Abstract
This phase 2 study (N = 116) evaluated single-agent vosaroxin, a first-in-class anticancer quinolone derivative, in patients ≥60 years of age with previously untreated unfavourable prognosis acute myeloid leukaemia. Dose regimen optimization was explored in sequential cohorts (A: 72 mg/m2 d 1, 8, 15; B: 72 mg/m2 d 1, 8; C: 72 mg/m2 or 90 mg/m2 d 1, 4). The primary endpoint was combined complete remission rate (complete remission [CR] plus CR with incomplete platelet recovery [CRp]). Common (>20%) grade ≥3 adverse events were thrombocytopenia, febrile neutropenia, anaemia, neutropenia, sepsis, pneumonia, stomatitis and hypokalaemia. Overall CR and CR/CRp rates were 29% and 32%; median overall survival (OS) was 7·0 months; 1-year OS was 34%. Schedule C (72 mg/m2) had the most favourable safety and efficacy profile, with faster haematological recovery (median 27 d) and lowest incidence of aggregate sepsis (24%) and 30-d (7%) and 60-d (17%) all-cause mortality; at this dose and schedule, CR and CR/CRp rates were 31% and 35%, median OS was 7·7 months and 1-year OS was 38%. Overall, vosaroxin resulted in low early mortality and an encouraging response rate; vosaroxin 72 mg/m2 d 1, 4 is recommended for further study in this population. Registered at www.clinicaltrials.gov: #NCT00607997.
Keywords: vosaroxin, topoisomerase-II inhibitor, acute myeloid leukaemia, elderly, newly diagnosed
Despite significant advances in the management of leukaemia, treatment of acute myeloid leukaemia (AML) in adults 60 years of age and older remains unsatisfactory. Older AML patients commonly display unfavourable disease characteristics, including antecedent haematological disorders (AHDs), treatment-related AML, unfavourable cytogenetic abnormalities and multidrug resistance (Pollyea et al, 2011). Rates of complete remission (CR) after standard induction therapy, typically 60–70% in younger patients, are <50% in patients >65 years of age (Appelbaum et al, 2006). Among older patients who achieve remission, 85% relapse within 3 years, with most surviving <1 year (Burnett et al, 2011). Treatment-related mortality among patients ≥70 years of age treated with intensive chemotherapy may be as high as 26% at 4 weeks (Kantarjian et al, 2006, 2010). Older patients considered to be high risk for toxicity or poor response often receive less intense chemotherapy regimens or supportive care; approximately 30% of older patients with AML receive standard induction regimens (Menzin et al, 2002). Given ongoing demographic shifts in the US (Vincent et al, 2010), the challenge of treating AML in older adults will continue to grow.
Vosaroxin is a first-in-class, non-anthracycline anticancer quinolone derivative that intercalates DNA and inhibits topoisomerase II, thereby inducing DNA damage, G2 cell-cycle arrest and apoptosis (Hawtin et al, 2010a). Vosaroxin causes site-selective DNA double-stranded breaks in G/C-rich sequences, similar to the DNA damage observed in prokaryotes treated with quinolone antibiotics (Hawtin et al, 2010a). Compared with classic topoisomerase II–inhibiting agents in current clinical use, the activity of vosaroxin is more specific, resulting exclusively from DNA intercalation and topoisomerase II inhibition (Hawtin et al, 2010a,b). Vosaroxin is minimally metabolized, thus avoiding formation of free radicals (Evanchik et al, 2009) or reactive oxygen species (Hawtin et al, 2010a) implicated in the cardiotoxicity of anthracyclines (Gewirtz, 1999; Minotti et al, 2004). In addition, vosaroxin is not a substrate of P-glycoprotein receptor–mediated efflux (Hoch et al, 2009), and its activity is independent of TP53 (Walsby et al, 2011), both potential mechanisms of drug resistance. Given these differentiating characteristics, vosaroxin may offer improved efficacy and safety over other topoisomerase II inhibitors.
In a phase 1b study in patients with relapsed or refractory AML, single-agent vosaroxin demonstrated an acceptable safety profile and encouraging clinical activity (Lancet et al, 2011). Vosaroxin was administered weekly for 3 weeks (maximum tolerated dose [MTD] 72 mg/m2) and twice-weekly for 2 weeks (MTD 40 mg/m2). The dose-limiting toxicity was oral mucositis (stomatitis); other toxicities included infection, febrile neutropenia and reversible gastrointestinal (GI) events.
Here, we report the results of the REVEAL-1 study (Response Evaluation of Vosaroxin in Elderly AML), a dose regimen optimization study assessing the efficacy and safety of single-agent vosaroxin in patients ≥60 years of age with newly diagnosed AML and an additional risk factor or adverse prognostic feature.
Patients and methods
Study design
This open-label, multicentre, phase 2 study evaluated the efficacy and safety of three vosaroxin treatment schedules in sequentially enrolled cohorts—Schedule A: vosaroxin 72 mg/m2 on days 1, 8, 15; Schedule B: vosaroxin 72 mg/m2 on days 1, 8; Schedule C: vosaroxin 72 mg/m2 (C72) or 90 mg/m2 (C90) on days 1, 4. The institutional review board at each centre approved the study protocol. All patients provided informed consent in accordance with the principles of the Declaration of Helsinki.
Patient eligibility
Briefly, patients ≥60 years of age with previously untreated de novo AML or secondary AML (with an AHD or following exposure to a potentially leukaemogenic agent), ≥20% blasts by bone marrow biopsy or aspiration, Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2 and no prior therapy for AML were eligible. Patients were also required to have at least one of the following adverse prognostic factors: age ≥70 years, AHD, ECOG PS 2 or intermediate or unfavourable karyotype (defined as any cytogenetic profile without the presence of t(8;21)(q22;q22); inv(16)(p13;q22) or t(16;16)(p13;q22); or t(15;17)(q22;q12) and variants). Patients were excluded if they had disseminated intravascular coagulation, active central nervous system involvement, other active malignancies or had been treated for AHD or with another investigational agent within 28 d of the first vosaroxin dose. See Supporting Information for a full list of inclusion and exclusion criteria.
Treatment
Patient cohorts were enrolled sequentially, first in Schedule A, which was the MTD for weekly dosing determined previously (Lancet et al, 2011). Based on a preliminary safety review of Schedule A, Schedule B was added. This dosing regimen eliminated the third vosaroxin dose, reducing the total dose administered per cycle and shortening the duration of treatment to 8 d. Subsequently, Schedule C was added, to assess whether dose intensification, relative to Schedule B, might improve antileukaemic activity without losing the tolerability of the 2-dose schedule; two doses (72 and 90 mg/m2) were evaluated for this schedule.
Vosaroxin was administered as a short (≤10 min) intravenous infusion. Patients could receive up to four treatment cycles. Patients with a reduction in bone marrow blast count or stable disease and no persistent, clinically significant nonhaematological adverse event (AE) after the first induction cycle were eligible for reinduction, to be initiated no earlier than day 15 and no later than day 57. Patients with CR, CR with incomplete platelet recovery (CRp), or CR with incomplete blood count recovery (CRi) after induction/reinduction therapy were eligible for 1–2 consolidation cycles.
Supportive care
Patients received transfusion of blood products in accordance with institutional guidelines. After a preliminary review of safety data for patients in Schedule A, the concomitant use of prophylactic antibiotics, antifungal agents and antiviral agents according to institutional protocols was encouraged in Schedule B and C cohorts. Use of growth factors according to national guidelines was permitted.
Safety and efficacy assessments
For each successive cohort, the efficacy and safety of the regimen at a particular dose and schedule were assessed. Safety was assessed at baseline and throughout the study to identify changes from baseline and determine the incidence and severity of AEs, which were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (v3.0) (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Bone marrow biopsy or aspiration was performed at screening, on day 15 (±1 d) of induction and reinduction cycles, at the time of haematological recovery (defined as absolute neutrophil count [ANC] >1000 cells/μl), and as clinically indicated. Responses were assessed based on International Working Group criteria (Cheson et al, 2003). Differences in efficacy and safety between cohorts were assessed qualitatively, with real-time, investigator-driven review.
Endpoints
The primary endpoint was the combined complete remission (CR + CRp) rate for each vosaroxin treatment schedule. Secondary endpoints were: safety; rates of 30- and 60-d all-cause mortality; leukaemia-free survival (LFS; the time from response to relapse or death from any cause); overall survival (OS; the time from first vosaroxin infusion to death from any cause); and pharmacokinetics (PK). The time to haematological recovery was defined as the time from treatment initiation to the earliest visit at which the ANC was >1·0 × 109/l.
PK studies
Plasma PK profiles were evaluated on day 1 for all four regimens and on day 4 for Schedules C72 and C90. Standard PK parameters, including maximum concentration (Cmax), terminal half-life (t1/2), area under the concentration-versus-time curve from time 0 to infinity (AUC0-inf), clearance (CL) and volume of distribution at steady state (Vss), were derived from plasma concentration-versus-time data. Renal excretion of vosaroxin and its metabolites, N-desmethyl-vosaroxin and O-desmethyl-vosaroxin, was estimated from urine samples. See the Supporting Information for a full description of PK studies.
Statistics
The safety population comprised all enrolled patients who received any amount of vosaroxin; all safety and efficacy analyses were performed in the safety population. In Schedules A and B, the study design was based on a single-arm Green-Dahlberg 2-stage design (Green & Dahlberg, 1992). A sample size of 30 patients in stage I and 25 additional patients in stage II provided 90% power (at a significance level of 0·05) to reject the null hypothesis that the probability of remission (P) is ≤0·30, if the true P is ≥0·50. Schedule C employed a single-stage design with sample size goals of 30 and 20 patients for the C72 and C90 cohorts, respectively (see the Supporting Information for a full description of sample size considerations for each cohort).
Descriptive statistics were used to summarize baseline patient characteristics, exposure, and safety and efficacy variables. Categorical and nominal variables were summarized by frequency and percentage. Continuous variables were summarized using standard summary statistics (N, mean, standard deviation, median, minimum and maximum). Ordinal variables were summarized by frequency distribution of scores and by summary statistics on the scores or shift tables, as appropriate. When appropriate, 95% confidence intervals (CIs) around point estimates were determined. Descriptive analyses of the time-to-event endpoints (LFS and OS) were performed using Kaplan–Meier methods.
Results
Patients and exposure
A total of 116 patients were enrolled across all schedules and doses and 113 received at least one dose of vosaroxin (safety population). Pretreatment characteristics of the patients are summarized in Table I. In all cohorts, the median patient age was ≥70 years and most patients (82%) had two or more adverse prognostic factors.
Table I.
Baseline patient and disease characteristics in patients treated with vosaroxin administered as a short (≤10 min) intravenous infusion
| Schedule A* | Schedule B† | Schedule C72‡ | Schedule C90§ | All patients | |
|---|---|---|---|---|---|
| Patients treated, n | 29 | 35 | 29 | 20 | 113 |
| Median age (range), years | 75 (61–89) | 75 (64–87) | 70 (61–84) | 78 (66–88) | 75 (61–89) |
| Gender, n (%) | |||||
| Male | 19 (66) | 23 (66) | 14 (48) | 17 (85) | 73 (65) |
| Female | 10 (35) | 12 (34) | 15 (52) | 3 (15) | 40 (35) |
| AML subtype,¶ n (%) | |||||
| With characteristic genetic abnormalities | 0 | 2 (6) | 2 (7) | 1 (5) | 5 (4) |
| With multilineage dysplasia | 12 (41) | 8 (23) | 6 (21) | 9 (45) | 35 (31) |
| Alkylating agent–related AML and MDS | 2 (7) | 2 (6) | 0 | 3 (15) | 7 (6) |
| Not otherwise categorized | 15 (52) | 22 (63) | 19 (66) | 7 (35) | 63 (56) |
| Missing | 0 | 1 (3) | 2 (7) | 0 | 3 (3) |
| Adverse prognostic factors, n (%) | |||||
| Age ≥70 years | 22 (76) | 27 (77) | 15 (52) | 17 (85) | 81 (72) |
| AHD | 11 (38) | 10 (29) | 7 (24) | 7 (35) | 35 (31) |
| ECOG PS 2 | 4 (14) | 4 (11) | 9 (31) | 3 (15) | 20 (18) |
| I/U cytogenetics | 27 (93) | 28 (80) | 28 (97) | 19 (95)** | 102 (90) |
| ≥2 risk factors | 25 (86) | 27 (77) | 24 (83) | 17 (85) | 93 (82) |
AHD, antecedent haematological disease; AML, acute myeloid leukaemia; ECOG PS, Eastern Cooperative Oncology Group performance status; I/U, intermediate/unfavourable; MDS, myelodysplastic syndrome.
72 mg/m2 on days 1, 8, and 15.
72 mg/m2 on days 1 and 8.
72 mg/m2 on days 1 and 4.
90 mg/m2 on days 1 and 4.
At diagnosis, by World Health Organization criteria (Vardiman et al, 2009).
Cytogenetic data are missing for one patient in this cohort.
All 113 treated patients completed at least one induction cycle and 26 (23%) completed two induction/reinduction cycles. Of the 30 patients (27%) who received consolidation, 12 received one and 18 received the per-protocol maximum of two consolidation cycles. Ninety-five patients (84%) discontinued study treatment, due to treatment failure (n = 50), death (n = 21), unacceptable AE (n = 6), disease relapse (n = 5), physician's decision (n = 5) or other reason (n = 8).
Efficacy
Overall, 33 (29%) and 36 (32%) patients achieved CR and CR/CRp, respectively. Responses were observed across all schedules; the highest CR/CRp rates were observed with Schedules A (41%) and C72 (35%) (Table II). Responses occurred in patients with all categories of risk factors, including those with ≥2 risk factors. Most remissions (28 of 36, 78%) occurred with one induction cycle; however, eight of 26 patients (31%) receiving a reinduction cycle subsequently achieved CR or CRp. The median times to ANC and to platelet count recovery among responders were 35 and 36 d, respectively. These times were longer with Schedule A (37 and 44 d) than with other schedules (B: 35 and 29 d; C72: 27 and 28 d; C90: 23 and 27 d).
Table II.
Outcomes with vosaroxin treatment in patients over 60 years of age with newly diagnosed AML (N = 113)
| Schedule A* (n = 29) | Schedule B† (n = 35) | Schedule C72‡ (n = 29) | Schedule C90§ (n = 20) | All patients (N = 113) | |
|---|---|---|---|---|---|
| Response, n (%)¶ | |||||
| CR | 12 (41) | 7 (20) | 9 (31) | 5 (25) | 33 (29) |
| CR + CRp | 12 (41) | 9 (26) | 10 (35) | 5 (25) | 36 (32) |
| Survival outcomes | |||||
| Median OS (95% CI), months | 8·6 (1·2–14·7) | 5·7 (1·9–10·1) | 7·7 (3·3–12·2) | 5·5 (1·8–11·1) | 7·0 (4·0–9·2) |
| Median LFS** (95% CI), months | 9·8 (2·4–15·7) | 10·9 (2·9–15) | 5·5 (1·5–7·3) | 5·8 (1·3–und) | 6·5 (4·9–9·8) |
| 1-year survival††, n (%) | 11 (38) | 11 (31) | 11 (38) | 5 (25) | 38 (34) |
| All-cause mortality | |||||
| 30-d (95% CI), % | 21 (9·9–40·4) | 8·6 (2·8–24·3) | 6·9 (1·8–24·9) | 10 (2·6–34·4) | 12 (6·8–19·0) |
| 60-d (95% CI), % | 38 (23·1–57·9) | 37 (23·5–55·2) | 17 (7·6–36·6) | 30 (14·7–54·9) | 31 (23·3–40·4) |
AML, acute myeloid leukaemia; CI, confidence interval; CR, complete remission; CRp, complete remission with incomplete platelet recovery; LFS, leukaemia-free survival; OS, overall survival; und, undefined.
72 mg/m2 on days 1, 8, and 15.
72 mg/m2 on days 1 and 8.
72 mg/m2 on days 1 and 4.
90 mg/m2 on days 1 and 4.
As assessed by the medical reviewer.
Calculated for patients with CR or CRp on study treatment.
All surviving patients had a minimum follow-up of 1 year.
The median OS in all patients was 7·0 months (95% CI, 4·0–9·2 months) (Fig1A); median OS was 8·6 months with Schedule A and 7·7 months with Schedule C72 (Table II). With a minimum follow-up of 1 year, the 1-year survival rate was 38% with both Schedules A and C72. Among responders (CR and CRp, n = 36), the median OS was 15·5 months (95% CI, 12·7–18·3 months) and the median LFS was 6·5 months (95% CI, 4·9–9·8 months) (Fig1B).
Figure 1.
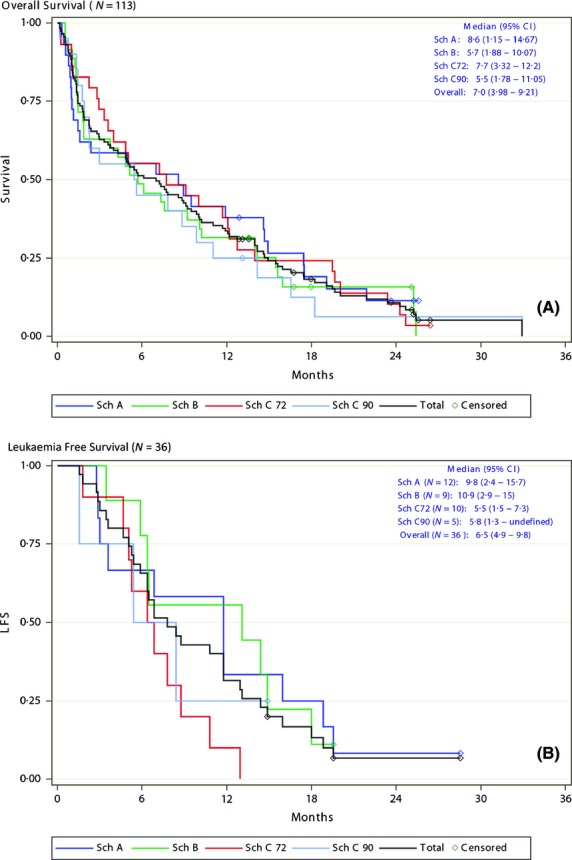
Survival outcomes in newly diagnosed acute myeloid leukaemia treated with vosaroxin. (A) Overall survival in the pooled population (N = 113). (B) Leukaemia-free survival in patients with CR/CRp (n = 36). Sch, schedule; CR, complete remission; CRp, complete remission with incomplete platelet recovery; 95% CI, 95% confidence interval.
Safety
Treatment-emergent AEs are summarized in Table III. Grade 3 or 4 AEs were predominantly haematologic and included thrombocytopenia (59%), febrile neutropenia (50%) and anaemia (49%). The incidence of grade 3/4 infections was higher with Schedule A (76%) than with the other 3 schedules (B: 57%; C72: 48%; C90: 55%). Grade ≥3 sepsis (aggregate of sepsis, bacteraemia, fungaemia and viraemia) occurred in 39% of patients; the frequency of sepsis was lowest in patients in the Schedule C72 cohort. The most frequent nonhaematological AEs of any grade included GI disorders (diarrhoea 74%, nausea 70%, oral mucositis/stomatitis 60%) and metabolic disorders (hypokalaemia 62%, anorexia 59%, hypomagneseamia 43%). The incidences of any-grade diarrhoea, oral mucositis/stomatitis, hypokalaemia and anorexia were lower with Schedules B and C than with Schedule A.
Table III.
Adverse events occurring as any grade in ≥20% or as grade ≥3 in ≥5% of all patients
| Grade | Schedule A* (n = 29) | Schedule B† (n = 35) | Schedule C72‡ (n = 29) | Schedule C90§ (n = 20) | All patients (N = 113) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any | ≥3 | Any | ≥3 | Any | ≥3 | Any | ≥3 | Any | ≥3 | |
| Haematological events, % | ||||||||||
| Febrile neutropenia | 48 | 35 | 66 | 60 | 62 | 55 | 50 | 45 | 58 | 50 |
| Thrombocytopenia | 45 | 45 | 69 | 69 | 69 | 62 | 60 | 60 | 61 | 59 |
| Anaemia | 41 | 38 | 54 | 46 | 66 | 55 | 60 | 60 | 55 | 49 |
| Neutropenia | 41 | 38 | 26 | 26 | 28 | 28 | 25 | 25 | 30 | 29 |
| Leucopenia | 7 | 7 | 14 | 14 | 0 | 0 | 5 | 5 | 7 | 7 |
| Non-haematological events, % | ||||||||||
| Cardiac disorders | ||||||||||
| Tachycardia | 38 | 0 | 40 | 0 | 17 | 0 | 25 | 5 | 31 | 1 |
| Gastrointestinal disorders | ||||||||||
| Diarrhoea | 83 | 7 | 77 | 6 | 69 | 0 | 65 | 10 | 74 | 5 |
| Stomatitis/oral mucositis | 72 | 28 | 57 | 11 | 55 | 28 | 55 | 25 | 60 | 22 |
| Nausea | 76 | 3 | 60 | 0 | 86 | 3 | 55 | 5 | 70 | 3 |
| Vomiting | 55 | 0 | 29 | 0 | 52 | 7 | 25 | 0 | 41 | 2 |
| Constipation | 41 | 0 | 37 | 0 | 17 | 0 | 20 | 0 | 30 | 0 |
| Oral pain | 7 | 3 | 14 | 3 | 17 | 10 | 30 | 15 | 16 | 7 |
| General disorders/administration site conditions | ||||||||||
| Peripheral oedema | 59 | 10 | 49 | 3 | 52 | 3 | 40 | 0 | 50 | 4 |
| Fatigue | 52 | 17 | 57 | 20 | 41 | 14 | 35 | 15 | 48 | 17 |
| Asthenia | 31 | 10 | 40 | 9 | 21 | 7 | 10 | 0 | 27 | 7 |
| Chills | 28 | 0 | 34 | 0 | 28 | 3 | 30 | 0 | 30 | 1 |
| Pyrexia | 17 | 0 | 26 | 3 | 24 | 0 | 35 | 0 | 25 | 1 |
| Infections and infestations | ||||||||||
| Aggregate sepsis¶ | 52 | 55** | 43 | 34** | 21 | 24 | 45 | 45** | 40 | 39** |
| Aggregate infections¶ | 45 | 21 | 37 | 6** | 45 | 10 | 35 | 20 | 41 | 13** |
| Aggregate pneumonia¶ | 41 | 31** | 40 | 34** | 38 | 28 | 35 | 25** | 39 | 30** |
| Metabolism and nutrition disorders | ||||||||||
| Anorexia | 72 | 28 | 51 | 9 | 59 | 10 | 55 | 0 | 59 | 12 |
| Hypokalaemia | 72 | 41 | 54 | 20 | 59 | 21 | 65 | 15 | 62 | 25 |
| Hypomagnesaemia | 52 | 3 | 40 | 0 | 48 | 0 | 30 | 0 | 43 | 1 |
| Hypophosphataemia | 45 | 24 | 23 | 3 | 21 | 7 | 25 | 15 | 28 | 12 |
| Dehydration | 28 | 14 | 20 | 0 | 10 | 3 | 25 | 15 | 20 | 7 |
| Hypocalcaemia | 28 | 14 | 14 | 6 | 31 | 10 | 20 | 10 | 23 | 10 |
| Hyperglycaemia | 10 | 7 | 20 | 6 | 21 | 10 | 15 | 5 | 17 | 7 |
| Nervous system disorders | ||||||||||
| Headache | 35 | 0 | 26 | 3 | 31 | 3 | 10 | 0 | 27 | 2 |
| Dizziness | 21 | 0 | 20 | 0 | 24 | 0 | 20 | 0 | 21 | 0 |
| Psychiatric disorders | ||||||||||
| Insomnia | 41 | 0 | 37 | 0 | 38 | 0 | 15 | 0 | 35 | 0 |
| Confusional state | 28 | 14 | 26 | 0 | 28 | 3 | 10 | 5 | 24 | 5 |
| Anxiety | 24 | 0 | 40 | 0 | 41 | 3 | 20 | 0 | 33 | 1 |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||
| Cough | 45 | 0 | 31 | 0 | 38 | 0 | 25 | 0 | 35 | 0 |
| Dyspnea | 38 | 24 | 40 | 9 | 38 | 3 | 25 | 15 | 36 | 12 |
| Rales | 35 | 0 | 23 | 0 | 7 | 0 | 15 | 0 | 20 | 0 |
| Epistaxis | 24 | 0 | 37 | 0 | 28 | 7 | 25 | 0 | 29 | 2 |
| Pleural effusion | 24 | 0 | 23 | 9 | 38 | 0 | 10 | 0 | 25 | 3 |
| Hypoxia | 10 | 7 | 17 | 14 | 21 | 3 | 10 | 0 | 15 | 7 |
| Skin and subcutaneous tissue disorders | ||||||||||
| Alopecia | 41 | 0 | 23 | 0 | 45 | 0 | 25 | 0 | 34 | 0 |
| Petechiae | 28 | 0 | 31 | 0 | 14 | 0 | 0 | 0 | 20 | 0 |
| Rash | 24 | 0 | 23 | 0 | 35 | 3 | 15 | 0 | 25 | 1 |
| Vascular disorders | ||||||||||
| Hypotension | 38 | 17 | 31 | 3 | 35 | 0 | 30 | 15 | 34 | 8 |
| Hypertension | 21 | 10 | 11 | 9 | 10 | 3 | 0 | 0 | 12 | 6 |
72 mg/m2 on days 1, 8, and 15.
72 mg/m2 on days 1 and 8.
72 mg/m2 on days 1 and 4.
90 mg/m2 on days 1 and 4.
Aggregate sepsis includes sepsis, bacteraemia, viraemia and fungaemia, and represents 32 preferred terms; aggregate infections represent 30 preferred terms; aggregate pneumonia represents 10 preferred terms.
Includes 1 or more grade 5 events. Grade 5 aggregate sepsis was observed with schedules A (n = 4), B (n = 3), and C90 (n = 2); grade 5 aggregate pneumonia was observed with Schedules A (n = 1), B (n = 1), and C90 (n = 2); grade 5 aggregate infection was observed with Schedule B (n = 1).
Ninety-one patients (81%) had ≥1 serious AE (SAE). The most common SAEs included pneumonia (24%), febrile neutropenia (21%) and oral mucositis/stomatitis (10%). Of 113 patients treated, 103 died over the study duration. Most deaths (78%; n = 80) were due to progressive disease (Table IV). Across the entire population, 30- and 60-d all-cause mortality rates were 12% and 31%, respectively. Early mortality was lowest with Schedule C72: 30- and 60-d mortality rates were 7% and 17%, respectively.
Table IV.
Causes of death in relapsed or refractory AML patients treated with vosaroxin and cytarabine
| Cause of death | Deaths within 30 d/60 d per end of follow-up, n | ||||
|---|---|---|---|---|---|
| Schedule A* (n = 29) | Schedule B† (n = 35) | Schedule C72‡ (n = 29) | Schedule C90§ (n = 20) | All patients (N = 113) | |
| Disease progression | 1/4/17 | 3/10/23 | 0/2/25 | 1/3/15 | 5/19/80 |
| Pneumonia | 1/2/2 | 0 | 0 | 0/1/1 | 1/3/3 |
| Sepsis | 1/1/1 | 0 | 0 | 1/1/2 | 2/2/3 |
| Cardiac arrest | 0 | 0/0/1 | 0 | 0 | 0/0/1 |
| Cardiac failure | 1/1/1 | 0 | 0 | 0 | 1/1/1 |
| Cardiomyopathy | 0 | 0 | 1/1/1 | 0 | 1/1/1 |
| Colon perforation | 1/1/1 | 0 | 0 | 0 | 1/1/1 |
| Hepatorenal failure | 0/1/1 | 0 | 0 | 0 | 0/1/1 |
| Lung cancer | 0 | 0/0/1 | 0 | 0 | 0/0/1 |
| Myocardial infarction | 0 | 0 | 1/1/1 | 0 | 1/1/1 |
| Pulmonary haemorrhage | 0 | 0/1/1 | 0 | 0 | 0/1/1 |
| Respiratory failure | 0 | 0 | 0 | 0/1/1 | 0/1/1 |
| Subdural haemorrhage | 0 | 0/1/1 | 0 | 0 | 0/1/1 |
| Unknown | 0/1/2 | 0/1/4 | 0/1/1 | 0 | 0/3/7 |
AML, acute myeloid leukaemia.
72 mg/m2 on days 1, 8, and 15.
72 mg/m2 on days 1 and 8.
72 mg/m2 on days 1 and 4.
90 mg/m2 on days 1 and 4.
PK analyses
Plasma PK profiles were evaluated in 33 patients; Table V summarizes PK parameters by patient cohort. After a single dose, plasma vosaroxin concentrations declined in a biphasic manner with a short initial distribution phase, followed by a prolonged elimination phase. Across all cohorts, the average t1/2 was approximately 20–28 h. The average total body CL was 3·2–4·9 l/h. The average Vss was approximately 108–139 l. Plasma vosaroxin exposure (AUC) increased approximately in proportion with the increase in dose between 72 and 90 mg/m2.
Table V.
PK parameters by schedule, dosing day, and dose
| Day | Schedule/dose (mg/m2) | N | Cmax* (ng/ml) | AUC0–72 h (h·ng/ml) | AUCinf (h·ng/ml) | CL (l/h) | Vss (l) | t½ (h) | MRTinf (h) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | |||
| 1 | A/72 | 10 | 3028 | 48·7 | 36 721 | 21·1 | 41 903 | 27·3 | 3·643 | 30·0 | 107·9 | 40·4 | 22·51 | 36·6 | 30·56 | 41·4 |
| B/72 | 8‡ | 2450 | 47·8 | 40 092 | 28·7 | 47 228 | 28·7 | 3·389 | 33·4 | 129·9 | 50·2 | 27·29 | 21·2 | 37·57 | 23·5 | |
| C/72 | 6 | 2865 | 47·8 | 37 401 | 13·9 | 44 848 | 13·0 | 3·235 | 15·2 | 116·6 | 28·5 | 28·27 | 32·1 | 37·16 | 36·8 | |
| C/90 | 8 | 3328 | 69·0 | 38 086 | 28·0 | 43 838 | 31·8 | 4·538 | 25·3 | 139·3 | 21·1 | 23·57 | 25·2 | 32·10 | 27·0 | |
| 4† | C/72 | 5 | 5792 | 114·9 | 35 125 | 23·8 | 40 539 | 26·9 | 4·387 | 30·3 | 137·3 | 39·5 | 23·52 | 27·6 | 31·83 | 35·7 |
| C/90 | 7§ | 4148 | 98·4 | 43 667 | 32·3 | 46 462 | 37·2 | 4·882 | 28·4 | 132·7 | 34·0 | 20·41 | 28·7 | 27·79 | 24·1 | |
PK, pharmacokinetic; AUC0-inf, area under the concentration-versus-time curve from time 0 to infinity; AUC0–72 h, AUC from time 0 to 72 h; CL, clearance; Cmax, maximum concentration; CV, coefficient of variation; MRTinf, mean residence time; t1/2, terminal half-life; Vss, volume of distribution at steady state.
Cmax for each patient occurred at the 5-min sampling time, except when the 5-min sample result was missing and for one patient for whom Cmax occurred at 15 min.
PK analysis conducted with a steady-state approach, where CL = CLss.
Nine patients were included in the determination of Cmax.
Eight patients each were included in the determination of Cmax and AUC0–72.
The drug accumulation ratio for day 4 to day 1 AUC0–72 h was 0·935 with vosaroxin 72 mg/m2 and 1·14 with vosaroxin 90 mg/m2. Over the range of CL values reported at the two dose levels studied, CL was independent of age. Vosaroxin CL on day 1 was significantly higher in men than in women (P = 0·0008), which may be related to a trend toward increased CL with greater body weight and greater body surface area. The average renal excretion of vosaroxin and its metabolites over 4 d was <5% of the total vosaroxin dose infused, indicating that vosaroxin CL is non-renal.
Discussion
REVEAL-1 evaluated the activity and tolerability of single-agent vosaroxin in previously untreated AML patients ≥60 years of age with ≥1 additional risk factor. Median patient age was 75 years and most patients (82%) had ≥2 risk factors. Complete remissions were observed across all treatment schedules and risk factors. Overall, vosaroxin produced a CR/CRp rate of 32%, with a 1-year OS rate of 34% (median OS 7·0 months). Although most remissions (78%) were achieved during the first induction cycle, 29% of patients who received a second induction cycle achieved CR or CRp, underscoring the value of reinduction.
Relative to weekly dosing for 3 weeks (Schedule A; days 1, 8, 15), tolerability of weekly dosing for 2 weeks (Schedule B; days 1, 8) appeared to be superior, with a notable decrease in the incidence of grade ≥3 sepsis (52% vs. 34%) and infections (21% vs. 6%), and reduced 30-d all-cause mortality (21% vs. 9%); however, the CR/CRp rate was lower with Schedule B than with Schedule A (26% vs. 41%) and 60-d all-cause mortality was comparable between Schedules B and A (37% vs. 38%). Although the number of patients in each cohort was too small to allow formal comparison, these observations suggest that elimination of the third weekly dose improved tolerability. However, the decreased dose intensity with Schedule B may have been associated with reduced antileukaemic activity.
The Schedule C72 cohort demonstrated the best risk-benefit profile, indicating that two doses of vosaroxin delivered over a short period of time may be most beneficial. With this schedule, time to recovery of both neutrophils and platelets was improved by more than 7 d compared with Schedules A and B, and the incidence of grade ≥3 aggregated sepsis was lowest (24%). The tolerability of Schedule C72 is particularly reflected in the low 30-d (7%) and 60-d (17%) all-cause mortality. In this cohort, CR and CR/CRp rates were 31% and 35%, median OS was 7·7 months and 1-year OS was 38%. Outcomes were not improved by increasing the Schedule C dose to 90 mg/m2, which was associated with increased incidences of grade ≥3 aggregate sepsis (45%) and 60-d mortality (30%).
The efficacy and mortality observed in this study, particularly in the Schedule C72 cohort, are consistent with the findings of two large, retrospective analyses of other chemotherapy induction regimens in previously untreated elderly patients with AML (Appelbaum et al, 2006; Kantarjian et al, 2006), despite the fact that patients included in REVEAL-1 were required to have at least one unfavourable prognostic factor in addition to age ≥60 years, making the REVEAL-1 cohort a poor-risk population. In a review of patients treated with intensive multi-agent chemotherapy at a single centre from 1980 to 2004, Kantarjian et al (2006) reported a CR rate of 45% in patients ≥65 years of age, with an 8-week all-cause mortality of 29%. Appelbaum et al (2006) compared treatment outcomes by age group in a retrospective analysis of five clinical studies; the CR rate for patients ≥66 years was 38% and the 30-d all-cause mortality was 19%.
A number of other agents are under investigation for the treatment of ageing patients with AML, including the cytotoxic agents decitabine (Blum et al, 2010; Kantarjian et al, 2012a) and sapacitabine (Kantarjian et al, 2012b). Decitabine demonstrated a generally acceptable toxicity profile in a phase 3 trial in patients ≥65 years with intermediate- or poor-risk cytogenetics (30- and 60-d mortality rates of 9% and 20% respectively), but was associated with a lower response rate (18% CR/CRp) and longer time to response (median 4·3 months) than observed in the current study (Kantarjian et al, 2012a); in a phase 2 study, low-dose decitabine over a 10-d period produced promising results (47% CR rate and 15% 8-week mortality), although other than age, patients were not required to have additional risk factors as in REVEAL (Blum et al, 2010). In a phase 2 study comparing three dose regimens of sapacitabine in patients ≥70 years, an overall CR/CRp/CRi rate of 15% was observed, with 30- and 60-d mortality rates of 13% and 26% (Kantarjian et al, 2012b). Each agent had an acceptable safety profile.
Because older patients often have comorbidities, the safety profile of an agent is a key consideration in treatment choice. Vosaroxin could therefore represent an important, efficacious and tolerable therapeutic option for patients ≥60 years of age with poor-risk AML for whom standard intensive chemotherapy regimens may not be suitable. Vosaroxin is a structurally novel agent with a well-validated mechanism of action and toxicities that should be both familiar and acceptable to physicians who treat this large and vulnerable patient population.
On the basis of the safety and efficacy results of this study, the recommended single-agent dose regimen of vosaroxin in this population is 72 mg/m2 on days 1, 4 (Schedule C72). Compared with other doses and schedules tested, this regimen is associated with more rapid haematological recovery; an improved AE profile, including decreased rates of aggregated sepsis and infections; and low 30- and 60-d all-cause mortality. In addition, response rates and OS were favourable with Schedule C72. Further evaluation of vosaroxin at this dose and schedule is warranted in a phase 3 randomized setting.
Acknowledgments
The authors thank Daniel L. Combs for performing PK analyses and David Arnold for statistical programming. The authors also thank Anna Lau, PhD, and Janis M. B. Leonoudakis, PhD, of Powered 4 Significance LLC for providing editorial assistance on the manuscript. Funding for manuscript development was provided by Sunesis Pharmaceuticals.
Author contributions
RKS contributed to study conception and design, data analysis and interpretation and manuscript writing. LDC contributed to study design and collection of data. MBM contributed to the collection and/or assembly of data, data analysis and interpretation, and manuscript writing. LJC contributed to collection and/or assembly of data, data analysis and interpretation. GCM contributed to study conception and design, collection and/or assembly of data, data analysis and interpretation and manuscript writing. JAF and RDL contributed to collection and/or assembly of data, data analysis and interpretation and manuscript writing. FR contributed to study conception and design, and collection of data. MAC, RMS, SRD, FT, WS, JM, PJS, SAS, and GB contributed to the collection and/or assembly of data. All authors critically reviewed the manuscript and approved the manuscript for submission.
Conflict of interest disclosure
RKS has consulted for and participated on a medical advisory committee for Sunesis, and reports grants and personal fees from Sunesis during the conduct of the study. RMS has served on a data and safety monitoring committee for other Sunesis- and Celgene-sponsored studies, on a steering committee for a Celgene-sponsored study, and on an ad hoc advisory board for Seattle Genetics and Celgene. WS was present for a VALOR advisory board in 2013. PJS reports support from Sunesis for clinical trial management during the conduct of the study; consultancy/speaker fees from Ariad, Incyte, and Novartis; and pending patents for anticancer technologies and shareholder interest in JSK Therapeutics and Lone Star Thiotherapies outside the submitted work. SAS reports advisory board/consultancy fees from Sunesis outside the submitted work. GCM reports employment at Sunesis from 2006 to 2011 outside the submitted work, and a use and methods patent application before leaving Sunesis employment. JAF reports employment at Sunesis from 2006 to 2013 and personal fees from Sunesis outside the submitted work. RDL reports personal fees from Sunesis during the conduct of the study and outside the submitted work. FR reports grants and personal fees from, and service as a member of advisory boards for Sunesis during the conduct of the study. LDC, MBM, MAC, SRD, FT, JM, LJC, GB have nothing to disclose.
Supporting Information
Data S1. Inclusion and exclusion criteria; Pharmacokinetic (PK) studies; Sample size considerations.
References
- Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE. Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, Liu S, Havelange V, Becker H, Schaaf L, Mickle J, Devine H, Kefauver C, Devine SM, Chan KK, Heerema NA, Bloomfield CD, Grever MR, Byrd JC, Villalona-Calero M, Croce CM. Marcucci G. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proceedings of the National Academy of Sciences USA. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett A, Wetzler M. Lowenberg B. Therapeutic advances in acute myeloid leukemia. Journal of Clinical Oncology. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Evanchik MJ, Allen D, Yoburn JC, Silverman JA. Hoch U. Metabolism of (+)-1,4-dihydro-7-(trans-3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiaz olyl)-1,8-naphthyridine-3-carboxylic acid (voreloxin; formerly SNS-595), a novel replication-dependent DNA-damaging agent. Drug Metabolism and Disposition. 2009;37:594–601. doi: 10.1124/dmd.108.023432. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Green SJ. Dahlberg S. Planned versus attained design in phase II clinical trials. Statistics in Medicine. 1992;11:853–862. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- Hawtin RE, Stockett DE, Byl JA, McDowell RS, Nguyen T, Arkin MR, Conroy A, Yang W, Osheroff N. Fox JA. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS One. 2010a;5:e10186. doi: 10.1371/journal.pone.0010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtin RE, Stockett DE, Wong OK, Lundin C, Helleday T. Fox JA. Homologous recombination repair is essential for repair of vosaroxin-induced DNA double-strand breaks. Oncotarget. 2010b;1:606–619. doi: 10.18632/oncotarget.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch U, Lynch J, Sato Y, Kashimoto S, Kajikawa F, Furutani Y. Silverman JA. Voreloxin, formerly SNS-595, has potent activity against a broad panel of cancer cell lines and in vivo tumor models. Cancer Chemotherapy and Pharmacology. 2009;64:53–65. doi: 10.1007/s00280-008-0850-3. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, O'Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J. Estey E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Ravandi F, O'Brien S, Cortes J, Faderl S, Garcia-Manero G, Jabbour E, Wierda W, Kadia T, Pierce S, Shan J, Keating M. Freireich EJ. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysak D, Minden M. Arthur C. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. Journal of Clinical Oncology. 2012a;30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Faderl S, Garcia-Manero G, Luger S, Venugopal P, Maness L, Wetzler M, Coutre S, Stock W, Claxton D, Goldberg SL, Arellano M, Strickland SA, Seiter K, Schiller G, Jabbour E, Chiao J. Plunkett W. Oral sapacitabine for the treatment of acute myeloid leukaemia in elderly patients: a randomised phase 2 study. The Lancet Oncology. 2012b;13:1096–1104. doi: 10.1016/S1470-2045(12)70436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet JE, Ravandi F, Ricklis RM, Cripe LD, Kantarjian HM, Giles FJ, List AF, Chen T, Allen RS, Fox JA, Michelson GC. Karp JE. A phase Ib study of vosaroxin, an anticancer quinolone derivative, in patients with relapsed or refractory acute leukemia. Leukemia. 2011;25:1808–1814. doi: 10.1038/leu.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzin J, Lang K, Earle CC, Kerney D. Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Archives of Internal Medicine. 2002;162:1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G. Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Pollyea DA, Kohrt HE. Medeiros BC. Acute myeloid leukaemia in the elderly: a review. British Journal of Haematology. 2011;152:524–542. doi: 10.1111/j.1365-2141.2010.08470.x. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A. Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- Vincent G, Velkoff V. Bureau UC. The Next Four Decades: The Older Population in the United States: 2010 to 2050. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. [Google Scholar]
- Walsby EJ, Coles SJ, Knapper S. Burnett AK. The topoisomerase II inhibitor voreloxin causes cell cycle arrest and apoptosis in myeloid leukemia cells and acts in synergy with cytarabine. Haematologica. 2011;96:393–399. doi: 10.3324/haematol.2010.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Inclusion and exclusion criteria; Pharmacokinetic (PK) studies; Sample size considerations.


