Abstract
Our previous study demonstrated that yuzu has an anti-platelet effect in rat blood. In the present study, we examined whether the anti-platelet effect of yuzu can be extended to human blood by investigating its ability to inhibit aggregations induced by various agonists in human platelet rich plasma (PRP). This study also investigated the underlying mechanism of yuzu focusing on ADP granule secretion, TXB2 formations, and PLCγ/Akt signaling. The results from this study showed that ethanolic yuzu extract (YE), and its components, hesperidin and naringin, inhibited human platelet aggregation in a concentration-dependent manner. YE, hesperidin and naringin also inhibited TXB2 formation and ADP release. The phosphorylation of PLCγ and Akt was significantly inhibited by YE, heperidin and naringin. Furthermore, we demonstrated that YE, heperidin and naringin has anti-platelet effects in rat ex vivo studies, and lower side effects in mice tail bleeding time studies. The results from this study suggest that YE, hesperidin and naringin can inhibit human platelet aggregation, at least partly through the inhibition of PLCγ and Akt, leading to a decrease in TXB2 formation and granule secretion.
Keywords: Yuzu, Hesperidin, Naringin, Platelet, Aggregation
INTRODUCTION
Platelet aggregation and the subsequent formation of blood clots are essential processes in hemostasis (Xia et al., 2012). Platelets can be activated abnormally in a pathological environment however, leading to thrombogenesis and the formation of occlusive thrombi. The circulating thrombi can occlude small blood vessels and subsequently cause various cardiovascular diseases including ischemic heart disease and stroke (Lee et al., 2014a). Therefore, the inhibition of platelet aggregation has been considered as a strategy to limit the progress of arterial thrombosis and platelet-associated cardiovascular diseases (Ruggeri, 2002).
To date, a number of anti-platelet drugs, such as clopidogrel and aspirin, have been developed and are used clinically to prevent thrombosis (Bassand, 2013). However, the use of these anti-platelet drugs is limited due to their associated side effects such as gastrointestinal bleeding and palpitation (Vaiyapuri et al., 2013). Therefore, the development of much safer and more effective anti-platelet agents is needed (Barrett et al., 2008).
Yuzu (Citrus junos sieb ex Tanaka) is a citrus fruit native to northeast Asia and can be found in Korea, China, and Japan. Citrus fruits contain dietary components such as hesperidin, naringin, luteolein, limonene, and vitamin C, which confer health benefits that include preventing cardiovascular disease and improving blood circulation. Yuzu also contains various flavonoids, such as hesperidin, naringin, rutin, rutin hydrate, narirutin, apigen-7-glucoside, quercetin, and tangeretin (Yang et al., 2013). Several studies have suggested that limonene from yuzu essential oil can be used to treat bronchial asthma due to its anti-inflammatory activities. Indeed, yuzu has been reported to inhibit the production of cytokines and reactive oxygen species, and to reduce eosinophil migration. Our previous study demonstrated that methanolic extract of yuzu has anti-platelet effects in rat blood (Yu et al., 2011). However, few studies has been reported about its underlying mechanism.
In this study, we evaluated whether the anti-platelet effect of yuzu and its components can be extended to human blood by examining whether they can inhibit aggregations induced by various agonists in human platelet rich plasma (PRP). We further investigated possible signaling mechanisms for their anti-platelet effects in human blood.
MATERIALS AND METHODS
Reagents
Ethanolic yuzu extract (YE) was obtained from Konkuk University (Seoul, Republic of Korea). Briefly, yuzu were harvested in the southern region of Korea, Goheung (Republic of Korea). Whole fruits, including seeds, pulp and skin, were extracted with 70% ethanol and dried to remove the solvent, then dissolved in saline for the experiments. Hesperidin, naringin, apyrase, acetylsalicylic acid (ASA), bovine serum albumin (BSA), fura 2-AM, β-nicotinamide adenine dinucleotide (reduced form, β-NADH) and pyruvic acid were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Collagen, thrombin, adenosine diphosphate (ADP), arachidonic acid (AA), and luciferin-luciferase reagent were purchased from Chrono-Log Co. (Harvertown, PA, USA). Antibodies against phospholipase Cγ (PLCγ), phosphor-PLCγ, PI3K/protein kinase B (Akt), and phospho-Akt were purchased from Cell Signaling (Danvers, MA, USA).
Animals
Animals were purchased from Samtako Laboratory Animal Center (Suwon, Korea). Sprague-Dawley (SD) rats (8 weeks, 220–240 g) and ICR mice (8 weeks, 34–40 g) were used for experiments. They were housed in a conventional animal facility with free access to food and water in a temperature- and relative humidity-controlled environment under a 12-h light/12-h dark schedule. The animals were acclimatized for a minimum of 7 days before experiments were performed. Animal study protocols conformed to guidelines in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the Committee on Animal Research at Ajou Medical Center, Ajou University.
Preparation of platelet rich plasma (PRP) from human blood
Human platelet suspensions were supplied by the Red Cross (Suwon, Korea). Human blood was collected from healthy human volunteers free of medications as indicated by the Red Cross guide. Human PRP were diluted with Tyrode’s solution (11.9 mM NaCl, 2.7 mM KCl, 2.1 mM MgCl2, 0.4 mM NaH2PO4, 11.9 mM NaHCO3, 11.1 mM glucose, and 3.5 mg/mL bovine serum albumin, pH 7.2).
Preparation of PRP from Rat
PRP from rat blood was prepared as described previously (Jung et al., 2002; Eskandariyan et al., 2014). Briefly, SD rats weighing 200–240 g were lightly anesthetized with diethyl ether. A volume of 8–10 mL of blood was collected from the abdominal aorta of each rat into a syringe containing 3.8% sodium citrate. The ratio of blood to 3.8% sodium citrate was adjusted to 1:9 v/v. After centrifugation at 150 g for 10 min at room temperature, the supernatant (PRP) was used for the aggregation study.
Platelet aggregation study
Platelet aggregation was performed by the previously described turbidimetric method using an aggregometer (Chrono-log, Havertown, USA) (Pyo et al., 2002; Kim et al., 2011). Briefly, PRP was incubated at 37°C for 5 min with different concentrations of samples (YE, hesperidin, and naringin) in the aggregometer while stirring at 1000 rpm. PRP was then stimulated with different aggregating agents at the following final concentrations: collagen, 2 μg/mL; thrombin, 0.4 U/mL; ADP, 10 μM; AA, 100 μM (Chrono-Log, USA). The extent of platelet aggregation was estimated by measuring the maximum height above the baseline reached by the aggregation curve within 5 min following stimulation.
ATP release assay
Detection of ATP release was performed as previously described (Flevaris et al., 2009). Washed platelets (WPs) were preincubated for 3 min at 37°C with various concentrations of samples and then stimulated with collagen. After the reaction was terminated, samples were centrifuged and supernatants were collected for the assay. ATP release was measured in a luminometer (GloMax 20/20, Promega, Madison, WI, USA) using an ATP assay kit (Biomedical Research Service Center, Buffalo, NY, USA).
Measurement of Thromboxane B2 (TXB2) Production
Measurement of TXB2 production was conducted using a TXB2 EIA kit (Cayman Chemical Co, Ann Arbor, MI, USA) as previously described (Cho et al., 2011; Seo et al., 2011). PRP (2×108 platelets/mL) was incubated for 3 min in the presence or absence of samples. Collagen (2 μg/mL) was added and the mixture was incubated at 37°C for 5 min with stirring. EDTA (10 mM) was added to stop TXB2 production. After centrifugation at 12,000 g for 3 min, the amount of TXB2 was measured according to the manufacturer’s instructions.
Western blot analysis
The immunoassay was performed as previously described (Endale et al., 2012). The WPs (2×108 platelets/ml) were stimulated with collagen (2 μg/ml) in the presence or absence of samples. Reactions were terminated by adding 10 mM EDTA and the platelets were centrifuged at 12,000 g for 3 min. After adding ice-cold platelet lysis buffer (1% NP40, 15 mM HEPES, 75 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium fluoride (NAF), 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 μg/ml aprotinin; pH 7.4), lysates were centrifuged at 13,000 g for 10 min at 4°C. The supernatants were collected and protein concentration was determined using a BCA protein assay (Thermo Scientific, IL, USA). Equal volumes of protein were resolved using SDS-PAGE (10%) and transferred to Polyvinylidene Difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% nonfat dry milk in TBS and incubated with the primary antibody diluted in TBS buffer (1:1000 ratio, 4°C overnight). Immunoblots were incubated with a horse radish peroxidase (HRP)-conjugated secondary antibody and the immune-reactive bands were visualized with a LAS 4000 (Fuji, Honshu, Japan) using a western blot detection system (WestZol, Intron, Seoul, Republic of Korea).
Ex vivo collagen-induced platelet aggregation study
SD rats were orally administered saline and samples, or ASA (50 mg/kg). Two hours later, rat blood samples were collected and the platelet aggregation study was performed as described above (Kim et al., 2011).
In vivo tail bleeding time study
Bleeding times were determined as previously described (Cho et al., 2008; Lee et al., 2014b). Male ICR mice, weighing 35–40 g, were used in this experiment. Mice were fasted for 24 h before experiments. For in vivo studies, the YE was suspended in saline and aspirin was suspended in 10% Tween 80-saline. ICR mouse were orally administered saline and YE (100 mg/kg), hesperidin (10 mg/kg), naringin (10 mg/kg), or ASA (50 mg/kg) once a day for 1 day, or for 7 days. Two hours after the last administration, mice were anesthetized with sodium pentobarbital (75 mg/kg, IP), and individually placed on a hotplate to maintain body temperature at 37°C. In each case, the tail was transected 3 mm from its tip with a razor blade, and then immersed in a 15-ml clear conical tube containing normal saline prewarmed to 37°C. Times to blood flow cessation (defined as no bleeding for 15 s) were measured. Data are expressed as means ± SEM.
Statistical analysis
All data are expressed as means ± SEMs of at least four different experiments. Statistical analysis was performed using one way ANOVA followed by Dunnett’s test or the paired t-test using Sigma Stat statistical software (San Rafael). Significance was accepted for p-values of <0.05.
RESULTS
Effect of YE on human platelet aggregation
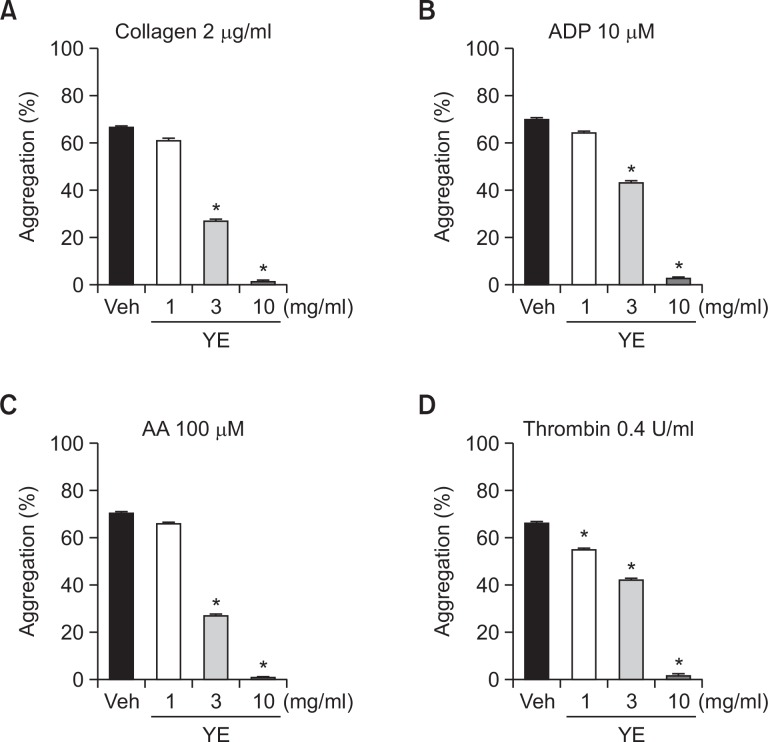
We evaluated the effect of YE on platelet aggregation induced by collagen, ADP, AA, and thrombin in human PRP. As shown in Fig. 1, YE (3, 10 mg/ml) significantly inhibited collagen-induced platelet aggregations (59.3 ± 2.0, 99.0 ± 0.5%, respectively), ADP-induced platelet aggregations (38.1 ± 3.0, 96.2 ± 1.2%, respectively), and AA-induced platelet aggregations (61.7 ± 0.7, 99.5 ± 0.5%, respectively) (Fig. 2A∼C). YE at concentrations of 1, 3, and 10 mg/ml also significantly inhibited thrombin-induced platelet aggregations (16.8 ± 2.8, 36.1 ± 1.5, 97.4 ± 1.9% respectively) (Fig. 2D).
Fig. 1.
The chemical structures of hesperidin and naringin. (A) Hesperetin 7-rhamnoglucoside (hesperidin). (B) 4′,5,7-Trihydroxyflavanone 7-rhamnoglucoside (naringin).
Fig. 2.
Inhibitory effects of ethanolic yuzu extract (YE) on human platelet aggregation. PRP was preincubated for 5 min with various concentrations of YE at 37°C before being aggregated with collagen 2 μg/ml (A), ADP 10 μM (B), AA 100 μM (C), and thrombin 0.4 U/ml (D). Data are expressed as means ± SEM (n=5). *p<0.05 vs. vehicle.
Effect of hesperidin on human platelet aggregation
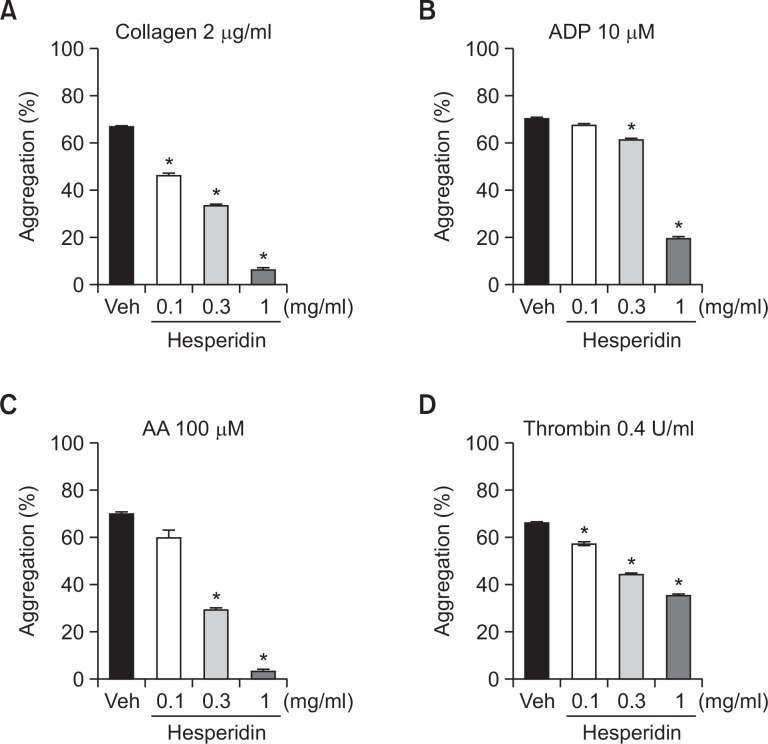
We evaluated the effect of hesperidin on platelet aggregation induced by collagen, ADP, AA, and thrombin in human PRP. Hesperidin at concentrations of 0.1, 0.3, and 1 mg/ml significantly inhibited collagen-induced platelet aggregations (30.4 ± 4.9, 50.4 ± 2.6, 90.6 ± 5.5%, respectively), and thrombin-induced platelet aggregations (13.2 ± 1.5, 33.0 ± 0.9, 46.1 ± 2.5%, respectively) respectively. (Fig. 3A, D). Hesperidin at concentrations of 0.3 and 1 mg/ml also significantly inhibited ADP-induced platelet aggregations (12.4 ± 1.4, 72.1 ± 2.2%, respectively), and AA-induced platelet aggregations (57.9 ± 1.6, 95.3 ± 2.4%, respectively) (Fig. 3B, C).
Fig. 3.
Inhibitory effects of hesperidin on human platelet aggregation. PRP was preincubated for 5 min with various concentrations of hesperidin at 37°C before being aggregated with collagen 2 μg/ml (A), ADP 10 μM (B), AA 100 μM (C), and thrombin 0.4 U/ml (D). Data are expressed as means ± SEM (n=5). *p<0.05 vs. vehicle.
Effect of naringin on human platelet aggregation
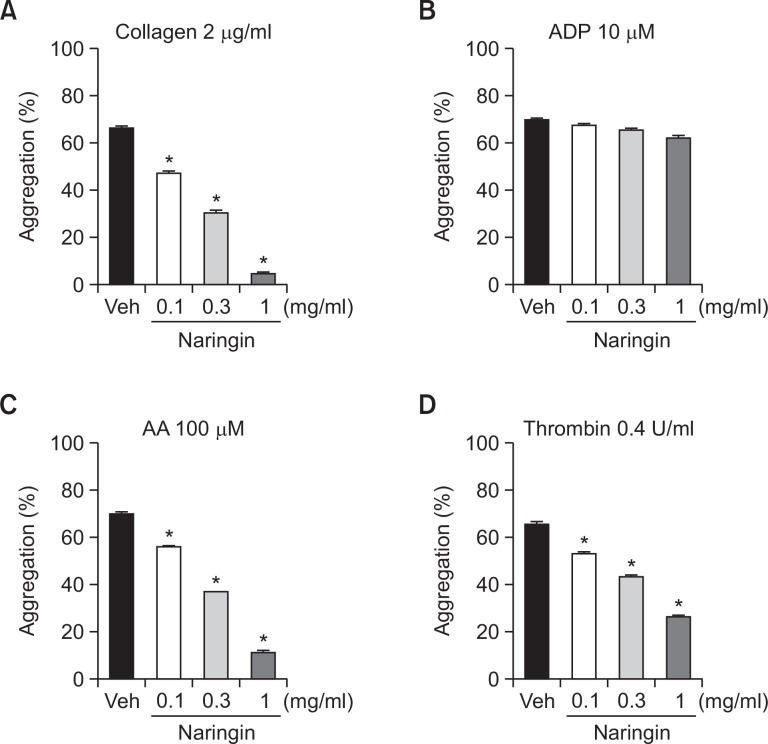
We evaluated the effect of naringin platelet aggregation induced by collagen, ADP, AA, and thrombin in human PRP. Naringin at concentrations of 0.1, 0.3, and 1 mg/ml significantly inhibited collagen-induced platelet aggregations (28.8 ± 1.9, 53.7 ± 0.8, 92.9 ± 1.6%, respectively), AA-induced platelet aggregations (20.0 ± 1.0, 46.8 ± 2.0, 83.7 ± 0.9%, respectively), and thrombin-induced platelet aggregations (19.3 ± 2.1, 34.0 ± 2.3, 59.9 ± 0.9%, respectively) (Fig. 4A, C, D). However, naringin did not inhibit ADP-induced platelet aggregation (Fig. 4B).
Fig. 4.
Inhibitory effects of naringin on human platelet aggregation. PRP was preincubated for 5 min with various concentrations of naringin at 37°C before being aggregated with collagen 2 μg/ml (A), ADP 10 μM (B), AA 100 μM (C), and thrombin 0.4 U/ml (D). Data are expressed as means ± SEM (n=5). *p<0.05 vs. vehicle.
Effect of YE, hesperidin, and naringin on dense granule secretion in human platelets
To investigate the mechanism underlying the anti-platelet effects of YE, hesperidin, and naringin, we evaluated their effects on ATP release, which is a well-known biomarker of granule secretion from activated platelets. Collagen-induced ATP release was significantly inhibited by 10 mg/ml of YE (85%), 1 mg/ml of hesperidin (77%) and 1 mg/ml of naringin (85%) (Fig. 5A).
Fig. 5.
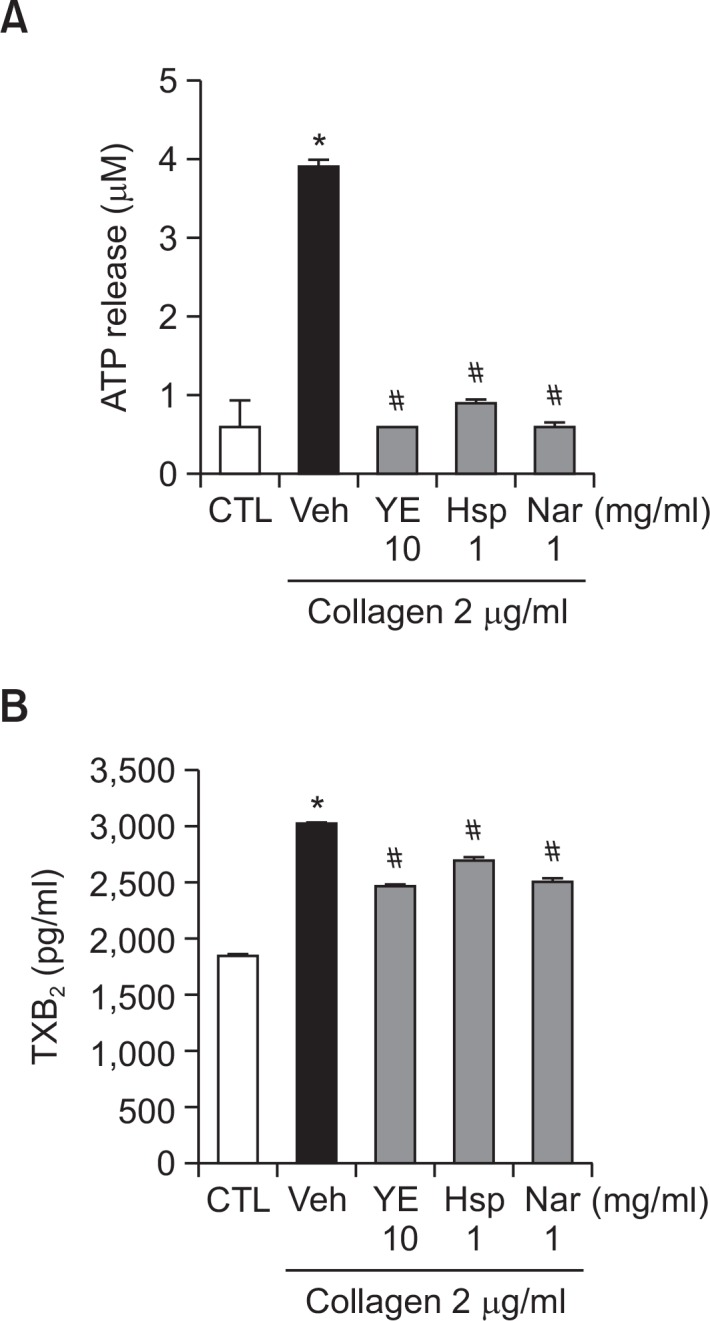
Inhibitory effects of ethanolic yuzu extract (YE) and its components on collagen-induced ATP release (A) and TXB2 formation (B). For ATP measurement, PRP was preincubated for 5 min with YE, hesperidin, and naringin at 37°C prior to the addition of a luciferin-luciferase reagent. After addition of the luciferin-luciferase reagent, PRP were stimulated with 2 μg/ml collagen. ATP release was measured using a lumi-aggregometer. To measure TXB2 formation, platelets were preincubated with YE (10 mg/ml), hesperidin (1 mg/ml), and naringin (1 mg/ml) for 3 min then stimulated with collagen (2 μg/mL) for 6 min. The amount of TXA2 was determined by measuring TXB2 concentrations using an EIA kit (n=8). Data are expressed as means ± SEM (n≥3). *p<0.05 vs. control. #p<0.05 vs. vehicle.
Effect of YE, hesperidin, and naringin on TXB2 formation in human platelets
To investigate another potential mechanism underlying the anti-platelet effects of YE, hesperidin, and naringin, we evaluated their effects on TXB2 formation. Collagen-induced TXB2 formation (3,034.2 ± 27.2) was significantly inhibited by 10 mg/ ml of YE (1,930.7 ± 23.5), 1 mg/ml of hesperidin (2,378.4 ± 57.5), and 1 mg/ml of naringin (2,014.0 ± 58.3) (Fig. 5B).
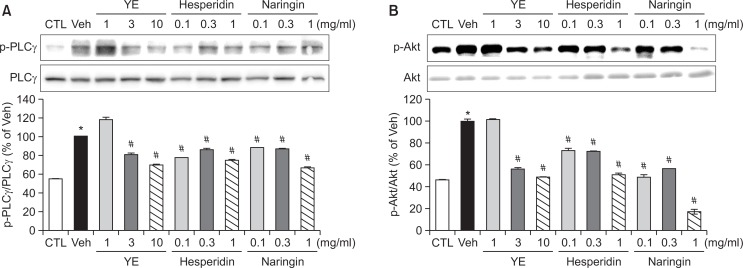
Effect of YE, hesperidin, and naringin on phosphorylation of PLCγ and Akt in human platelet
As a candidate for the signaling pathways, we evaluated the phosphorylation of proteins in the collagen-induced pathway. YE (3, 10 mg/ml), hesperidin (0.1, 0.3, 1 mg/ml), and naringin (0.1, 0.3, 1 mg/ml) significantly inhibited the phosphorylation of Akt and PLCγ (Fig. 6).
Fig. 6.
Effects of ethanolic yuzu extract (YE) and its components on PLCγ, and Akt phosphorylation. Washed platelets (WPs) were preincubated for 5 min with YE, hesperidin, and naringin at 37°C before aggregation with collagen 2 μg/ml. 10 mM EDTA was added to terminate the reaction and the platelets were lysed. The phosphorylation levels of PLCγ (A), and Akt (B) were determined by western blotting as described in Materials and Methods. Data are expressed as means ± SEM (n=5). *p<0.05 vs. control. #p<0.05 vs. vehicle.
Effect of YE on platelet aggregation ex vivo
We also evaluated the effect of YE on collagen-induced platelet aggregation. Oral administration of 100 mg/kg YE, as well as 10 mg/kg hesperidin and naringin significantly inhibited collagen-induced platelet aggregation (29.8 ± 1.8, 24.3 ± 1.9, 23.4 ± 0.7% respectively). However, the effects were less than that of ASA (40.4 ± 2.1%) (Fig. 7).
Fig. 7.
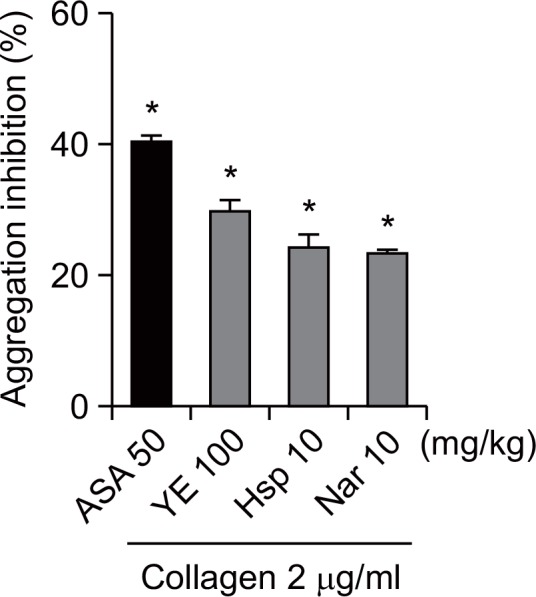
Inhibitory effects of ethanolic yuzu extract (YE) and its components on platelet aggregation ex vivo. YE (100 mg/kg), hesperidin (10 mg/kg), naringin (10 mg/kg), or aspirin (50 mg/kg) was administered orally. Two hours after administration, rat blood samples were collected. PRP was stimulated with collagen 2 μg/ml. Data are expressed as means ± SEM (n≥5). *p<0.05 vs. vehicle.
Effect of YE on bleeding time
We also determined bleeding times in ICR mice to evaluate the side effects of YE. Bleeding time (66.2 ± 4.7 s) in mice was prolonged by oral administration of 100 mg/kg YE (127.4 ± 5.1 s), and 10 mg/kg hesperidin (120.0 ± 2.3 s) and naringin (121.2 ± 2.5 s). However, the extent of prolongation was significantly less than that of ASA (197.0 ± 10.1 s) (Fig. 8).
Fig. 8.
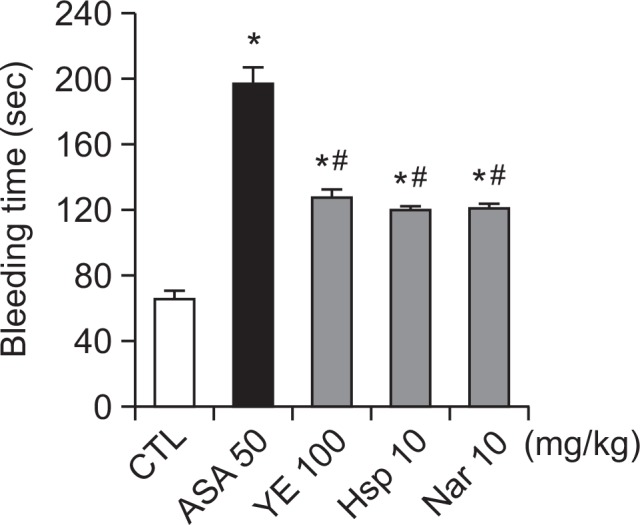
Effects of ethanolic yuzu extract (YE) and its components on mouse tail vein bleeding times. YE (100 mg/kg), hesperidin (10 mg/kg), naringin (10 mg/kg), or aspirin (50 mg/kg) was administered orally to SD rats. Two hours after administration, bleeding time was determined as described in Materials and Methods. Data are expressed as means ± SEM (n≥10). *p<0.05 vs. control. #p<0.05 vs. aspirin.
DISCUSSION
The present study, demonstrates for the first time that YE can attenuate aggregation of human platelets and that it also inhibits TXA2 production, granule secretion and phosphorylation of the platelet aggregation related proteins, Akt and PLCγ.
Platelets have the functions of activation, aggregation, adhesion and secretion (Xia et al., 2012). Under pathologic conditions, platelets bind with extracellular matrix, such as collagen or thrombin generated at the site of injury, leading to platelet activation and thrombus formation (Yu et al., 2011). While our previous study indicated that the methanolic extract of yuzu induces inhibitory effects on rat platelets (Yu et al., 2011), the results from the present study confirmed that the anti-platelet potential of YE and its components can be extended to the human blood by showing that the aggregation of human PRP was significantly inhibited by YE, hesperidin and naringin in a concentration-dependent manner.
The mechanisms responsible for platelet activation and increased platelet aggregation have been reported to involve various signaling pathways that depend on agonists. Indeed, the responses of platelets to collagen stimulation are known to be mediated via glycoprotein VI (GPVI) and integrin a2b1, while the responses to thrombin are largely mediated through G-protein coupled protease-activated receptors (PARs). Collagen induces platelet activation through Src family kinase mediated Fc receptor g-chain tyrosine phosphorylation followed by Syk and linker for activation of T cells (LAT) tyrosine phosphorylation. Activated LAT and PI3K lead to 1,2-diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3) release, PKC activation, and Ca2+ mobilization through tyrosine phosphorylation of PLCγ2 (Endale et al., 2012). The PLCγ/PKCδ/ extracellular signal-regulated kinases (ERK) and/or the p38 mitogen-activated protein kinases (p38) signaling pathway are activated in addition to PI3K/protein kinase B (Akt) signaling. Akt, PKCδ, ERK and p38 are common molecules in platelet activation signaling pathways, whereas PLCγ is a molecule that plays a specific role in the collagen-induced platelet activation pathway (Liou et al., 2012). Upon exposure to activating agonists, such as collagen and thrombin, platelets liberate AA and the liberated AA is converted by cyclooxygenase and TXA2 synthase into TXA2. ADP is known to be secreted from dense granules in activated platelets. The α-granules contain many proteins such as fibrinogen, von Willebrand factor, and P-selectin. Dense granules contain molecules such as ADP, ATP, and calcium (Vaiyapuri et al., 2013). When ADP is released, it binds to ADP receptors, such as P2Y1 and P2Y12, in an autocrine and paracrine manner, leading to platelet activation and aggregation. P2Y1 receptor activation regulates Ca2+-dependent signaling, whereas P2Y12 receptor activation regulates integrin αIIbβ3. The results from our study showed that YE has a greater effect on granule secretion and TXB2 formation, while hesperidin and naringin have stronger effects on the phosphorylation of PLCγ and Akt. To determine whether the anti-platelet effects of YE and its components are maintained in vivo, we evaluated the effects of YE, hesperidin, and naringin on rat platelet aggregation ex vivo after oral administration. YE and its components had anti-platelet effects ex vivo. We then evaluated the side effects of YE and its components by performing a bleeding time assay. YE and its components prolonged bleeding time, but the extent of prolongation was less than that of ASA. These results suggest that YE intake can prevent uncontrolled platelet activation with fewer side effects than ASA.
In conclusion, our findings from this study suggest that YE and its components induce a significant anti-platelet activity in human blood and therefore, may have therapeutic potential for the prevention of platelet-associated cardiovascular diseases.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No.PJ009074), Rural Development Administration, Republic of Korea.
REFERENCE
- Barrett NE, Holbrook L, Jones S, Kaiser WJ, Moraes LA, Rana R, Sage T, Stanley RG, Tucker KL, Wright B, Gibbins JM. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154:918–939. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassand JP. Current antithrombotic agents for acute coronary syndromes: focus on bleeding risk. Int J Cardiol. 2013;163:5–18. doi: 10.1016/j.ijcard.2011.10.104. [DOI] [PubMed] [Google Scholar]
- Cho H-J, Choi S-A, Kim C-G, Jung T-S, Hong J-H, Rhee M-H, Park H-J, Park H-J. Spinach saponin-enriched fraction inhibits platelet aggregation in cAMP- and cGMP-dependent manner by decreasing TXA2 production and blood coagulation. Biomol Ther. 2011;19:218–223. doi: 10.4062/biomolther.2011.19.2.218. [DOI] [Google Scholar]
- Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endale M, Lee WM, Kamruzzaman SM, Kim SD, Park JY, Park MH, Park TY, Park HJ, Cho JY, Rhee MH. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol. 2012;167:109–127. doi: 10.1111/j.1476-5381.2012.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandariyan Z, Esfahani Zadeh M, Haj Mohammad Ebrahim Tehrani K, Mashayekhi V, Kobarfard F. Synthesis of thio-ether derivatives of quinazoline-4-one-2-thione and evaluation of their antiplatelet aggregation activity. Arch Pharm Res. 2014;37:332–339. doi: 10.1007/s12272-013-0192-5. [DOI] [PubMed] [Google Scholar]
- Flevaris P, Li Z, Zhang G, Zheng Y, Liu J, Du X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood. 2009;113:893–901. doi: 10.1182/blood-2008-05-155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Kim MH, Lee SH, Baik EJ, Park SW, Moon CH. Antithrombotic effect of onion in streptozotocin-induced diabetic rat. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:453–458. doi: 10.1054/plef.2002.0373. [DOI] [PubMed] [Google Scholar]
- Kim H, Oh SJ, Liu Y, Lee MY. A comparative study of the anti-platelet effects of cis- and trans-resveratrol. Biomol Ther. 2011;19:201–205. doi: 10.4062/biomolther.2011.19.2.201. [DOI] [Google Scholar]
- Lee DS, Kim TH, Jung YS. Inhibitory effect of allyl isothiocyanate on platelet aggregation. J Agric Food Chem. 2014a;62:7131–7139. doi: 10.1021/jf4041518. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Bae JS. Antiplatelet, anticoagulant, and profibrinolytic activities of baicalin. Arch Pharm Res. 2014b doi: 10.1007/s12272-014-0410-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Liou JT, Mao CC, Liu FC, Lin HT, Hung LM, Liao CH, Day YJ. Levobupivacaine differentially suppresses platelet aggregation by modulating calcium release in a dose-dependent manner. Acta Anaesthesiol. Taiwan. 2012;50:112–121. doi: 10.1016/j.aat.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Pyo MK, Lee Y, Yun-Choi HS. Anti-platelet effect of the constituents isolated from the barks and fruits of Magnolia obovata. Arch Pharm Res. 2002;25:325–328. doi: 10.1007/BF02976634. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- Seo EJ, Lee DU, Kwak JH, Lee SM, Kim YS, Jung YS. Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J Ethnopharmacol. 2011;135:48–54. doi: 10.1016/j.jep.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Vaiyapuri S, Ali MS, Moraes LA, Sage T, Lewis KR, Jones CI, Gibbins JM. Tangeretin regulates platelet function through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Arterioscler Thromb Vasc Biol. 2013;33:2740–2749. doi: 10.1161/ATVBAHA.113.301988. [DOI] [PubMed] [Google Scholar]
- Xia Q, Wang X, Xu DJ, Chen XH, Chen FH. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thromb Res. 2012;130:409–414. doi: 10.1016/j.thromres.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Hwang JT, Kwon DY, Kim MJ, Kang S, Moon NR, Park S. Yuzu extract prevents cognitive decline and impaired glucose homeostasis in beta-amyloid-infused rats. J Nutr. 2013;143:1093–1099. doi: 10.3945/jn.112.173401. [DOI] [PubMed] [Google Scholar]
- Yu HY, Park SW, Chung IM, Jung YS. Anti-platelet effects of yuzu extract and its component. Food Chem Toxicol. 2011;49:3018–3024. doi: 10.1016/j.fct.2011.09.038. [DOI] [PubMed] [Google Scholar]