Abstract
Although coffee is known to have antioxidant, anti-inflammatory, and antitumor properties, there have been few reports about the effect and mechanism of coffee compounds in colorectal cancer. Heat shock proteins (HSPs) are molecular chaperones that prevent cell death. Their expression is significantly elevated in many tumors and is accompanied by increased cell proliferation, metastasis and poor response to chemotherapy. In this study, we investigated the cytotoxicity of four bioactive compounds in coffee, namely, caffeine, caffeic acid, chlorogenic acid, and kahweol, in HT-29 human colon adenocarcinoma cells. Only kahweol showed significant cytotoxicity. Specifically, kahweol increased the expression of caspase-3, a pro-apoptotic factor, and decreased the expression of anti-apoptotic factors, such as Bcl-2 and phosphorylated Akt. In addition, kahweol significantly attenuated the expression of HSP70. Inhibition of HSP70 activity with triptolide increased kahweol-induced cytotoxicity. In contrast, overexpression of HSP70 significantly reduced kahweol-induced cell death. Taken together, these results demonstrate that kahweol inhibits colorectal tumor cell growth by promoting apoptosis and suppressing HSP70 expression.
Keywords: Kahweol, Apoptosis, Heat shock protein 70, Colorectal cancer
INTRODUCTION
Colorectal cancer is one of the most common human malignancies with a high rate of mortality (Grivicich et al., 2007). Most colorectal cancers are due to lifestyle factors and increasing age, with only a small number of cases due to genetic causes. The majority of patients are diagnosed at an advanced stage so that chemotherapy is required (Grivicich et al., 2007).
Many studies have shown that coffee consumption in humans is associated with a reduced incidence of a wide variety of cancers, including colon cancer (Giovannucci, 1998; Larsson and Wolk, 2007; Cardenas et al., 2011; Yu et al., 2011). Coffee has been reported to exert its preventive effects against oxidative stress and DNA damage (Lee and Jeong, 2007). Animal studies also support the hypothesis that coffee has chemoprotective effects (Wurzner et al., 1977; Stalder et al., 1990; Cavin et al., 2002). The epidemiology of colorectal cancer provides the most supportive evidence of a potential coffee-dependent protection (Cavin et al., 2002).
Coffee beans contain more than a thousand of compounds (Spiller, 1984). One compound in coffee that may be responsible for these benefits is kahweol. It is fat-soluble compounds known as diterpene molecules, which are present in oil derived from Arabica coffee beans (Lee et al., 2012). It has been reported that kahweol possess antioxidant and antitumor activities (Lee and Jeong, 2007; Kim et al., 2009; Oh et al., 2009). Although kahweol has been shown to induce apoptosis in human breast tumor cells (Cardenas et al., 2014), the underlying mechanism in colorectal cancer cells is not known.
Heat shock proteins (HSPs) are molecular chaperones that prevent cell death. They are transiently induced by stress in normal cells and are often constitutively overexpressed in tumors (Kim et al., 2013). HSPs have been classified into the HSP60, HSP70, HSP90, and the small HSP family based on their molecular weight. It has been shown that especially, HSP70 contributes to cell survival and resistance to chemotherapy via multiple anti-apoptotic functions in colorectal cancer cells (Sarto et al., 2000; Garrido et al., 2001; Takayama et al., 2003). It has been also reported that inducible HSP70 interferes the anticancer drug-induced apoptosis (Rashmi et al., 2004). Furthermore, HSP70 RNA interference (RNAi) significantly suppressed HT-29 human colorectal cancer cell growth both in vitro and in vivo (Zhong et al., 2011). Therefore, inhibiting the expression and function of HSP70 might offer a potential anticancer therapeutic strategy in the treatment of cancers.
In this study, we assessed the antitumor effects of four major components of coffee and elucidated the effects of one of them, kahweol, on apoptosis and HSP70 expression in human colorectal cancer cells.
MATERIALS AND METHODS
Cell culture and chemicals
HT-29 human colorectal cancer cells, which were obtained from the Korean Cell Line Bank, were cultured in Dulbecco’s modified Eagle’s medium (Hyclone Laboratories, Inc., Logan, UT, USA) with 10% fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in a 5% CO2 humidified atmosphere. Caffeine, chlorogenic acid, caffeic acid, and triptolide were purchased from Sigma-Aldrich (St. Louis, MO, USA) and kahweol was acquired from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). These compounds were dissolved in dimethylsulfoxide (DMSO) and added to cell cultures at the desired concentrations.
Microscopic analysis
The HT-29 cells were exposed to the indicated concentrations (control, 100 μM, and 200 μM) of kahweol, and morphological changes were monitored for 6 h. The photographs were taken with a phase contrast microscope (Olympus equipped with Canon camera) at 100× magnification.
Transient transfection of heat shock protein 70
HSP70 was transiently transfected into HT-29 cells using LT-1 (Mirus Bio, Madison, WI, USA) transfection reagent according to the manufacturer’s protocol. Two days after transfection, cells were treated with kahweol or vehicle control, respectively, for 24 h.
Cell viability assay
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. First, HT-29 cells (5×105) were incubated in 12-well plates and treated with various concentrations of kahweol for 20 h at 37°C. Subsequently, MTT (5 mg/mL) was added to each well and incubated for 1 h. Finally, the resulting formazan crystals were dissolved in DMSO and the optical density of each well was measured at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The results were expressed as a percentage of surviving cells over control cells.
Lactate Dehydrogenase (LDH) activity assay
Release of lactate dehydrogenase (LDH) is an in vitro marker of cellular toxicity. HT-29 cells were treated with various concentrations of kahweol for 20 h, and then the supernatant was used to assay LDH activity, according to the manufacturer’s instruction (Roche Diagnostic Systems, Montclair, NJ, USA).
Western blot analysis
HT-29 cells were cultured with the aforementioned compounds for 16 h, and then cell lysates were prepared with lysis buffer. Equal amounts of protein were separated on 10% sodium dodecyl sulfate-polyacrylamide gels. Then, proteins were transferred to Hypond polyvinylidene fluoride membranes (Amersham Biosciences, Piscataway, NJ, USA) and blocked in 5% skim milk in Tris-buffered saline with 0.1% Tween-20 for 1 h at room temperature. Antibodies against cleaved caspase-3 (1:500; Cell Signaling Technology, Danvers, MA, USA), cleaved poly (ADP-ribose) polymerase (PARP) (1:1,000), Bcl-2 (1:1,000), phosphorylated Akt (1:1,000), total Akt (1:1,000), HSP70 (1:1,000), and β-actin (1:2,500; Sigma-Aldrich, Saint Louis, MO, USA) were diluted in 5% skim milk.
Statistical analysis
All data are expressed as means ± standard deviation (SD). Statistical significance was analyzed by two-tailed Student’s t-test (SPSS version 12.0, SPSS, Inc., Chicago, IL, USA). Data with values of p<0.05 were considered as statistically significant. Single (*), double (**) and triple (***) marks represent statistical significance in p<0.05, p<0.01 and p<0.001, respectively.
RESULTS
Kahweol suppresses cell viability in HT-29 cells
Given the previous reports that coffee beans possess anti-tumor effects (Giovannucci, 1998; Larsson and Wolk, 2007), cytotoxic property of active compounds of coffee beans such as caffeic acid, caffeine, chlorogenic acid, and kahweol was examined in HT-29 human colorectal cancer cells (Fig. 1). Noticeable changes in the viability of HT-29 cells were not observed up to 200 micromolar concentrations of caffeic acid, caffeine, and chlorogenic acid. In contrast, kahweol exhibited a significant cytotoxic effect in HT-29 cells in a concentration-dependent manner.
Fig. 1.
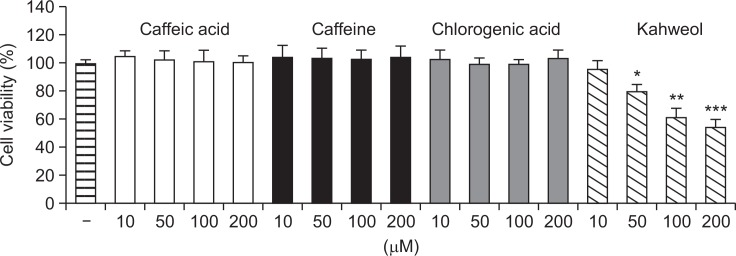
Cytotoxic profiles of bioactive compounds from coffee beans in HT-29 human colorectal cells. HT-29 cells were incubated with caffeic acid, caffeine, chlorogenic acid, or kahweol for 24 h and cell viability was examined using MTT assay. Results are expressed as the mean ± SD of three or more independent experiments. *p<0.05, **p<0.01, and ***p<0.001 vs. control.
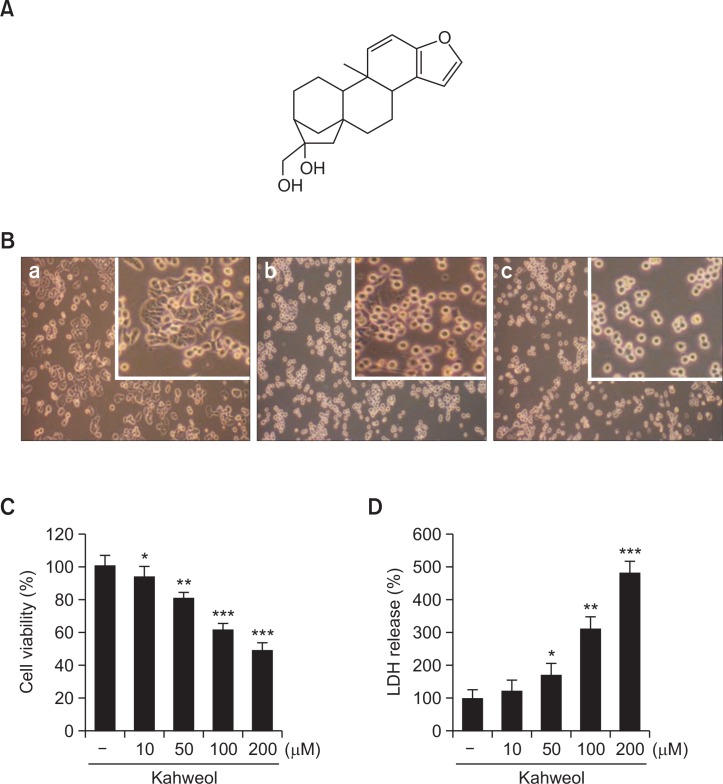
Kahweol induces morphological changes and exhibits cytotoxicity in HT-29 cells
In accordance with the cytotoxicity result in Fig. 1, kahweol (Fig. 2A) induced cell rounding and detachment in a concentration-dependent manner (Fig. 2B). No morphological changes were observed in cells treated with vehicle control (Fig. 2Ba). In contrast, cell rounding and detachment were observed in kahweol-treated cells in a concentration-dependent manner (100 μM (Fig. 2Bb) and 200 μM (Fig. 2Bc). To quantitatively measure the amount of kahweol-induced cytotoxicity in HT-29 cells, MTT and LDH release assays were carried out. Kahweol significantly suppressed the viability of HT-29 cells and increased the LDH release in concentration-dependent manners (Fig. 2C, D).
Fig. 2.
Chemical structure of kahweol (A) and effects of kahweol on the morphology (B) and cytotoxicity (C, D) in HT-29 human colorectal cancer cells. B. Cells were treated with kahweol at the indicated concentrations (control (a), 100 μM (b), and 200 μM (c)) for 6 h, and then morphological changes were assessed using an inverted phase contrast microscope. Images are shown at 100× magnification. C, D. Cells were treated with kahweol for 24 h, and then cell viability was measured by MTT (C) and lactate dehydrogenase release (D) assays. Results are expressed as the mean ± SD of three or more independent experiments. *p<0.05, **p<0.01, and ***p<0.001 vs. control.
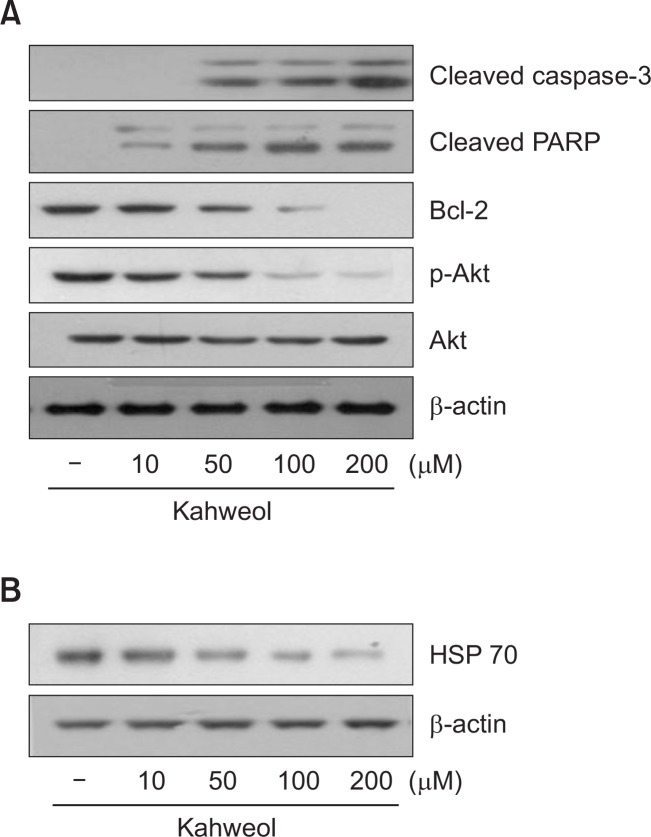
Kahweol activates caspase-3 and suppresses anti-apoptotic factors in HT-29 cells
To understand how kahweol suppresses the cell viability of HT-29 cells, we examined its effects on the expression levels of several key proteins involved in apoptosis. Kahweol increased the expression of activated caspase-3, a pro-apoptotic enzyme, and cleaved PARP in a concentration-dependent manner (Fig. 3A). In contrast, kahweol significantly decreased the expression of two anti-apoptotic proteins, Bcl-2 and phosphorylated Akt, in a concentration-dependent manner (Fig. 3A).
Fig. 3.
Effect of kahweol on the expression of apoptosis-related proteins (A) and HSP70 (B) in HT-29 cells. Cells were treated with the indicated concentrations of kahweol for 16 h, and then the expression levels of cleaved caspase-3, poly (ADP-ribose) polymerase (PARP), Bcl-2, phosphorylated Akt (p-Akt), total Akt, and HSP70 were determined by Western blot analysis. β-actin was used as a loading control.
Kahweol attenuates heat shock protein 70 expression in HT-29 cells
Since HSPs are often overexpressed in tumors and promote their growth (Grivicich et al., 2007; Kim et al., 2013), we examined the effect of kahweol on the expression level of HSP70 in HT-29 cells. Kahweol significantly suppressed HSP70 expression in a concentration-dependent manner (Fig. 3B).
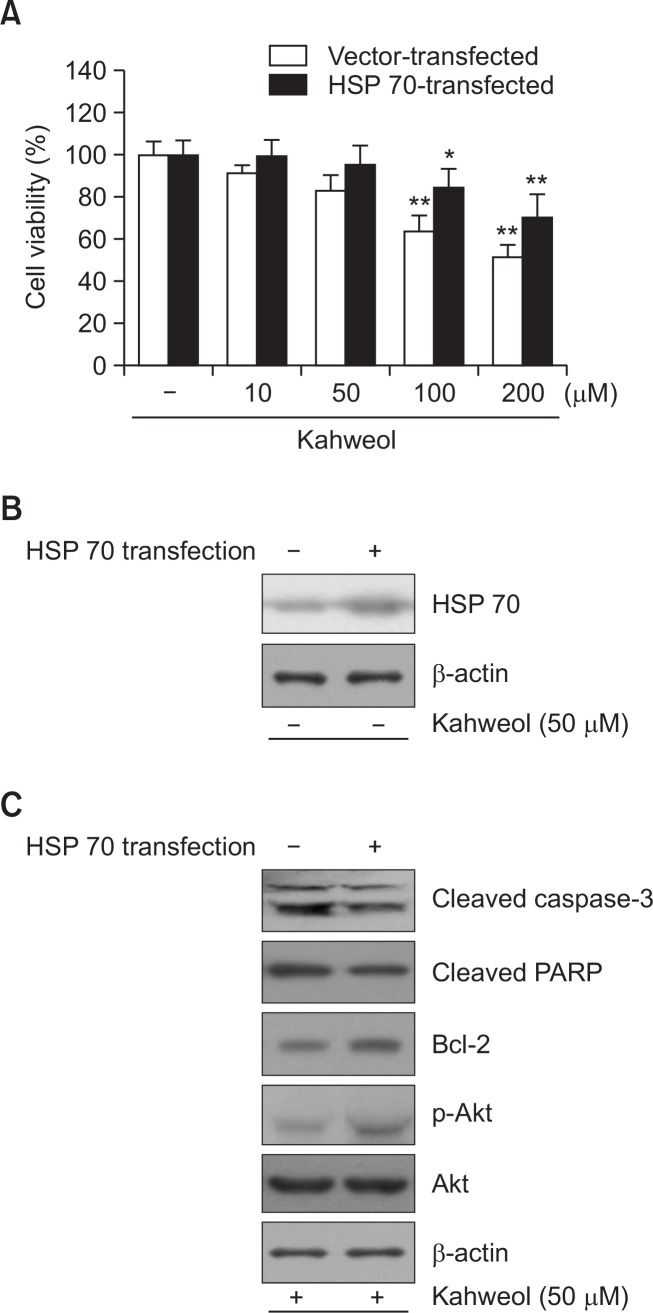
Heat shock protein 70 activity influences kahweol-induced cytotoxicity in HT-29 cells
To determine the importance of HSP70 in kahweol-induced cytotoxicity, we measured the viability of HT-29 cells with either downregulated or upregulated HSP70 activity in HT-29 cells treated with kahweol. Pretreatment with triptolide, a HSP70 inhibitor, increased kahweol-induced cytotoxicity (Fig. 4), whereas transient overexpression of HSP70 significantly reduced cytotoxicity (Fig. 5A). Increased expression of HSP70 was observed with transient transfection of HSP70 construct (Fig. 5B). Expression of HSP70 significantly decreased the expression of activated caspase-3 and cleaved PARP and increased the expression of Bcl-2 and phosphorylated Akt (Fig. 5C). Collectively, these results suggest that HSP70 plays an important role in kahweol-induced cytotoxicity.
Fig. 4.
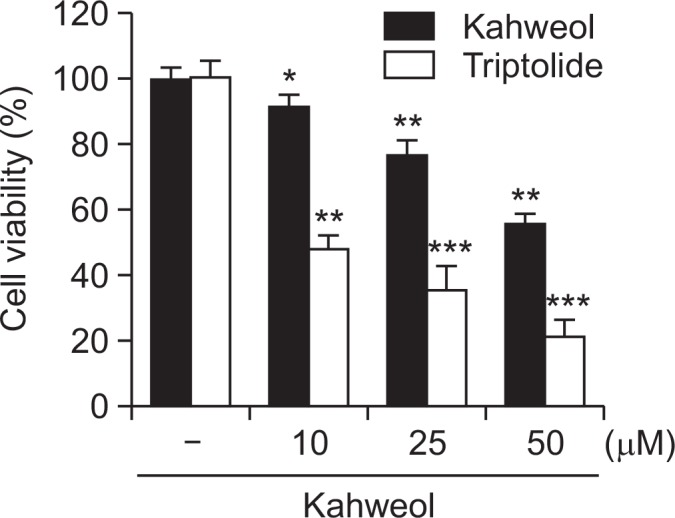
Effects of triptolide, a HSP70 inhibitor, on cell viability in kahweol-treated HT-29 cells. Cells were pretreated with triptolide (10 μM), and then incubated with the indicated concentrations of kahweol for 48 h, and then cell viability was measured by MTT. Results are expressed as the mean ± SD of three or more independent experiments. *p<0.05, **p<0.01, and ***p<0.001 vs. control.
Fig. 5.
Effect of HSP70 overexpression on kahweol-induced apoptitic cytotoxicity in HT-29 cells. A. Cells were transiently transfected with HSP70, and then incubated with the indicated concentrations of kahweol for 24 h. Cell viability was measured by MTT. B. The expression level of HSP70 was determined by Western blot analysis. Compared with cells transfected with vehicle (control), HSP70 expression was significantly elevated in HSP70-transfected cells. C. HSP70 overexpression significantly reduced the expression of caspase-3 and PARP and increased the expression of Bcl-2 and p-Akt. β-actin was used as a loading control. Results are expressed as the mean ± SD of three or more independent experiments. *p<0.05, and **p<0.01 vs. control.
DISCUSSION
Previous studies revealed that coffee consumption had the chemopreventive effect in colorectal cancer, and several bioactive compounds showed the pharmacological effectiveness (Vitaglione et al., 2012; Isshiki et al., 2013). The present study demonstrated that kahweol, the coffee deterpene, significantly suppressed the viability of HT-29 human colorectal cancer cells. Kahweol inhibits HT-29 cell growth via caspase-mediated apoptosis. Kahweol also significantly attenuated the expression of HSP70. Inhibition of HSP70 function with triptolide potentiated kahweol-induced cytotoxicity. And, over-expression of HSP70 lessened the kahweol-induced cytotoxicity in HT-29 cells, suggesting that HSP70 plays a significant role in kahweol-induced cytotoxicity in HT-29 cells.
Among several coffee compounds, kahweol has been reported to possess antitumor and chemoprotective properties in many studies (Cavin et al., 2002; Um et al., 2010; Cardenas et al., 2014). Although kahweol induces cell death in various human tumor cells such as lung, breast, and leukemia, its underlying mechanism by which kahweol exerts its pro-apoptotic effects has not been clearly demonstrated in human colorectal cancer cells. In the present study, kahweol showed cytotoxicity in HT-29 colon cancer cells and exhibited increased activation of caspase-3 and concurrent suppression of anti-apoptotic components such as Bcl-2 and phosphorylated Akt in HT-29 cells. Our results are consistent with previous reports that kahweol activates pro-apoptotic caspases, such as caspases 3/7 and 9, in human breast tumor cells (Cardenas et al., 2014) and inhibits anti-apoptotic proteins, such as Bcl-2 and phosphorylated Akt, in human leukemia cells (Oh et al., 2009).
It has been reported that HSPs are often overexpressed in tumors and promote their growth (Grivicich et al., 2007; Kim et al., 2013; Singh and Suri, 2014). Especially, HSP70 is often constitutively overexpressed in human colorectal cancer cells (Grivicich et al., 2007; Kim et al., 2013), and high expression of HSP70 is associated with resistance to chemotherapy (Sarto et al., 2000; Garrido et al., 2001; Takayama et al., 2003). Furthermore, knockdown of HSP70 exhibited significant reduction in cell growth (Singh and Suri, 2014). Both in vivo and in vitro animal studies showed that inhibition of HSP70 has antitumor effect against colon cancer (Gurbuxani et al., 2001; Schmitt et al., 2006). The present data demonstrated that treatment of kahweol significantly suppressed the expression of HSP70 in a concentration-dependent manner in HT-29 cells. The finding that triptolide, a HSP70 inhibitor, increases kahweol-induced cytotoxicity is in agreement with its ability to inhibit the growth and metastatic potential of various cancers, including colon cancer (Yang et al., 2003; Johnson et al., 2011). Triptolide is known to inhibit the growth and metastasis of various cancer cells, including colon cancer in in vivo studies and HSP70 inhibition is considered to play an important role in antitumor effect of triptolide (Yang et al., 2003; Johnson et al., 2011). Furthermore, our observation that HSP70 overexpression reduces the expression of pro-apoptotic factors and increases the expression of anti-apoptotic factors suggests that HSP70 suppresses kahweol-induced cell death in HT-29 cells. This is consistent with previous reports that inhibition of HSP70 has antitumor effects against colon cancer (Gurbuxani et al., 2001; Schmitt et al., 2006). Overexpression of HSP70 also exhibited increased phosphorylation of Akt. Previously, it has been reported that HSP70 plays an essential role in the activation of phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway in endothelial cells (Shiota et al., 2010). Further studies are necessary to provide an exact mechanism by which HSP70 regulates Akt phosphorylation in HT-29 cells. Another limitation of the present study is that cytotoxic effect of kahweol might be limited to in HT-29 colon cancer cells. The examination of antitumor activity of kahweol in multiple colon cancer cell models including HCT116 and SW480 cells would provide extensive information of kahweol in colon cancers. Therefore, it is necessary in the future. The present data strongly suggest that HSP70 plays an important role in kahweol-induced cytotoxicity of HT-29 cells. Since HSP70 interferes with chemotherapy-induced apoptosis (Rashmi et al., 2004), a combination of kahweol and a HSP70 inhibitor may be an effective therapeutic agent for colorectal cancer. However, due to the in vitro nature of this study, further research in animal or preclinical studies is needed to confirm our findings, elucidate additional mechanisms of kahweol activity, and investigate its clinical potential.
In conclusion, we have shown that kahweol significantly inhibits colorectal tumor cell growth by promoting apoptosis. Kahweol exerts its pro-apoptotic properties presumably by suppressing HSP70 expression. The present study strongly suggests that kahweol might be a potential antitumor agent as a HSP70 inhibitor in colorectal cancers. However, the exact mechanism by which kahweol suppresses HSP70 expression may need further studies.
Acknowledgments
This study was supported by a 2012 research grant from Kangwon National University.
REFERENCES
- Cardenas C, Quesada AR, Medina MA. Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PLoS One. 2011;6:e23407. doi: 10.1371/journal.pone.0023407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Quesada AR, Medina MA. Insights on the antitumor effects of kahweol on human breast cancer: decreased survival and increased production of reactive oxygen species and cytotoxicity. Biochem Biophys Res Commun. 2014;447:452–458. doi: 10.1016/j.bbrc.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol. 2002;40:1155–1163. doi: 10.1016/S0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Meta-analysis of coffee consumption and risk of colorectal cancer. Am J Epidemiol. 1998;147:1043–1052. doi: 10.1093/oxfordjournals.aje.a009398. [DOI] [PubMed] [Google Scholar]
- Grivicich I, Regner A, Zanoni C, Correa LP, Jotz GP, Henriques JA, Schwartsmann G, da Rocha AB. Hsp70 response to 5-fluorouracil treatment in human colon cancer cell lines. Int J Colorectal Dis. 2007;22:1201–1208. doi: 10.1007/s00384-007-0307-x. [DOI] [PubMed] [Google Scholar]
- Gurbuxani S, Bruey JM, Fromentin A, Larmonier N, Parcellier A, Jäättelä M, Martin F, Solary E, Garrido C. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene. 2001;20:7478–7485. doi: 10.1038/sj.onc.1204948. [DOI] [PubMed] [Google Scholar]
- Isshiki M, Ohta H, Tamura H. Coffee reduces SULT1E1 expression in human colon carcinoma Caco-2 cells. Biol Pharm Bull. 2013;36:299–304. doi: 10.1248/bpb.b12-00902. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wang X, Evers BM. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J Surg Res. 2011;168:197–205. doi: 10.1016/j.jss.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Hwang YP, Jeong HG. Kahweol blocks STAT3 phosphorylation and induces apoptosis in human lung adenocarcinoma A549 cells. Toxicol Lett. 2009;187:28–34. doi: 10.1016/j.toxlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Kim JA, Kim Y, Kwon BM, Han DC. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J Biol Chem. 2013;288:28713–28726. doi: 10.1074/jbc.M113.488346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Lee KA, Chae JI, Shim JH. Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma. J Biomed Sci. 2012;19:60. doi: 10.1186/1423-0127-19-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Jeong HG. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol Lett. 2007;173:80–87. doi: 10.1016/j.toxlet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Oh JH, Lee JT, Yang ES, Chang JS, Lee DS, Kim SH, Choi YH, Park JW, Kwon TK. The coffee diterpene kahweol induces apoptosis in human leukemia U937 cells through down-regulation of Akt phosphorylation and activation of JNK. Apoptosis. 2009;14:1378–1386. doi: 10.1007/s10495-009-0407-x. [DOI] [PubMed] [Google Scholar]
- Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis. 2004;25:179–187. doi: 10.1093/carcin/bgh001. [DOI] [PubMed] [Google Scholar]
- Sarto C, Binz PA, Mocarelli P. Heat shock proteins in human cancer. Electrophoresis. 2000;21:1218–1226. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1218::AID-ELPS1218>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Maingret L, Puig PE, Rerole AL, Ghiringhelli F, Hammann A, Solary E, Kroemer G, Garrido C. Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res. 2006;66:4191–4197. doi: 10.1158/0008-5472.CAN-05-3778. [DOI] [PubMed] [Google Scholar]
- Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funase Y, Miura K, Iwao H. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol. 2010;30:491–497. doi: 10.1161/ATVBAHA.109.193631. [DOI] [PubMed] [Google Scholar]
- Singh S, Suri A. Targeting the testis-specific heat-shock protein 70-2 (HSP70-2) reduces cellular growth, migration, and invasion in renal cell carcinoma cells. Tumour Biol. 2014;35:12695–12706. doi: 10.1007/s13277-014-2594-5. [DOI] [PubMed] [Google Scholar]
- Spiller MA. The chemical components of coffee. Prog Clin Biol Res. 1984;158:91–147. [PubMed] [Google Scholar]
- Stalder R, Bexter A, Wurzner HP, Luginbuhl H. A carcinogenicity study of instant coffee in Swiss mice. Food Chem Toxicol. 1990;28:829–837. doi: 10.1016/0278-6915(90)90056-S. [DOI] [PubMed] [Google Scholar]
- Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- Um HJ, Oh JH, Kim YN, Choi YH, Kim SH, Park JW, Kwon TK. The coffee diterpene kahweol sensitizes TRAIL-induced apoptosis in renal carcinoma Caki cells through down-regulation of Bcl-2 and c-FLIP. Chem Biol Interact. 2010;186:36–42. doi: 10.1016/j.cbi.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Vitaglione P, Fogliano V, Pellegrini N. Coffee, colon function and colorectal cancer. Food Funct. 2012;3:916–922. doi: 10.1039/c2fo30037k. [DOI] [PubMed] [Google Scholar]
- Wurzner HP, Lindstrom E, Vuataz L, Luginbuhl H. A 2-year feeding study of instant coffees in rats. II. Incidence and types of neoplasms. Food Cosmet Toxicol. 1977;15:289–296. doi: 10.1016/S0015-6264(77)80199-5. [DOI] [PubMed] [Google Scholar]
- Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- Yu X, Bao Z, Zou J, Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96. doi: 10.1186/1471-2407-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong MA, Zhang H, Qi XY, Lu AG, You TG, Gao W, Guo XL, Zhou ZQ, Yang Y, Wang CJ. ShRNA-mediated gene silencing of heat shock protein 70 inhibits human colon cancer growth. Mol Med Rep. 2011;4:805–810. doi: 10.3892/mmr.2011.528. [DOI] [PubMed] [Google Scholar]