Abstract
Conjugated linoleic acids (CLA) are a family of isomers of linoleic acid. CLA increases growth arrest and apoptosis of human colorectal cancer cells through an isomer-specific manner. ATF3 belongs to the ATF/CREB family of transcription factors and is associated with apoptosis in colorectal cancer. The present study was performed to investigate the molecular mechanism by which t10, c12-CLA stimulates ATF3 expression and apoptosis in human colorectal cancer cells. t10, c12-CLA increased an apoptosis in human colorectal cancer cells in dose dependent manner. t10, c12-CLA induced ATF3 mRNA and luciferase activity of ATF3 promoter in a dose-dependent manner. The responsible region for ATF3 transcriptional activation by t10, c12-CLA is located between −147 and −1850 of ATF3 promoter. mRNA stability of ATF3 was not affected by t10, c12-CLA treatment. t10, c12-CLA increases GSK3β expression and suppresses IGF-1-stimulated phosphorylation of Akt. The knockdown of ATF3 suppressed expression of GSK3β and NAG-1 and PARP cleavage. The results suggest that t10, c12-CLA induces apoptosis through ATF3-mediated pathway in human colorectal cancer cells.
Keywords: Conjugated linoleic acid, Activating transcription factor 3, Colon cancer
INTRODUCTION
Colorectal cancer is the third leading cause of cancer-related death in the United States (Jemal et al., 2011). There are many different and converging pathways for apoptosis or survival of cancer cells (Negrini et al., 2010). The PI3K/Akt pathway, a key regulator of cell growth and survival, is commonly dysregulated in human colon cancers (Lim and Counter, 2005). In particular, Akt has been implicated as an important prognostic factor in human cancer development and progression (West et al., 2003; West et al., 2004).
Aberrant activation of Akt leads to increase the inactive GSK3β, which is downstream target kinase of Akt in colorectal cancer (Cross et al., 1995; Agarwal et al., 2005). GSK3β is a multifunctional serine (Ser)/threonine (Thr) kinase and play an important role in a number of biological processes as mechanistic link between the canonical Wnt signaling and survival of cancer cells (Jope and Bijur, 2002; Doble and Woodgett, 2003; Forde and Dale, 2007). Therefore, Akt/GSK3β pathway has emerged as an important and attractive therapeutic target for colon cancer therapy.
Recent studies have provided strong evidences that many dietary compounds including curcumin (Kumar et al., 2003), ellagic acid (Umesalma and Sudhandiran, 2011), resveratrol (Aziz et al., 2006; Shukla and Singh, 2011), and conjugated linoleic acid (CLA) (Lee et al., 2006) (Cho et al., 2009) targeted Akt/GSK3β pathway in different types of cancer. CLAs are positional and geometric isomers of conjugated dienoic derivatives of linoleic acid, an n-6 polyunsaturated fatty acid. The naturally occurring dietary source of CLA is in the lipid fraction of meat of rumen origin, milk, and dairy products (Roche et al., 2002) because it is biosynthesized in the process of microbial fermentation in the rumen of ruminant animals (Chin et al., 1994). The most abundant isoform of CLA in foods is cis-9, trans-11 (c9, t11) CLA, while synthetically prepared CLA contained the both c9, t11-CLA and trans-10, cis-12 (t10, c12) CLA in similar amounts. This preparation of CLA has been often used to determine its bioactivities previously (Dilzer and Park, 2012). In in vivo studies, the CLA has been shown to alter the carcinogen-induced tumorigenesis in rodent models (Ha et al., 1990; Ip et al., 1994; Belury, 2002; Lee et al., 2005; Dilzer and Park, 2012).
In terms of anticancer mechanisms of CLA, it has been shown that CLA induce apoptosis and suppress cell cycle progression due to suppress PI3K/Akt and ERK signaling cascade in cancer cell lines (Kim et al., 2003). Previously, our laboratory also demonstrated that t10, c12-CLA modulated colorectal cancer cells survival and proliferation (Lee et al., 2006). The proposed mechanisms include inhibition of Akt signaling and subsequent activation of proapoptotic proteins, non-steroidal anti-inflammatory drugs (NSAIDs)-activated gene-1 (NAG-1) (Lee et al., 2006).
NAG-1 is a member of the TGF-β superfamily (Baek et al., 2001) and NAG-1 expression leads to an inhibition of primary tumor growth in carcinogen-driven colorectal cancer (Baek et al., 2006) and lung cancer in vivo (Cekanova et al., 2009). ATF3 is a member of the activating transcription factor/ cAMP-responsive element binding protein (ATF/CREB) family of transcription factors (Hai and Curran, 1991). ATF3 gene expression is elevated by a variety of stress signals, including carcinogens as well as chemotherapy (Chen et al., 1996; Ameri et al., 2007). These data suggest that ATF3 could be regarded as selective and a promising molecular target for chemoprevention and chemotherapy. However, the molecular mechanisms that facilitate these effects are still unclear. Here, we report that the presence of t10, c12-CLA results in an increase of ATF3 expression through transcriptional upregulation in the promoter of ATF3 gene and subsequently activates GSK3β and NAG-1 in human colon cancer cell lines.
MATERIALS AND METHODS
Materials and DNA
Human colorectal adenocarcinoma cells, HT-29, Lovo, and HCT-116 were purchased from American Type Culture Collection (Manassas, VA, USA). Antibodies for ATF3 were purchased from Santa Cruz (Santa Cruz, CA, USA). Antibodies for GSK3β, PARP, actin, phospho-AKT (Ser473) and total AKT were purchased from Cell Signaling (Beverly, MA, USA). The trans-10, cis-12 CLA (t10, c12-CLA) was purchased from Natural Lipids (Hovdebygda, Norway). The t10, c12-CLA preparation was 94 % pure, with 2% cis-9, trans-11 isomer (c9, t11-CLA) and 3% other conjugated linoleic acid isomers. IGF-1 was purchased from BD Biosciences (Bedford, MA, USA). Human ATF3 promoter constructs were previously reported (Lee et al., 2010a). All chemicals were purchased from Fisher Scientific, unless otherwise specified.
Cell culture and treatment
HT-29, Lovo, and HCT-116 cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/ streptomycin antibiotics mixture at 37°C in 5% CO2 Incubator. The cells were plated in 6 well culture dishes (for Western blot and RT-PCR) or 12 well culture dish (for luciferase assay) and grown until cells were 80–90% confluent. Then, the cells were incubated with t10, c12-CLA in serum-free media at indicated doses and times in Figure legends. t10, c12-CLA was prepared and added to serum-free media as described previously (Lee et al., 2006). Bovine serum albumin (BSA) without CLA was used as vehicle.
Transient transfection and assay of luciferase activity
The cells were plated in 12-well plates at the concentration of 2×105 cells/well and ATF3 promoter construct were transfected using Lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. DNA mixtures containing 0.5 μg of ATF3 promoter and 0.05 μg of pRL-null vector were transfected in serum-free media for 5 h and the cells were incubated with serum-containing media overnight. Then, the transfected cells were exposed to different doses of t10, c12-CLA for 24 h. The media was removed and cell lysates were obtained using 1X luciferase lysis buffer, and luciferase activity was measured using a dual luciferase assay kit (Promega, Madison, WI, USA).
RNA interference
HCT-116 cells were transfected with control or ATF3 small interference RNA (siRNA; Santa Cruz; Cat# sc-29757) at a concentration of 100 nM using TransIT-TKO transfection reagent (Mirus, Madison, WI, USA), as described previously (Yamaguchi et al., 2004). After 24 h transfection, the cells were treated with vehicle or t10, c12-CLA (50 μM) for 24 h for Western blot.
Measurement of apoptosis
Apoptosis was assayed using Cell Death Detection ELISAPLUS Kit (Roche Diagnostics, Indianapolis, IN, USA) as we previously described (Jeong et al., 2013). This kit detects qualitatively and quantitatively the amount of cleaved DNA/ histone complexes (nucleosomes). Briefly, the cells were treated with t10, c12-CLA for 24 h and cytosolic extracts and immunoreagent were mixed and incubated for 2 h at room temperature. After washing with the incubation buffer, 100 μl of ABTS solution was added and incubated for 20 min. The absorbance was recorded at 405 nm and 490 nm in an enzyme-linked immunosorbent assay plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
Western analysis
Cell lysates were extracted by incubating the cells with radioimmunoprecipitation assay (RIPA) buffer for 30 min on ice. To prevent protein degradation, the RIPA buffer was supplemented with protease inhibitors (1 mM PMSF, 5 μg/ml aprotinin and 5 μg/ml leupeptin) and phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF) and centrifuged at 12,000 rpm for 5 min at 4°C. The protein concentration was determined by the BCA protein assay (Pierce, Rockford, IL, USA) using BSA as the standard. The proteins (30 μg) were separated on SDS-PAGE and transferred to nitrocellulose membranes (Osmonics, Minnetonka, MN, USA). The membranes were incubated with a specific primary antiserum in Tris buffered saline (TBS) containing 0.05% Tween-20 (TBS-T) and 5% non-fat dry milk at 4°C overnight. After three washes with TBS-T for 30 min, the blots were incubated with horse radish peroxidase (HRP)-conjugated IgG for 1 h at room temperature, visualized using ECL (Amersham Biosciences, Piscataway, NJ, USA) and quantified by Gel Doc 2000 system and Image Lab Software (Bio-Rad Laboratories, Hercules, CA, USA).
Isolation and analysis of RNA
Total RNA was isolated from cells using Trizol Reagent (Invitrogen), according to the manufacturer’s instructions. RNA samples with an OD260/OD280 ratio greater than 1.8 were used for semi-quantitative RT-PCR. One μg of total RNA was used to produce cDNA using the Verso cDNA synthesis Kit (Thermo Scientific, Pittsburgh, PA, USA). PCR was performed using one μL of cDNA mixture and the PCR Master Mix solution (Promega, Madison, WI, USA) according to the manufacturer’s instruction. The sequence of the oligonucleotide primers were as follows; ATF3 sense (5′-gtttgaggattttgctaacctgac-3′) and antisense (5′-agctgcaatcttatttctttctcgt-3′); GAPDH sense (5′-acccagaagactgtggatgg-3′ and antisense (5′-ttctagacggcaggtcaggt-3′). The PCR products were run on 1.5% agarose gels containing ethidium bromide dye and photographed. A densitometric analysis was performed using Image J software (National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis of ATF3 promoter assay was performed with Student’s unpaired t-test, with statistical significance set at *p<0.05.
RESULTS
The t10, c12-CLA stimulates expression of ATF3 and apoptosis
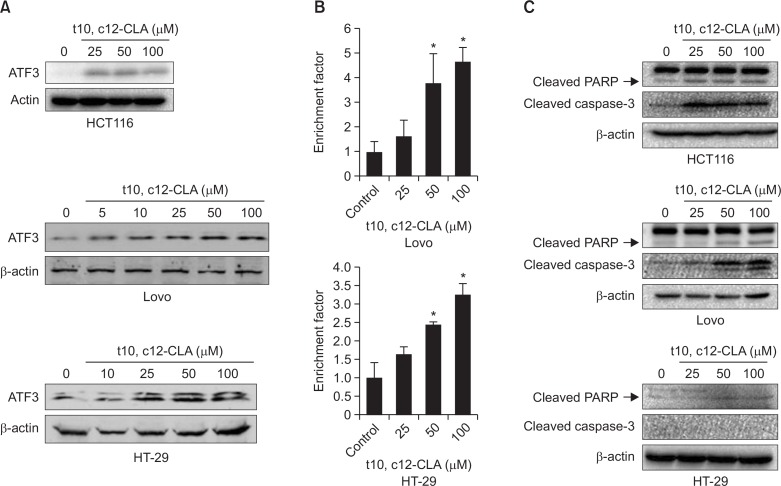
Our previous study demonstrated that CLA increased growth arrest and apoptosis of human colorectal cancer cells through isomer-specific manner (Lee et al., 2006). We also found that t10, c12-CLA increased expression of tumor suppressor gene, NAG-1 through ATF3-mediated pathway. To further extend our understand on anti-cancer mechanisms of t10, c12-CLA, we tested effect of t10, c12-CLA on expression of ATF3 in a well-established human colorectal cancer cell lines including HT-29, Lovo and HCT-116. As shown in Fig. 1A, t10, c12-CLA stimulated expression of ATF3 protein in dose-dependent manner in all cell lines we tested. Since we observed that t10, c12-CLA increased apoptosis of HCT-116 cells (Lee et al., 2006), we measured apoptosis in HT-29 and Lovo cells. As shown in Fig. 1B, treatment of t10, c12-CLA for 24 h increased apoptosis in dose-dependent manner in LoVo and HT-29 cells. Next, we also measured the cleavage of PARP and caspase3, a hallmark of apoptosis (Wang et al., 1997). t10, c12-CLA resulted in an increase of cleaved PARP and caspase 3 which is positively correlated with ATF3 expression (Fig. 1), although there is no change in caspase-3 cleavage in HT-29 cells.
Fig. 1.
t10, c12-CLA increases ATF3 expression and apoptosis in different human colorectal cancer cells. (A, C) HCT116, LoVo and HT-29 cells were incubated with media containing indicated concentrations of t10, c12-CLA for 24 h. Western analysis was performed for ATF3, cleaved PARP, caspase-3, and actin as described in Methods. Data represent one experiment. (B) Apoptosis of LoVo and HT-29 cells were analyzed with Cell Death Detection ELISAPLUS Kit (Roche Diagnostics). *p<0.05 versus vehicle-treated cells.
The t10, c12-CLA increase ATF3 expression through transcriptional upregulation of ATF3 gene
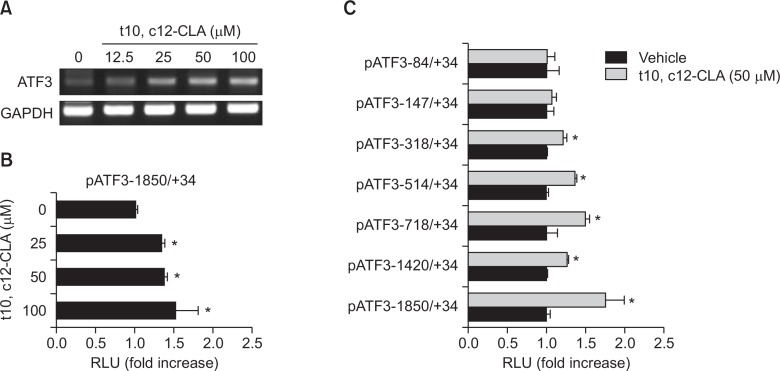
To test whether t10, c12-CLA affects the transcriptional activity of the ATF3 gene, we measured mRNA of ATF3 gene in the cells treated with different doses of t10, c12-CLA. As shown in Fig. 2A, t10, c12-CLA treatment increase ATF3 mRNA in dose-dependent manner. This result is consistent with Western blot analysis, indicating that increased protein expression is caused by increased transcription of ATF3 gene in HCT-116 cells treated with t10, c12-CLA.
Fig. 2.
t10, c12-CLA increases transcriptional activity of ATF3 gene. (A) HCT-116 cells were treated with different doses of t10, c12-CLA for 24 h. Total RNA was isolated and RT-PCR was performed as described in Methods. Data represent one experiment. (B) The pATF3-1850/+34 construct (0.5 μg) was co-transfected with pRL-null vector (0.05 μg). The cells were treated with indicated doses of t10, c12-CLA for 24 h and then luciferase activity was measured as described in Methods. Values are expressed as mean ± SD of 3 replicates. *p<0.05 versus vehicle-treated cells. (C) HCT116 cells were transfected with indicated ATF3 deletion promoter constructs (0.5 μg) with pRL-null vector (0.05 μg). The cells were treated with vehicle or 50 μM of t10, c12-CLA for 24 h and luciferase activity was measured. Values are expressed as mean ± SD of 3 replicates. *p<0.05 versus vehicle-treated cells.
To observe if increased mRNA is associated with transcriptional upregulation, we analyzed the promoter activity using luciferase construct containing ATF3 promoter (pATF3 −1850/+34). The ATF3 promoter construct were transfected into HCT-116 cells and treated with 0, 25, 50, and 100 μM of t10, c12-CLA for 24h. The luciferase activities were measured to assess the transactivation for ATF3 promoter. The t10, c12-CLA treatment resulted in the significant induction of luciferase activity in the cells treated with t10, c12-CLA (Fig. 2B).
Next, we performed the promoter assay using different size of the ATF3 promoter clones to identify the promoter region responsible for the transcriptional upregulation of the ATF3 gene by t10, c12-CLA. We transfected seven different size of the ATF3 promoter (pATF3-84/+34, pATF3 −147/+34, pATF3 −318/+34, pATF3 −514/+34, pATF3 −718/+34, pATF3 −1420/+34, and pATF3 −1850/+34) into the HCT-116 cells and incubated for 24 h with and without 50 μM t10, c12-CLA. As shown in Fig. 2C, t10, c12-CLA showed 1.2, 1.4, 1.5, 1.3, and 1.8 fold increase of transactivation of ATF3 in pATF3 −318/+34, pATF3 −514/+34, pATF3 −718/+34, pATF3 −1420/+34, and pATF3 −1850/+34 transfected cells, respectively. However, t10, c12-CLA did not change luciferase activity of pATF3 −147/+34 and pATF3 −84/+34 transfected cells. These data indicate that t10, c12-CLA probably affects the promoter region between −147 bp and −1850 bp for ATF3 gene transcription.
The t10, c12-CLA does not regulate ATF3 RNA stability
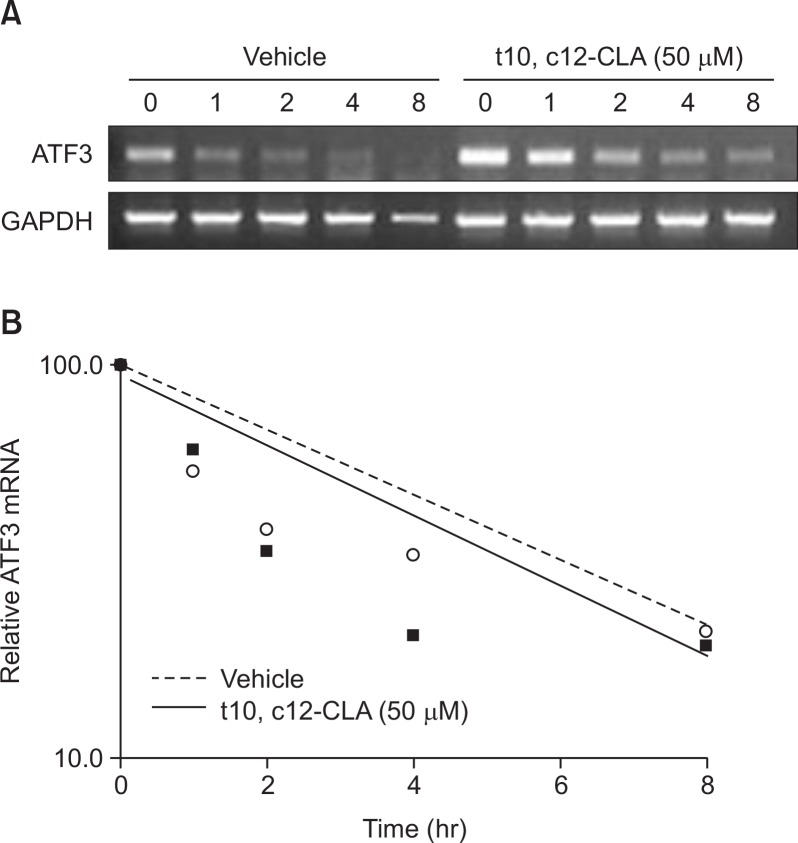
Since the increase of ATF3 mRNA (Fig. 2A) is more prominent than the increase of the promoter activity (Fig. 2B, C), we hypothesized that t10, c12-CLA may affect the post-transcriptional regulation. To determine whether the treatment of t10, c12-CLA influence the mRNA stability, HCT-116 cells were treated with either vehicle or 50 μM of t10, c12-CLA for 24 h and then exposed to actinomycin D (inhibitor of RNA synthesis) for 0, 1, 2, 4 or 8 h. mRNA of ATF3 was compared using semi-quantitative RT-PCR to compare the half-lives of the ATF3 mRNA. The results indicate that half-life of ATF3 mRNA is the same between vehicle- and t10, c12-CLA-treated cells (Fig. 3A, B). These results suggest that t10, c12-CLA does not affect ATF3 mRNA stability.
Fig. 3.
t10, c12-CLA does not influence ATF3 stability (A) HCT-116 cells were treated with vehicle or t10, c12-CLA for 24 h, and subsequently co-treated with actinomycin D. At the indicated times, total RNAs were isolated and mRNA was examined by RT-PCR. (B) The band signals were quantified with Scion Image software. The relative level of ATF3 mRNA (relative to the level of GAPDH) was calculated and the results plotted as the % of the mRNA level present at time 0 of actinomycin D treatment. Data represent one experiment.
The t10, c12-CLA modulates Akt phosphorylation and GSK3β expression, and ATF3 mediates t10, c12-CLA-stimulated expression of GSK3β and NAG-1, and apoptosis
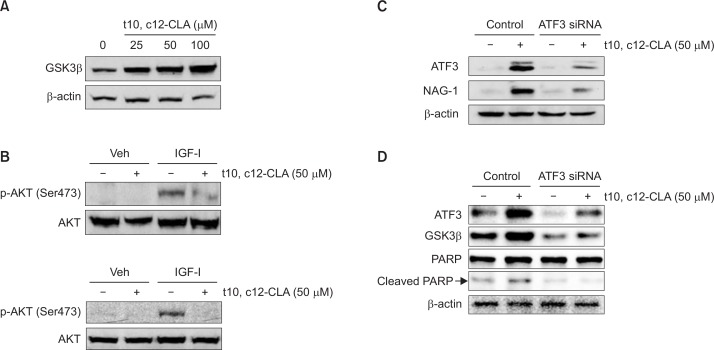
GSK3β is an important mediator of PI3K/Atk pathway and modulator of apoptosis. Akt is also an apoptotic regulator that is elevated in cancer. In previous study, we found that Akt/GSK3β axis mediates t10, c12-CLA-stimulated apoptosis (Lee et al., 2006). ATF3 directly upregulated the transactivation of NAG-1 gene and both are the downstream target of GSK3β (Yamaguchi et al., 2004; Lee et al., 2006). Here, we found that treatment of t10, c12-CLA increased expression of GSKβ in dose-dependent manner (Fig. 4A) while it did not affect phosphorylation of GSKβ (data not shown).
Fig. 4.
Dependency of ATF3 in t10, c12-CLA-induced GSK3β and NAG-1 expression, and apoptosis (A) HCT116 cells were exposed to different doses of t10, c12-CLA for 24 h and Western blot was performed GSK3β and actin. (B) The HCT116 (upper) and HT-29 (lower) cells were treated with vehicle or t10, c12-CLA (50 μM) for 24 h and stimulated with IGF-1 (100 ng/mL). Western analysis was performed using antibodies for phospho-Akt (Ser473), total Akt and actin. (C, D) HCT-116 cells were transfected with control or ATF3 siRNA (100 nM) for 24 h and treated with t10, c12-CLA (50 μM) for 24 h. Western analysis was performed for ATF3, NAG-1, GSK3β, PARP, and actin. Data represent one experiment.
IGF-I is one of the most potent natural activators of the AKT signaling. The receptor for IGF-I (IGF-IR) is expressed in both normal and colon cancer cells, including HCT116 (Donovan and Kummar, 2008). We tested if t10, c12-CLA inhibited IGFI-stimulated Akt phosphorylation. We observed repression of IGF-1-stimulated Akt phosphorylation in the cells treated with t10, c12-CLA (Fig. 4B).
Next we tested the effect of the ATF3 status on the expression of NAG-1 and apoptosis. HCT116 cells were transiently transfected with control siRNA and ATF3 siRNA and then treated with 50 μM of t10, c12-CLA. As shown in Fig. 4C, the transfection of ATF3 siRNA dramatically repressed t10, c12-CLA-stimulated expression of NAG-1, confirming our previous claim that ATF3 is an upstream molecule of NAG-1 transactivation. In addition, the knockdown of ATF3 ameliorated t10, c12-CLA-stimulated cleavage of PARP, implying that ATF3 mediates t10, c12-CLA-induced apoptosis (Fig. 4D). Interestingly, we found that the ATF3 knockdown led to the inhibitions of t10, c12-CLA-stimulated GSK3β expression (Fig. 4D). These data suggest that ATF3 might act as GSK3β regulator in human colorectal cancer cells.
DISCUSSION
In our previous study, we proposed a novel molecular mechanism of anti-cancer activity of t10, c12-CLA. It includes increased expression of a novel proapoptotic protein, NAG-1 through AKT/GSK3β pathway (Lee et al., 2006). Another interesting finding is that ATF3 may mediate t10, c12-CLA-induced NAG-1 expression. However, the effect of t10, c12-CLA on ATF3 expression and potential link of ATF3 to other signaling components such as Akt and GSK3β and subsequent apoptosis remains unanswered. Therefore, we raise an important question regarding the underlying molecular mechanisms of ATF3 activation by t10, c12-CLA. Here, we demonstrate direct evidence that t10, c12-CLA increase ATF3 expression through transcriptional upregulation of ATF3 gene and ATF3 mediate t10, c12-CLA-stimulated NAG-1 expression and apoptosis in human colorectal cancer cells.
The transcriptional regulation of ATF3 gene is mediated by several mechanisms involving other CREB/ATF transcription factors and C/EBPs (Lee et al., 2010a, 2013). In fact, some dietary compounds, including tolfenamic acid and diindolylmethane, induce ATF3 transcription through ATF2 or ATF4-dependent mode. Unlikely tolfenamic acid and diindolylmethane, responsible promoter region for ATF3 transcription by t10, c12-CLA is localized between −147 and −1859 region, implying that another transcriptional mechanism might be involved in transactivation of ATF3 gene by t10, c12-CLA. However, the increase of ATF3 mRNA (Fig. 2A) is more prominent than the increase of the promoter activity (Fig. 2B, C), suggesting involvement of post-transcriptional regulation. So we compared mRNA stability. The results indicate that half-life of ATF3 mRNA is the same between vehicle- and t10, c12-CLA-treated cells. For this inconsistency, we speculate that the promoter region we used in this study may not include responsible cisacting elements responsible for transcriptional activity of ATF3 gene. And this also suggests that ATF3 gene regulation by t10, c12-CLA is different from that by other compounds we reported previously.
Deregulation of PI3K/AKT pathways by specific inhibitors triggers a cascade of cellular responses of apoptosis in numerous cancer cells. The current results suggest that inhibition of AKT phosphorylation may be a responsible mechanism by which t10, c12-CLA activates ATF3 expression.
Interesting finding is that knockdown of ATF3 gene ameliorates t10, c12-CLA-stimulated NAG-1 expression. This result is in agreement with the previous result that overexpression of ATF3 increased NAG-1 promoter activity (Lee et al., 2006), confirming our previous results that ATF3 is an upstream regulator of NAG-1 gene.
Another interesting finding of our current study is a dose-dependent increase of GSK3β expression (Fig. 4A). GSK3β is one of the primary target genes of AKT pathway and mediates apoptotic signals (Jope and Bijur, 2002; Doble and Woodgett, 2003; Forde and Dale, 2007). Increased GSK3β expression was also observed in capsaicin treated human colorectal cancer cells (Lee et al., 2010b), suggesting that increased expression of GSK3β could be a common phenomenon in cancer cells exposed to anti-cancer compounds. In fact, cellular level of GSK3β is highly associated with process of apoptosis and by modulation of expression of numerous target transcription factors (Bijur and Jope, 2001).
In our previous study, the knockdown of GSK3β inhibited t10, c12-CLA-mediated ATF3 induction (Lee et al., 2006). However, current study supports that ATF3 might be one of upstream gene of GSK3β. Another study indicated that GSK3β directly associate with ATF3 and form big complex proteins (Lee et al., 2010b). Further study is required to explain the inconsistency and obtain more detailed information on how ATF3 interact with GSK3β in response to dietary compounds including t10, c12-CLA and capsaicin.
Among the two major CLA isomers, the cis-9, trans-11 CLA (c9, t11-CLA) is the primary isomer found in meat and dairy foods. The t10, c12 CLA is present in small quantity in foods but synthetically prepared CLA contains significant amounts of this isomer, approximately 35–45% along with about the same quantity of the c9, t11-CLA. Anti-cancer activity of CLA shows isomer specific in human colorectal cancer. In our previous study, we observed that t10, c12-CLA, but not c9, t11-CLA, increased apoptosis and expression of ATF3 and GSK3β in human colorectal cancer cells (Lee et al., 2006). Further studies will be required to address how CLAs show the positional isomer dependence in terms of carcinogenesis.
The current data demonstrate that t10, c12-CLA increases transcription of ATF3 gene which might be associated with multiple signaling components including Akt, GSK3β and NAG-1 in human colorectal cancer cells.
Acknowledgments
This work was supported by a research grant from start-up funds from University of Maryland to S-H Lee.
REFERENCES
- Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- Ameri K, Hammond EM, Culmsee C, Raida M, Katschinski DM, Wenger RH, Wagner E, Davis RJ, Hai T, Denko N, Harris AL. Induction of activating transcription factor 3 by anoxia is independent of p53 and the hypoxic HIF signalling pathway. Oncogene. 2007;26:284–289. doi: 10.1038/sj.onc.1209781. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–908. [PubMed] [Google Scholar]
- Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J Biol Chem. 2001;276:37436–37442. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, Baek SJ. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev. Res. (Phila) 2009;2:450–458. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996;16:1157–1168. doi: 10.1128/mcb.16.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SF, Storkson JM, Liu W, Albright KJ, Pariza MW. Conjugated linoleic acid (9,11- and 10,12-octadecadienoic acid) is produced in conventional but not germ-free rats fed linoleic acid. J Nutr. 1994;124:694–701. doi: 10.1093/jn/124.5.694. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Kwon GT, Park JH. trans-10, cis-12 Conjugated linoleic acid induces depolarization of mitochondrial membranes in HT-29 human colon cancer cells: a possible mechanism for induction of apoptosis. J. Med. Food. 2009;12:952–958. doi: 10.1089/jmf.2009.0056. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Dilzer A, Park Y. Implication of conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci Nutr. 2012;52:488–513. doi: 10.1080/10408398.2010.501409. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan EA, Kummar S. Role of insulin-like growth factor-1R system in colorectal carcinogenesis. Crit Rev Oncol Hematol. 2008;66:91–98. doi: 10.1016/j.critrevonc.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha YL, Storkson J, Pariza MW. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990;50:1097–1101. [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–1215. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jeong JB, Choi J, Lou Z, Jiang X, Lee SH. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int Immunopharmacol. 2013;16:184–190. doi: 10.1016/j.intimp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Jope RS, Bijur GN. Mood stabilizers, glycogen synthase kinase-3 beta and cell survival. Mol. Psychiatry. 2002;7(Suppl 1):S35–45. doi: 10.1038/sj.mp.4001017. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J Nutr. 2003;133:2675–2681. doi: 10.1093/jn/133.8.2675. [DOI] [PubMed] [Google Scholar]
- Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl ester: a curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: potential for prostate cancer management. Neoplasia. 2003;5:255–266. doi: 10.1016/S1476-5586(03)80057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Lee HJ, Cho HY, Kim YJ. Role of the conjugated linoleic acid in the prevention of cancer. Crit Rev Food Sci Nutr. 2005;45:135–144. doi: 10.1080/10408690490911800. [DOI] [PubMed] [Google Scholar]
- Lee SH, Bahn JH, Whitlock NC, Baek SJ. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene. 2010a;29:5182–5192. doi: 10.1038/onc.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Krisanapun C, Baek SJ. NSAID-activated gene-1 as a molecular target for capsaicin-induced apoptosis through a novel molecular mechanism involving GSK3{beta}, C/ EBP{beta}, and ATF3. Carcinogenesis. 2010b;31:719–728. doi: 10.1093/carcin/bgq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Min KW, Zhang X, Baek SJ. 3,3′-diindolyl-methane induces activating transcription factor 3 (ATF3) via ATF4 in human colorectal cancer cells. J Nutr Biochem. 2013;24:664–671. doi: 10.1016/j.jnutbio.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yamaguchi K, Kim JS, Eling TE, Safe S, Park Y, Baek SJ. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–981. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Roche HM, Noone E, Sewter C, Mc Bennett S, Savage D, Gibney MJ, O’Rahilly S, Vidal-Puig AJ. Isomer-dependent metabolic effects of conjugated linoleic acid: insights from molecular markers sterol regulatory element-binding protein-1c and LXRalpha. Diabetes. 2002;51:2037–2044. doi: 10.2337/diabetes.51.7.2037. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- Umesalma S, Sudhandiran G. Ellagic acid prevents rat colon carcinogenesis induced by 1, 2 dimethyl hydrazine through inhibition of AKT-phosphoinositide-3 kinase pathway. Eur J Pharmacol. 2011;660:249–258. doi: 10.1016/j.ejphar.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K, Wagner EF. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI200316147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–451. doi: 10.1158/0008-5472.CAN-03-3241. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Lee SH, Eling TE, Baek SJ. Identification of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway. J Biol Chem. 2004;279:49617–49623. doi: 10.1074/jbc.M408796200. [DOI] [PubMed] [Google Scholar]