Abstract
Cardiovascular disease is a broad term describing disease of the heart and/or blood vessels. The main blood vessel supplying the body with oxygenated blood is the aorta. The aorta may become affected in diseases such as atherosclerosis and aneurysm. Researchers investigating these diseases would benefit from direct observation of the aorta to characterize disease progression as well as to evaluate efficacy of potential therapeutics. The goal of this protocol is to describe proper isolation and excision of the aorta to aid investigators researching cardiovascular disease. Isolation and excision of the aorta allows investigators to look at gross morphometric changes as wells as allowing them to preserve and stain the tissue to look at histologic changes if desired. The aorta may be used for molecular studies to evaluate protein and gene expression to discover targets of interest and mechanisms of action. This technique is superior to imaging modalities as they have inherent limitations in technology and cost. Additionally, primary isolated cells from a freshly isolated and excised aorta can allowing researchers to perform further in situ and in vitro assays. The isolation and excision of the aorta has the limitation of having to sacrifice the animal however, in this case the benefits outweigh the harm as it is the most versatile technique in the study of aortic disease.
Keywords: Medicine, Issue 93, Cardiovascular, aorta, murine, isolation, surgery, excision, anatomy
Introduction
The aorta plays a pivotal role in cardiovascular function supplying the body with oxygenated blood and similar to others portions of the body, is susceptible to disease. Common aortic diseases include atherosclerosis and aneurysm, which are the results of genetic and environmental factors including diet, smoking and sedentary lifestyle1. Atherosclerosis is the deposition of a calcium- and lipid-based plaque to the aortic wall typically found in patients with chronic hyperlipidemia2. Aneurysms are characterized by degradation of the structural components of the aorta and thinning of the vessel wall, followed by an increased diameter which could ultimately lead to rupture3.
Animal models are important tools used to study the mechanism of disease and efficacy of potential treatments. Common animal models used in cardiovascular research, specifically research investigating vascular and metabolic disorders include genetically modified mice to alter lipid levels such as apolipoprotein E gene knockout and low density lipoprotein receptor gene knockout mice4. The majority of methods to evaluate mechanisms of disease and efficacy of therapy would include the isolation and excision of the aorta.
Once the aorta is localized and isolated, morphometric analysis can be determined, such as presence of aneurysm, typically defined as a greater than 50% increase in diameter5. After necessary in situ analysis is complete, the aorta can be excised for further analysis. An excised aorta can be flash frozen to conduct molecular studies such as protein and/or gene expression assays or fixed using 4% paraformaldehyde and later embedded, sectioned and stained for histological analysis. Histological analysis of the aorta can demonstrate common features of aortic disease such as structural degradation, plaque formation, and infiltration of leukocytes6,7. Additionally, an excised aorta can be used to isolate primary cell lines including endothelial and smooth muscle cells which can subsequently be used for a variety of in vitro studies7.
Currently, there are no other methods to provide this in-depth characterization of aortic disease as well as provide researchers with tools to further study cardiovascular disease. Imaging modalities such as magnetic resonance imaging, computed tomography and ultrasonography are the closest methods to morphologically evaluate the aorta, however this is difficult in small animals and obtaining a device with adequate technology is expensive8. Immortalized cell lines can be purchased to investigate potential mechanisms of disease and efficacy of therapies, however the artificial nature of these cells are limiting in studying the effects on the cell life cycle and apoptosis9.
The overall goal of this manuscript is to demonstrate the sterile isolation and excision of the murine aorta in the investigation of cardiovascular disease.
Protocol
All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee of the University of Cincinnati and in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
1. Preparing the Mouse
Euthanize by exposing the animal to a supratherapeutic dose of anesthetic, isoflurane inhaled to effect. Verify primary euthanasia via toe pinch as a noxious stimulus. Secondary method of euthanasia, diaphragm cutting, will occur in an upcoming step. Alternative methods may be used granted euthanasia is obtained.
Sanitizie the external surface of the mouse by spraying the fur along the abdominal region, where the initial cuts will be made, with 70% ethanol to the point of moisture so that the fur is wet with ethanol and any loose/dry hairs do not enter the region.
Lay the mouse out on a surgical board in the supine position with upper and lower appendages extended outward.
Secure appendages to the board using surgical tape.
2. Isolation of the Heart and Aorta
After proper positioning of the mouse, use forceps to locate and isolate abdominal skin just inferior to the xiphoid process of the sternum.
Create tension by lifting the skin straight up and use scissors to remove the superficial skin, exposing the superior portion of the peritoneum and inferior portion of the thoracic cavity.
Enter the peritoneal cavity by lifting the xiphoid process and making lateral incisions just inferior to the process along the subcostal margins.
Dissect up into the thoracic cavity, going through the diaphragm, being sure not to lacerate the heart or any major blood vessels.
Extend the lateral incisions made in step 2.3 cranially to remove the anterior portion of the ribcage. If necessary, free the heart from the anterior chest wall using blunt dissection.
Clean the chest cavity of extraneous blood and fluid by using sterile gauze to absorb material. Once the area is more visible, remove the lungs to better expose the heart and aorta.
3. Perfusion of Heart and Aorta
NOTE: To obtain blood via cardiac puncture, do so just prior to this step.
Fill a 10 cc syringe with 10 ml of sterile ice cold 1x phosphate buffer solution (PBS), and attach a 25 gauge needle to the syringe.
Gently insert the needle into the left ventricle of the heart.
Cut the right atrium to alleviate pressure buildup from perfusion. Perfuse the contents of the syringe into the mouse over 2 - 3 min.
Use sterile gauze at the opening in the right atrium to absorb perfusion fluid.
4. Isolation and Excision of the Aorta
Upon completion of perfusion, use sterile gauze to absorb any remaining fluid clouding the field of view within the thoracic cavity.
Expose the gastrointestinal contents by cutting caudally through the abdominal wall, extending the incision to the suprapubic area. Extend the incision further towards the lower limbs bilaterally to create skin flaps which can be pinned down or excised.
Remove the lobes of the liver, pancreas, stomach, spleen, and intestines to better visualize the aorta. Take care when dissecting near the perirenal region, as the aorta is superficial at the branches of the renal arteries. NOTE: Perform this step in a precise and matriculate manner as the gastrointestinal tract is rich with bacteria which increases the propensity for contamination.
Rinse the area with 1x PBS and remove all fluid by absorption with a sterile gauze.
Using sterile microscissors and microforceps, separate the aorta from the spine dorsally and the esophagus ventrally. It is best to use blunt dissection and utilizing a dissection microscope for this. NOTE: In isolating the aorta, start caudally at the iliac bifurcation and move cranially cleaning and separating the aorta from the dorsal surface of the cavity or start cranially at the carotids and brachiocephalic arteries dissecting caudally. Either method is appropriate and is up to the researchers comfort.
Remove the perivascular adipose tissue using fine microscissors being sure not to remove a portion of the aortic wall. This is essential to minimize the potential for fibroblast contamination when culturing aortic smooth muscle cells.
Representative Results
Upon completion of the procedure, there will be an intact aorta originating from the heart, descending into the thoracic and abdominal cavities with the renal arteries still attached (Figure 1A). From here, the aorta can be imaged in situ to quantify morphometric changes which are diagnostic in the study of abdominal aortic aneurysms (Figure 1B and 1C). Subsequently, the aorta can be removed, fixed and stained to look at histological changes. A general and common stain of the aorta is the hematoxylin and eosin stain (Figure 2A and 2B). Additionally, the structural integrity of the aorta can be qualitatively quantified using Verhoff van Geison staining to look at the elastin bands (Figure 2C and 2D). Instead of obtaining a histological analysis, the investigator may flash freeze the aorta for subsequent protein and ribonucleic acid expression. The final option for the investigator is to use the aorta to obtain primary cell isolation. These cells can be grown in culture (Figure 3A) and used for a variety of in vitro studies including viability studies (Figure 3B) and protein localization (Figure 3C).
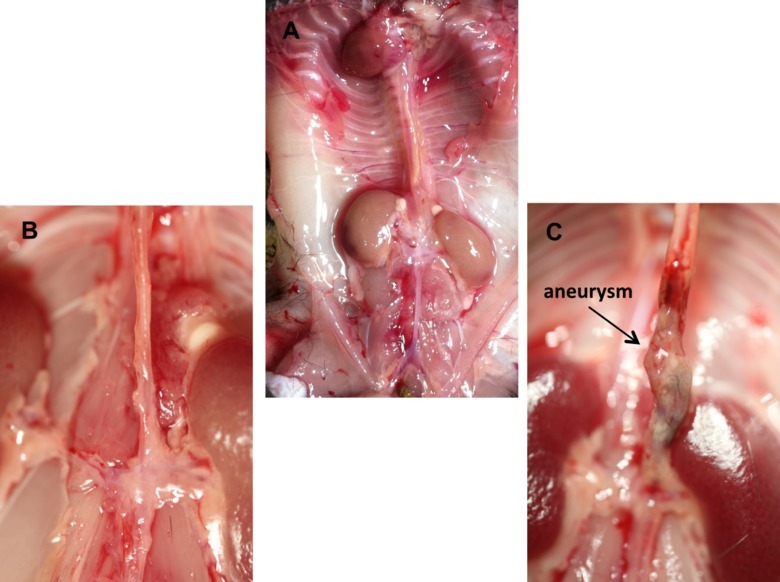 Figure 1. In situ representative images of murine aorta. (A) Depiction of the normal mouse anatomy after the isolation of the aorta including the heart and kidneys. (B) Magnified image of a normal intact murine abdominal aorta. (C) Magnified image of a diseased abdominal aortic with an aneurysm in the suprarenal region. Please click here to view a larger version of this figure.
Figure 1. In situ representative images of murine aorta. (A) Depiction of the normal mouse anatomy after the isolation of the aorta including the heart and kidneys. (B) Magnified image of a normal intact murine abdominal aorta. (C) Magnified image of a diseased abdominal aortic with an aneurysm in the suprarenal region. Please click here to view a larger version of this figure.
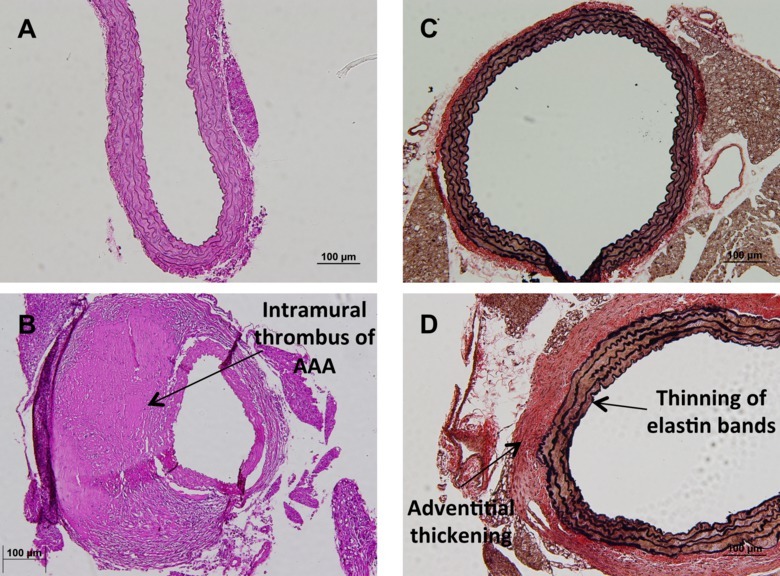 Figure 2. Histological analysis of murine aorta. (A) Representative image of a normal hematoxylin and eosin stain of a healthy suprarenal aorta at 20X magnification. (B) Representative image of an aneurismal suprarenal aorta with intramural thrombus after hematoxylin and eosin stain at 20X magnification. (C) Representative image of a Verhoff van Geison stain, highlighting the elastin bands of a normal suprarenal aorta at 10X magnification. (D) Representative image of a Verhoff van Geison stain highlighting the elastin bands of a suprarenal aorta developing disease noted by the thinning and striating of elastin bands and thickening of the adventitial layer. Please click here to view a larger version of this figure.
Figure 2. Histological analysis of murine aorta. (A) Representative image of a normal hematoxylin and eosin stain of a healthy suprarenal aorta at 20X magnification. (B) Representative image of an aneurismal suprarenal aorta with intramural thrombus after hematoxylin and eosin stain at 20X magnification. (C) Representative image of a Verhoff van Geison stain, highlighting the elastin bands of a normal suprarenal aorta at 10X magnification. (D) Representative image of a Verhoff van Geison stain highlighting the elastin bands of a suprarenal aorta developing disease noted by the thinning and striating of elastin bands and thickening of the adventitial layer. Please click here to view a larger version of this figure.
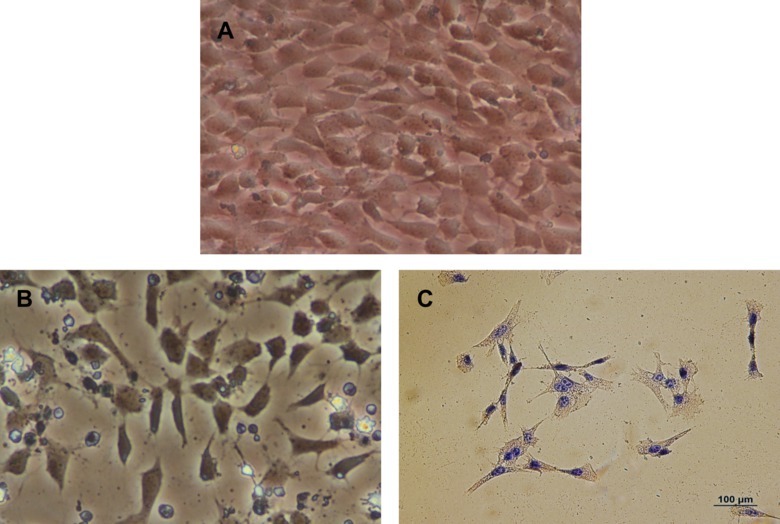 Figure 3. Primary aortic myocytes cultured from aorta. (A) Representative image of primary aortic myocytes in culture at 20X magnification obtained from the abdominal aorta. (B) Representative image of primary aortic myocytes during a Trypan blue exclusion assay after a 500 µM treatment of H2O2 at 20X magnification obtained from the abdominal aorta. (C) Representative image of an immunohistochemistry stain for alpha actin in primary isolate aortic smooth muscle cells from the abdominal aorta. Please click here to view a larger version of this figure.
Figure 3. Primary aortic myocytes cultured from aorta. (A) Representative image of primary aortic myocytes in culture at 20X magnification obtained from the abdominal aorta. (B) Representative image of primary aortic myocytes during a Trypan blue exclusion assay after a 500 µM treatment of H2O2 at 20X magnification obtained from the abdominal aorta. (C) Representative image of an immunohistochemistry stain for alpha actin in primary isolate aortic smooth muscle cells from the abdominal aorta. Please click here to view a larger version of this figure.
Discussion
Aortic disease can lead to significant systemic pathology and possibly death in severe cases. Treatment options are currently limited therefore continued research in the field is necessary10. Murine models are ideal to further study disease progression and potentially therapeutic options4. Therefore, the successful completion of the method described in this manuscript will provide investigators with the tools to measure disease progression and drug efficacy.
This technique can be modified in several ways in order to optimize the results. The use of surgical loupes or a dissecting microscope can aid in the visualization of the murine anatomy and vasculature details and when removing surrounding adipose tissue from the aorta. Additionally, the use of dedicated surgical utensils for specific tasks can increase the longevity of tools; for example, the use of scissors for cutting skin and separate scissors to cut through the ribs would be ideal. The use of a small gauged needle when perfusing the heart is preferred as this will allow the tissue to close around the puncture as opposed to a larger gauge needle where a hole will remain.
After the aorta is successfully removed, it can be utilized for a variety of assays. The aorta can be used for molecular studies such as enzyme activity, protein expression and gene expression profiles. Additionally, the aorta can be fixed for histologic analysis where in situ assays can be utilized as well as characterization of the aortic morphology. Finally, one can isolate smooth muscle and/or endothelial cells from a freshly harvest aorta for in vitro studies with these primary isolated cells.
The major limitation to isolation and excision of the aorta is that the animal must be sacrificed, especially in the cases of longitudinal studies. Currently, there is no single technique comparable to isolation and excision of the murine aorta. Small animal imaging technologies is becoming more advanced and approaching characterization capabilities of direct observation however it continues to have limitations including cost8. Immortalized cells lines can be used for in vitro studies however immortal cells are not as useful as primary isolated cells for in vitro assays especially for viability studies9. Isolation and excision of the murine aorta is currently the most versatile technique that can be used in the study of aortic disease.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors have no acknowledgments.
References
- Go AS, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Harker L. Hyperlipidemia and atherosclerosis. Science. 1976;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Clouse WD, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24(3):429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- Pleumeekers HJ, et al. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol. 1995;142(12):1291–1299. doi: 10.1093/oxfordjournals.aje.a117596. [DOI] [PubMed] [Google Scholar]
- Brophy CM, Reilly JM, Smit GJ, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991;5(3):229–233. doi: 10.1007/BF02329378. [DOI] [PubMed] [Google Scholar]
- Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2001;23(4):185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- Weiss RG. Imaging the murine cardiovascular system with magnetic resonance. Circ Res. 2001;88(6):550–551. doi: 10.1161/01.res.88.6.550. [DOI] [PubMed] [Google Scholar]
- Graham EL, Balla C, Franchino H, Melman Y, del Monte F, Das S. Isolation culture, and functional characterization of adult mouse cardiomyoctyes. J Vis Exp. 2013. p. 50289. [DOI] [PMC free article] [PubMed]
- Wassef M, Upchurch GR, Jr, Kuivaniemi H, Thompson RW, Tilson MD3rd. Challenges and opportunities in abdominal aortic aneurysm research. J Vasc Surg. 2007;45(1):192–198. doi: 10.1016/j.jvs.2006.09.004. [DOI] [PubMed] [Google Scholar]


