) and 25 TD children (8 girls and 17 boys, age range = 5.1–10.2 years; Table1) were studied (final samples, after exclusion). Autistic children were recruited from habilitation centers in Uppsala and Stockholm and had a clinical community diagnosis of ASD (Autistic Disorder, Asperger's Syndrome, or Pervasive Developmental Disorder—Not Otherwise Specified) according to DSM–IV (American Psychiatric Association, 1994). We selectively recruited children judged by their clinical psychologist at the habilitation center to have an IQ above 70 (subsequently confirmed by formal testing). The TD group was recruited from birth records from the same geographical area as the ASD group. None of the TD children had relatives (including second degree) with ASD. None of the children in this study had major medical conditions (e.g., epilepsy). In all children, we assessed intelligence using the Wechsler Intelligence Scales (Wechsler, 1967/2002, 2003), autistic symptomatology using the Social Responsiveness Scale (SRS; Constantino, 2005; Constantino & Gruber, 2009), and socioeconomic status using an in-house form (Table1). Recruited families were predominantly Caucasian.
Table 1.
Study Group Characteristics, Final Samples (Mean/Standard Deviation)
| Measure | TD (N = 25) | ASD (N = 10) | Pairwise comparison (p valuea) |
|---|---|---|---|
| Age (years) | 6.9/1.2 | 6.7/1.7 | .659 |
| WPPSI–III/WISC–IV total | 101/13 | 104/19 | .740 |
| WPPSI–III/WISC–IV verbal | 104/12 | 102/20 | .460 |
| WPPSI–III/WISC–IV nonverbal | 106/14 | 110/19 | .645 |
| SES (arbr. unit) | 48/6 | 45/11 | .292 |
| SRS-total (T score) | 42/5 | 76/12 | < .001 |
Note. ASD = autism spectrum disorder; TD = typically developing; WPPSI = Wechsler Preschool and Primary Scale of Intelligence; WISC = Wechsler Intelligence Scale for Children; SES = socioeconomic status; SRS = Social Responsiveness Scale.
Independent samples t test.
Two participants (one with ASD) were excluded because of technical failure and seven (four with ASD) because of procedural error. Table1 summarizes participant characteristics (final samples after exclusion). Parents provided written consent, and the study was approved by the Local Ethics Committee in Stockholm and conducted in accordance with the standards specified in the 1964 Declaration of Helsinki. As compensation for their participation, the family received a gift voucher (value ∼25 euro).
Procedure
After familiarization, the participants were brought to the study room with their caregivers. The room included as few distractions as possible (e.g., white curtains covered the walls and technical equipment, except for the eye tracker itself). To resemble a typical classroom environment, two colorful illustrations were hung on a wall behind the experimenter, separated from the head by ∼5 visual degrees. This setting was chosen to increase the ecological validity of the study, keeping both social and nonsocial stimuli within the field of view of the child and the stimulus area covered by the eye tracker.
The experimenter (C.C. or M.J.) and the child were seated in chairs, facing each other. The experimenters were young adult males, one with blond hair and a short beard and the other with dark brown hair and no beard. The eye tracker (Tobii TX300; 60 Hz; Tobii Technology, Stockholm, Sweden) was placed on a table approximately 70 cm in front of and 30 degrees below the participant's line of vision. A camera provided video recordings of the stimulus area from a perspective slightly below that of the child (snapshot; Figure1a). This video was used to define events and areas of interest (AOIs) for subsequent analysis of eye movements. A portable calibration surface with five holes (calibration points) was built to calibrate the eye tracker, using the procedure required by the eye-tracker software (Tobii Studio, Tobii Technology, Stockholm, Sweden). During calibration, a thin object was moved sequentially from hole to hole, accompanied by verbal instruction to look at it. The calibration surface was placed where the face of the experimenter would be located during the subsequent experiment. The calibration procedure was repeated until a good calibration was obtained, which was checked online via a monitor placed behind the child.
Figure 1.
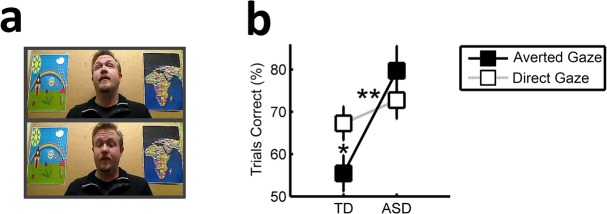
Stimuli and results. (a) On each trial of the digit-span test, the experimenter looked either at the child (direct gaze trials, top) or downward to the test protocol (averted gaze trials, bottom; note that camera angle deviates slightly from child's point of view). (b) The experimenter's gaze direction affected cognitive performance differently in the two groups. In children with typical development (TD), digit-span performance was worse in the averted gaze condition than the direct gaze condition. The children with autism spectrum disorder (ASD) performed equally well in the two conditions. The two groups differed only in the averted gaze condition. Error bars represent the standard error of the mean. *p < .05. **p < .01.
The participants were given the digit-span task, similar to the one used in Wechsler Intelligence Scale for Children, Fourth Edition (WISC–IV; Wechsler, 2003). The digits were given at a rate of one digit per second, in accordance with the WISC–IV test manual. First, it was ensured that the child understood the task. Then, her or his maximum digit span was established by gradually giving longer series of digits (start level = two digits). Maximum digit span was defined as correctly reporting back at least one of two given series at a certain level. Maximum digit-span performance did not differ between groups (ASD: M = 4.2, SD = 0.4; TD: M = 4.4, SD = 0.1, p = 397, Mann–Whitney U test). Thereafter, the actual experiment started, consisting of 6–12 blocks (depending on the child's engagement) of four trials each. The groups did not differ in total number of trials (ASD: M = 32.2, SD = 6.9, TD: M = 34.8, SD = 5.0), t(35) = 1.203, ns. The blocks alternated between easy (two digits per trial) and difficult blocks (trials with the child's maximum level). Only the difficult blocks were analyzed further (the easy blocks functioned as breaks), unless otherwise specified.
On each trial, the experimenter either looked at the child (direct gaze condition) or looked down toward the test protocol (averted gaze condition; Figure1a). This trial-to-trial alternation was also applied when maximum digit span was established. This assured that the maximum digit span was not influenced by the child's potential preference for any of the two conditions. The order of direct gaze and averted gaze trials and of easy and difficult blocks was balanced across participants and groups. Intelligence testing was conducted after the eye-tracking session. After controlling for age, the correlation between the maximum level of performance during the experiment and the children's total IQ score was 0.594 (p < .001, partial correlation).
Data Reduction
We used the Tobii Studio software (Tobii, Stockholm, Sweden) to code events on the basis of the audio and video recording obtained during the experiment and to export text files with the event codes along with eye-tracking data (expressed as pixel coordinates of the scene video). These events included information about condition, phase, and difficulty level. On the basis of previous reports, we divided each trial into an encoding phase (when the experimenter communicates the digit series) and an answering phase (Doherty-Sneddon et al., 2012). These phases reflect qualitatively different task demands.
In-house computer programs written in MATLAB (MathWorks Inc., Natick, MA) were used to (a) define a dynamic AOI covering the face of the experimenter (defined throughout the scene video recording, individually for each participant), (b) calculate the duration of looking in the face AOI relative to the time spent looking at the whole stimulus area, (c) calculate the vertical distance to the center of the face AOI for all data falling within the AOI (normalized to AOI height), and (d) produce summary statistics for the gaze data for the different conditions (e.g., direct vs. averted gaze trials).
We avoided defining small AOIs covering specific face parts, due to the risk that small AOIs would not produce accurate results in a live eye-tracker setting when the model occasionally moves away from the calibrated stimulus surface (e.g., toward or away from the child). Therefore, we chose to use the vertical coordinate of the gaze within the face AOI as a proxy for eye versus mouth looking (Falck-Ytter, von Hofsten, Gillberg, & Fernell, 2013). The face AOI was an ellipse, covering the whole face of the experimenter. The center of the face AOI was defined as the tip of the nose of the experimenter.
As observers typically look most at the eyes and the mouth when looking at a face (Rice, Moriuchi, Jones, & Klin, 2012; de Wit, Falck-Ytter, & von Hofsten, 2008), the vertical position within the face is likely to primarily reflect the relative distribution of gaze toward these two locations. To evaluate the degree of correspondence between looking performance across the two contexts, we correlated the vertical gaze position within the face in the live context with the eye to mouth index (EMI; looking duration at eyes divided by looking duration at both eyes and mouth) during free viewing of static happy faces (each showed for 4 s, 4 different face stimuli, 3 repetitions of stimulus, 12 trials in total; eye tracking conducted with Tobii T120, at 60 Hz, on the same day as the other tasks). The stimuli were taken from the Karolinska Directed Emotional Faces (Lundqvist, Flykt, & Öhman, 1998).
Statistical Analysis
Cognitive performance data were analyzed using nonparametric tests for distributional reasons. To evaluate our main prediction for cognitive performance, we calculated the difference score between the percentage of correct trials in the direct gaze and in the averted gaze conditions, and compared this measure between groups (this is analogous to testing the interaction effect in a 2 × 2 analysis of variance with group and condition as factors).
Unless otherwise specified, remaining data fulfilled criteria for parametric testing (normality; homogeneity of variance). N for individual tests corresponds to global N (Table1) unless otherwise stated. Alpha level was .05 (two-tailed) throughout.
Results
Cognitive Performance
Consistent with our prediction, the difference score (% trials correct in direct gaze condition minus % trials correct in the averted gaze condition) was significantly different in the two groups (p = .008, Mann–Whitney U test; Figure1b). The TD group performed worse on the digit-span task when the experimenter looked down at the test protocol than when he looked directly at them (p = .049; Wilcoxon signed-rank test). No such effect was found in the ASD group. The groups differed only in the averted gaze condition, in which the ASD group performed better than the TD group (p = .003, Mann–Whitney U test). To rule out sex as a confounder for this main result, we excluded all girls from the TD group except for the 2 most closely matching the 1 girl in the ASD group on total IQ (resulting in 19 TD children, of which 2 were girls; Rice et al., 2012). Again, we found that the direction of the experimenter's gaze differentially modulated the cognitive performance of the two groups (p = .045, Mann–Whitney U test).
In the TD group, the total T score on the Social Responsiveness Scale correlated negatively with the difference score for cognitive performance (r = −.477, p = .016, Spearman's rho), suggesting that typical children with least autistic traits were most affected by the experimenter's gaze direction. This relation was not found in the clinical group. In light of recent studies linking eye movements and arousal in social contexts to language (Norbury et al., 2009; Stagg, Davis, & Heaton, 2013), we also correlated the difference score with verbal IQ. No significant relations were found either for the full sample or in separate groups.
Eye Movements
A general linear model (GLM) with condition (direct gaze, averted gaze), phase (encoding, answering), and group entered as factors showed that irrespective of group, the children looked less at the experimenter's face (relative to the whole scene) during the answering phase than during the encoding phase, F(1, 33) = 39.245, p < .001, partial eta squared = .543, a result consistent with previous reports (Doherty-Sneddon et al., 2012). Also, the children looked less at the experimenter in the averted gaze condition compared to the direct gaze condition, F(1, 33) = 26.652, p < .001, partial eta squared = .447, which also is consistent with previous research (Freeth et al., 2013). No other main effects or interactions were found (p ≥ .313).
The above measure reflects the looking duration in the face relative to the time spent looking at the whole stimulus area. However, because the children's gaze aversion could involve eye movements outside the stimulus area (and thus the tracking space of the eye tracker), we repeated the above analysis using a modified dependent measure: the looking duration in the face AOI relative to the duration of the phase. This measure does not take into account where the child was looking when he or she was not looking at the face, and is analogous to the gaze aversion measure used by Doherty-Sneddon et al. (2012). Using this measure, the above pattern of results was replicated. There were strong main effects of phase and condition, and no main or interaction effect involving the group factor. With this measure, we also checked whether task difficulty (maximum digit span contrasted with easy “break” trials with only two digits) moderated the looking duration in the face, as found previously by Doherty-Sneddon et al. As expected, a GLM with group and difficulty entered as factors confirmed less looking at the face during difficult trials than easy trials, F(1, 33) = 7.261, p = .011, partial eta squared = .180, and no effect involving the group factor.
For the vertical gaze position within the face of the experimenter, we found a main effect of phase only, F(1, 33) = 17.598, p < .001, partial eta squared = .348, indicating that children, irrespective of group and condition, looked higher up in the face of the experimenter during the answering phase than the encoding phase. No other main effects or interactions were found (p ≥ .441). Descriptive statistics for these analyses are in Table2.
Table 2.
Gaze Data: Descriptive Statistics
| Group | Measure | Condition | Phase | M | SD |
|---|---|---|---|---|---|
| TD | Looking duration in face AOI (%)a | Direct gaze | Encoding | 82.50 | 17.55 |
| Answering | 60.21 | 22.64 | |||
| Averted gaze | Encoding | 71.88 | 16.41 | ||
| Answering | 49.79 | 17.86 | |||
| Vertical position in faceb | Direct gaze | Encoding | −16.47 | 14.62 | |
| Answering | −8.94 | 14.08 | |||
| Averted gaze | Encoding | −14.06 | 14.11 | ||
| Answering | −7.83 | 16.29 | |||
| ASD | Looking time in face AOI (%) | Direct gaze | Encoding | 78.06 | 15.84 |
| Answering | 60.01 | 20.88 | |||
| Averted gaze | Encoding | 64.05 | 22.32 | ||
| Answering | 42.56 | 23.51 | |||
| Vertical position in face | Direct gaze | Encoding | −6.91 | 17.45 | |
| Answering | 0.73 | 17.71 | |||
| Averted gaze | Encoding | −9.34 | 18.18 | ||
| Answering | 1.64 | 13.37 |
Note. ASD = autism spectrum disorder; TD = typically developing; AOI = areas of interest.
Refers to the percentage of looking duration in the face AOI relative to looking duration anywhere in the stimulus area.
Refers to the vertical distance from the fixation point to the center of the face AOI (only for gaze data falling within the face AOI). Possible values are in the range −50 to 50, where negative values indicate fixations below the center of the AOI and positive values indicate fixations above the center of the AOI (a value of 50 would indicate looking at the top of the face AOI). The center of the face AOI was defined as the tip of the nose of the experimenter.
In the TD group, looking higher up in the face in the live context was associated with having higher EMI during observation of faces presented on a computer monitor (r = .412, p = .041; Spearman's rho; see the Method section for further details). In the ASD group the corresponding relation was not found (r = −.55, p = .881; Spearman's rho). On average, during observation of static faces on the computer screen, the ASD sample looked in the eye AOI (relative to the face AOI) 64.0% of the time (SD = 7.2%), and in the mouth AOI 28.4% of the time (SD = 8.6%). The corresponding figures for the TD group were 63.6% (SD = 17.9%) and 34% (SD = 16.5%). As can be seen, although both groups looked predominantly at these two key areas and the means were strikingly similar, the spread was higher in the typical children. Indeed, the spread of the EMI collected during the screen-based eye-tracking task was significantly different between groups, F(33) = 4,410, p = .043; Levene's test. In contrast, the spread in the vertical measure collected during live eye tracking was similar across groups (see Table2).
Discussion
The present study shows that eye contact affects nonsocial cognition differently in ASD. Typical children performed best when being looked at, but no such effect was found in the clinical group. This suggests that nonautistic children underachieve when the experimenter looks away. The groups differed in the averted gaze condition only, in which the ASD group outperformed the TD group. Cognitive performance did not differ between groups in the direct gaze condition. The finding that typical children performed worse when the experimenter averted his gaze is consistent with a study by Fullwood and Doherty-Sneddon (2006), which indicated reduced performance during an averted gaze condition relative to both a direct gaze condition and a condition with no visual information at all. The lack of differences in terms of eye movements suggests that the difference in cognitive performance between groups in this study cannot be explained by different overt attention to faces or eyes.
The finding that eye contact affects cognition differently in the two child groups has important practical implications. IQ is a maximum performance measure that requires optimal conditions to be assessed. Conversely, if testing conditions are suboptimal, the test results will not correctly reflect the person's true maximal performance. The study suggests that optimal conditions for maximum memory performance may differ between children in systematic ways. Specifically, our findings suggest that for some children, consistent, high use of eye contact may be important. Because IQ tests are used frequently to assist important decisions about children's life (e.g., school placement), understanding how contextual and individual factors influence performance is a high priority. The current study also motivates more research into the use of eye contact in school settings, as it suggests that, at least for some children, eye contact may boost processing of new information.
Although the current study cannot point to the exact mechanism, the results indicate that when typical children (in particular those with low SRS scores) see the experimenter look down, this distracts their attention from the task. As noted in the Introduction, Hietanen et al. (2008) found significantly less approach motivation and autonomic arousal during averted gaze than direct gaze in TD individuals in a live setting. Kylliainen et al. (2012) found approach-related tendencies (left-sided frontal alpha-band EEG asymmetry) in typical children during observation of faces with open eyes (compared to closed eyes), but neither approach nor avoidance tendencies in children with ASD in the same condition. It is not unlikely that these differences in motivation could lead to different attentional capacity and cognitive performance in the child groups.
The children with ASD showed no sign of negatively valenced overarousal (Dalton et al., 2005). First, they looked at the other person just as much as the nonautistic group. Second, they performed just as well during direct gaze trials as the other group. Finally, the ASD group did not perform significantly worse in the direct gaze than the averted gaze conditions. These negative results are particularly striking when one considers the fact that the social interactions were live, with the young child facing an experiment leader who they did not know from before.
Altered performance on a nonsocial task like the one used in the current work is easier to explain with arousal models than with the altered subcortical route model (see the Introduction and Kelley & Gorham, 1988; Senju & Johnson, 2009a), which predicts selective effects on social brain functions. On the other hand, because the information to be remembered was transmitted from a face, the subcortical route could still play a role in this context. Of relevance for this discussion is a recent study testing the subcortical route hypothesis in a sample of infant siblings at high risk for ASD (Elsabbagh et al., 2013). The study failed to support this model—infants with later emerging ASD oriented to faces in a face pop out task (supposedly measuring the integrity of the subcortical route) just as quickly as children who did not develop ASD. Relatedly, a recent study found robust orienting to first-order configural information from faces in adults with ASD (Shah, Gaule, Bird, & Cook, 2013). Against this background, it does not seem likely that the current results reflect a dysfunction in this specific subcortical circuit in ASD.
As noted in the Introduction, the natural pedagogy account (Csibra & Gergely, 2009) specifies that cues of communicative intent like direct gaze should selectively enhance memory for information that is kind relevant and generalizable (e.g., object identity). According to this theory, these cues should not enhance memory for episodic facts that are only relevant for the here and now (e.g., object location). The task in the current experiment was to report back a series of random digits in the same order as they were given by the experimenter. Because this information is episodic rather than kind relevant and generalizable, facilitation during direct gaze trials cannot easily be explained by the natural pedagogy account (Csibra & Gergely, 2009). In fact, one could argue that this theory predicts negative influence by direct gaze on performance in this context (Nemeth et al., 2013). However, in order to properly test this theory, memory for episodic information and kind-relevant information should be tested simultaneously. In typical development, eye contact is expected to facilitate memory of kind relevant information at the expense of episodic information (Yoon et al., 2008). This modification could be a future development of the current work.
As expected from previous research, the children looked less at the face during answering than encoding phases of the task, and during difficult compared to easy trials (Doherty-Sneddon et al., 2012). This pattern has been interpreted as evidence for a functional role of gaze aversion—people look away to reduce processing load when trying to solve a task (Doherty-Sneddon & Phelps, 2005; Doherty-Sneddon et al., 2002). However, eye movements differ as a function of difficulty even in nonvisual information retrieval tasks (Ehrlichman, Micic, Sousa, & Zhu, 2007), and it is possible that gaze aversion is a by-product of cognitive processing rather than a strategy to disengage from task-irrelevant visual information. Direct gaze is arguably a more information-rich stimulus than averted gaze, and we found that during direct gaze trials the children spontaneously looked more at the adult than during averted gaze trials. This may be functional depending on the utility of the information, but critically, the magnitude of this enhancement did not change as a function of whether the face transmitted task-critical information (encoding phase) or not (answering phase). Despite this lack of modulation at the level of eye movements, being looked at caused the TD children to perform better, not worse, on the cognitive task. Thus, while the current study replicates several previous empirical findings (Doherty-Sneddon et al., 2012; Freeth et al., 2013; Fullwood & Doherty-Sneddon, 2006), it does not support the interpretation that gaze aversion reflects a functional process that serves to optimize (minimize) cognitive load.
Interestingly, we found that children looked lower in the face in the encoding than in the answering phase. This result may reflect a functional behavior, as mouth-related information is expected to be most relevant when attending to speech. Conversely, looking higher up (at the eyes) may be particularly useful during the answer phase, when the child may be searching for subtle cues of nonverbal feedback to her responses.
We found that looking higher up in the face during the live interaction was associated with looking more at the eyes relative to the mouth when observing images of faces. This supports our assumption that the vertical face looking measure is related to whether the observer looks at eyes or the mouth. This finding also suggest generalizability of looking patterns from an active task in a dynamic live context to a passive task during observation of static emotional faces shown on a computer monitor. It points to the possibility that individual differences in preference for particular face parts exist in children, and that these have some stability across contexts that entail substantial differences on both transmitting and receiving sides. Curiously, this correlation was found only in the TD children. In the ASD group, the correlation was nonsignificant. One likely factor behind this negative result was the limited spread in the ASD group in the screen-based task. That is, while the spread in the vertical measure from live eye tracking was similar across groups (Table2), the spread in the EMI in the screen-based eye-tracking task was significantly smaller in the ASD group than in the TD group. This pattern motivates further comparisons of performance across contexts.
As already noted, the vertical face looking measure is only a proxy for eye versus mouth looking. Thus, it remains a possibility that some children looked high up in the face, but still not directly in the eyes. However, both previous work and our present data from the screen-based task suggest that children with ASD and children with typical development look predominantly at the eyes and mouth when looking at faces (e.g., Rice et al., 2012). Thus, the most parsimonious interpretation is that the vertical measure predominantly measures eyes versus mouth looking in the current live task as well.
The correlation between the magnitude of the eye contact effect and SRS scores in the TD group is consistent with the view that ASD may be the extreme end of a phenotypic and etiological continuum (Lundstrom et al., 2012; Robinson et al., 2011; Ronald, Larsson, Anckarsater, & Lichtenstein, 2011; see also Chen & Yoon, 2011). However, we found no correlation in the ASD group, which may reflect low statistical power. Alternatively, the relation between the level of autistic traits and sensitivity to eye contact could be nonlinear.
The study has some limitations. First, the ASD group was small, and independent replication is desirable. Second, the conclusions are based on one cognitive task only. Although we have argued for the particular importance of this task given its role in IQ testing and its contribution to the total IQ score, future studies should include different tasks. In particular, it would be interesting to see how the effect generalizes to kind-relevant information and nonverbal information. Finally, in addition to manipulating the type of information to be processed, future studies would benefit from using electrophysiological measures like EEG and measurement of galvanic skin responses during cognitive testing. Such data would speak more directly to applicability of the various models discussed in this article.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bölte S, editor; Hallmayer J, editor. Autism spectrum conditions. FAQs on autism, Asperger syndrome, and atypical autism answered by international experts. Cambridge, MA: Hogrefe; 2010. [Google Scholar]
- Chen FS. Yoon JMD. Brief report: Broader autism phenotype predicts spontaneous reciprocity of direct gaze. Journal of Autism and Developmental Disorders. 2011;41:1131–1134. doi: 10.1007/s10803-010-1136-2. doi: 10.1007/s10803-010-1136-2. [DOI] [PubMed] [Google Scholar]
- Constantino JN. Social Responsiveness Scale: Preschool version for 3-year-olds, research version. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN. Gruber CP. Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2009. [Google Scholar]
- Csibra G. Gergely G. Trends in Cognitive Sciences. 2009;13:148–153. doi: 10.1016/j.tics.2009.01.005. doi: 10.1016/j.tics.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH. Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–526. doi: 10.1038/nn1421. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit T, Falck-Ytter T. von Hofsten C. Young children with autism spectrum disorder look differently at positive versus negative emotional faces. Research in Autism Spectrum Disorders. 2008;2:651–659. doi: 10.1016/j.rasd.2008.01.004. [Google Scholar]
- Doherty-Sneddon G, Bruce V, Bonner L, Longbotham S. Doyle C. Development of gaze aversion as disengagement from visual information. Developmental Psychology. 2002;38:438–445. doi: 10.1037//0012-1649.38.3.438. [PubMed] [Google Scholar]
- Doherty-Sneddon G. Phelps FG. Gaze aversion: A response to cognitive or social difficulty? Memory & Cognition. 2005;33:727–733. doi: 10.3758/bf03195338. doi: 10.3758/bf03195338. [DOI] [PubMed] [Google Scholar]
- Doherty-Sneddon G, Riby DM. Whittle L. Gaze aversion as a cognitive load management strategy in autism spectrum disorder and Williams syndrome. Journal of Child Psychology and Psychiatry. 2012;53:420–430. doi: 10.1111/j.1469-7610.2011.02481.x. doi: 10.1111/j.1469-7610.2011.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman H, Micic D, Sousa A. Zhu J. Looking for answers: Eye movements in non-visual cognitive tasks. Brain and Cognition. 2007;64:7–20. doi: 10.1016/j.bandc.2006.10.001. doi: 10.1016/j.bandc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Gliga T, Pickels A, Hudry K, Charman T, Johnson M BASIS Team. The development of face orienting in infants at-risk for autism. Behavioural Brain Research. 2013;251:147–154. doi: 10.1016/j.bbr.2012.07.030. doi: 10.1016/j.bbr.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T BASIS Team. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. doi: 10.1016/S0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T, Bölte S. Gredebäck G. Eye tracking in early autism research. Journal of Neurodevelopmental Disorders. 2013;5:1–13. doi: 10.1186/1866-1955-5-28. doi: 10.1186/10.1186/1866-1955-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter T, von Hofsten C, Gillberg C. Fernell E. Visualization and analysis of eye movement data from children with typical and atypical development. Journal of Autism and Developmental Disorders. 2013;43:2249–2258. doi: 10.1007/s10803-013-1776-0. doi: 10.1007/s10803-013-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeth M, Foulsham T. Kingstone A. What affects social attention? Social presence, eye contact and autistic traits. PLoS One. 2013;8(1):e53286. doi: 10.1371/journal.pone.0053286. doi: 10.1371/journal.pone.0053286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry R. Smith GF. The effects of feedback and eye contact on performance of a digit-coding task. Journal of Social Psychology. 1976;96:145–146. [Google Scholar]
- Fullwood C. Doherty-Sneddon G. Effect of gazing at the camera during a video link on recall. Applied Ergonomics. 2006;37:167–175. doi: 10.1016/j.apergo.2005.05.003. doi: 10.1016/j.apergo.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Gredebäck G, Fikke L. Melinder A. The development of joint visual attention: A longitudinal study of gaze following during interactions with mothers and strangers. Developmental Science. 2010;13:839–848. doi: 10.1111/j.1467-7687.2009.00945.x. doi: 10.1111/j.1467-7687.2009.00945.x. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P. Johnson MH. Neural correlates of eye-gaze detection in young children with autism. Cortex. 2005;41:342–353. doi: 10.1016/s0010-9452(08)70271-5. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Leppanen JM, Peltola MJ, Linna-Aho K. Ruuhiala HJ. Seeing direct and averted gaze activates the approach-avoidance motivational brain systems. Neuropsychologia. 2008;46:2423–2430. doi: 10.1016/j.neuropsychologia.2008.02.029. doi: 10.1016/j.neuropsychologia.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kaplan HS, Hooper PL. Gurven M. The evolutionary and ecological roots of human social organization. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3289–3299. doi: 10.1098/rstb.2009.0115. doi: 10.1098/rstb.2009.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DH. Gorham J. Effects of immediacy on recall of information. Communication Education. 1988;37:198–207. [Google Scholar]
- Krolak-Salmon P, Henaff MA, Vighetto A, Bertrand O. Mauguiere F. Early amygdala reaction to fear spreading in occipital, temporal, and frontal cortex: A depth electrode ERP study in human. Neuron. 2004;42:665–676. doi: 10.1016/s0896-6273(04)00264-8. doi: 10.1016/s0896-6273(04)00264-8. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Braeutigam S, Hietanen JK, Swithenby SJ. Bailey AJ. Face- and gaze-sensitive neural responses in children with autism: A magnetoencephalographic study. European Journal of Neuroscience. 2006;24:2679–2690. doi: 10.1111/j.1460-9568.2006.05132.x. doi: 10.1111/j.1460-9568.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- Kylliainen A. Hietanen JK. Skin conductance responses to another person's gaze in children with autism. Journal of Autism and Developmental Disorders. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Wallace S, Coutanche MN, Leppanen JM, Cusack J, Bailey AJ. Hietanen JK. Affective-motivational brain responses to direct gaze in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2012;53:790–797. doi: 10.1111/j.1469-7610.2011.02522.x. doi: 10.1111/j.1469-7610.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- Laidlaw KEW, Foulsham T, Kuhn G. Kingstone A. Potential social interactions are important to social attention. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5548–5553. doi: 10.1073/pnas.1017022108. doi: 10.1073/pnas.1017022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A. Öhman A. Karolinska Directed Emotional Faces—KDEF. 1998. CD ROM, Department of Clinical Neuroscience, Psychology section, Karolinska Institutet. [Google Scholar]
- Lundstrom S, Chang Z, Rastam M, Gillberg C, Larsson H, Anckarsater H. Lichtenstein P. Autism spectrum disorders and autistic like traits similar etiology in the extreme end and the normal variation. Archives of General Psychiatry. 2012;69:46–52. doi: 10.1001/archgenpsychiatry.2011.144. doi: 10.1001/archgenpsychiatry.2011.144. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Turcsik AB, Farkas G. Janacsek K. Social communication impairs working-memory performance. Applied Neuropsychology: Adult. 2013;20:211–214. doi: 10.1080/09084282.2012.685134. doi: 10.1080/09084282.2012.685134. [DOI] [PubMed] [Google Scholar]
- Norbury CF, Brock J, Cragg L, Einav S, Griffiths H. Nation K. Eye-movement patterns are associated with communicative competence in autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 2009;50:834–842. doi: 10.1111/j.1469-7610.2009.02073.x. doi: 10.1111/j.1469-7610.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- Otteson JP. Otteson CR. Effect of teachers gaze on childrens story recall. Perceptual and Motor Skills. 1980;50:35–42. [Google Scholar]
- Pellicano E. Macrae CN. Mutual eye gaze facilitates person categorization for typically developing children, but not for children with autism. Psychonomic Bulletin & Review. 2009;16:1094–1099. doi: 10.3758/PBR.16.6.1094. doi: 10.3758/pbr.16.6.1094. [DOI] [PubMed] [Google Scholar]
- Rice K, Moriuchi JM, Jones W. Klin A. Parsing heterogeneity in autism spectrum disorders: Visual scanning of dynamic social scenes in school-aged children. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:238–248. doi: 10.1016/j.jaac.2011.12.017. doi: 10.1016/j.jaac.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risko EF, Laidlaw K, Freeth M, Foulsham T. Kingstone A. Social attention with real versus reel stimuli: Toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience. 2012;6:143. doi: 10.3389/fnhum.2012.00143. doi: 10.3389/fnhum.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happe F. Ronald A. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Archives of General Psychiatry. 2011;68:1113–1121. doi: 10.1001/archgenpsychiatry.2011.119. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Larsson H, Anckarsater H. Lichtenstein P. A twin study of autism symptoms in Sweden. Molecular Psychiatry. 2011;16:1039–1047. doi: 10.1038/mp.2010.82. doi: 10.1038/mp.2010.82. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Bouret S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Senju A. Csibra G. Gaze following in human infants depends on communicative signals. Current Biology. 2008;18:668. doi: 10.1016/j.cub.2008.03.059. doi: 10.1016/j.cub.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Senju A. Johnson MH. Atypical eye contact in autism: Models, mechanisms and development. Neuroscience and Biobehavioral Reviews. 2009a;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Senju A. Johnson MH. The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences. 2009b;13:127–134. doi: 10.1016/j.tics.2008.11.009. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Yaguchi K. Hasegawa T. Deviant gaze processing in children with autism: An ERP study. Neuropsychologia. 2005;43:1297–1306. doi: 10.1016/j.neuropsychologia.2004.12.002. doi: 10.1016/j.neuropsychologia.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Senju A, Yaguchi K, Tojo Y. Hasegawa T. Eye contact does not facilitate detection in children with autism. Cognition. 2003;89:B43–B51. doi: 10.1016/s0010-0277(03)00081-7. doi: 10.1016/s0010-0277(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Shah P, Gaule A, Bird G. Cook R. Robust orienting to protofacial stimuli in autism. Current Biology. 2013;23:R1087–R1088. doi: 10.1016/j.cub.2013.10.034. doi:10.1016/j.cub.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg SD, Davis R. Heaton P. Associations between language development and skin conductance responses to faces and eye gaze in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43:2303–2311. doi: 10.1007/s10803-013-1780-4. doi: 10.1007/s10803-013-1780-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preshcool and Primary Scale of Intelligence: Third Edition (WPPSI-III) San Antonio, TX: Harcourt Assessment; 2002. (Original work published 1967) [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for children. 4th ed. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- Yoon JMD, Johnson MH. Csibra G. Communication-induced memory biases in preverbal infants. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13690–13695. doi: 10.1073/pnas.0804388105. doi: 10.1073/pnas.0804388105. [DOI] [PMC free article] [PubMed] [Google Scholar]


