Abstract
This study investigated the possible effects and molecular mechanisms of diallyl disulfide (DADS) against cyclophosphamide (CP)-induced hemorrhagic cystitis (HC) in rats. Inflammation response was assessed by histopathology and serum cytokines levels. We determined the protein expressions of nuclear transcription factor kappa-B (NF-κB), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumor necrosis factor-α (TNF-α), oxidative stress, urinary nitrite-nitrate, malondialdehyde (MDA), and 8-hydroxy-2’-deoxyguanosine (8-OHdG). Finally, we studied the involvement of mitogen-activated protein kinases (MAPKs) signaling in the protective effects of DADS against CP-induced HC. CP treatment caused a HC which was evidenced by an increase in histopathological changes, proinflammatory cytokines levels, urinary nitrite-nitrate level, and the protein expression of NF-κB, COX-2, iNOS, TNF-α, p-c-Jun N-terminal kinase (JNK), and p-extracellular signal regulated kinase (ERK). The significant decreases in glutathione content and glutathione-S-transferase and glutathione reductase activities, and the significant increase in MDA content and urinary MDA and 8-OHdG levels indicated that CP-induced bladder injury was mediated through oxidative DNA damage. In contrast, DADS pretreatment attenuated CP-induced HC, including histopathological lesion, serum cytokines levels, oxidative damage, and urinary oxidative DNA damage. DADS also caused significantly decreased the protein expressions of NF-κB, COX-2, iNOS, TNF-α, p-JNK, and p-ERK. These results indicate that DADS prevents CP-induced HC and that the protective effects of DADS may be due to its ability to regulate proinflammatory cytokines production by inhibition of NF-κB and MAPKs expressions, and its potent anti-oxidative capability through reduction of oxidative DNA damage in the bladder.
Keywords: Cyclophosphamide, Hemorrhagic cystitis, Diallyl disulfide, Oxidative damage, MAPKs, NF-κB
INTRODUCTION
Cyclophosphamide (CP), an oxazaphosphorine alkylating agent, is widely used in the treatment of various human malignancies (Jurado et al., 2008). However, hemorrhagic cystitis (HC) is a major potential toxicity and dose-limiting side effect of CP, which has been a main problem for physicians during chemotherapy.
Although the exact mechanism of CP-induced HC is still not fully understood, chemical-induced oxidative stress is believed to be a major contributing factor to its HC probably related to differential metabolism of CP into acrolein (Bhatia et al., 2006; Kiuchi et al., 2009; Tripathi and Jena, 2010). Reactive oxygen species (ROS) trigger the activation of critical signaling molecules and then initiate signaling transduction (Ray et al., 2012). Furthermore, ROS can activate multiple signaling pathways that influence the cytotoxicity observed in affected cells, including nuclear transcription factor kappa-B (NF-κB) and the phosphorylation cascades leading to the activation of mitogen-activated protein kinases (MAPKs), thus modulating a number of different steps in the inflammatory cascade (Kim et al., 2007). MAPKs and NF-κB have important activities as mediators of cellular responses to extracellular signals, and are thought to play an important role in the regulation of proinflammatory molecules on cellular responses (Kim et al., 2007; Song et al., 2014).
MAPKs, which include extracellular signal regulated kinase (ERK), c-jun N-terminal kinase (JNK), and p38 subfamilies, are important regulatory proteins through which various extracellular signals are transduced into intracellular responses. They regulate inflammatory proteins as well as immune responses and expression of various cytokines, e.g., interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (Hommes et al., 2003). Previous studies demonstrated that CP-induced HC leads to ERK activation in urinary bladder (Corrow and Vizzard, 2007). Evidences confirmed that activation of JNK and p38 MAPKs may be involved in the mechanism of CP-induced HC (Song et al., 2014).
NF-κB belongs to the Rel family of transcriptional activator proteins, which has a critical role mediating inflammation, apoptosis, and growth in inflammatory disease. Modulation of NF-κB modifies the expression of genes involved in the inflammatory process, including inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and proinflammatory cytokines (Cuesta et al., 2010). A previous study also reported that NF-κB is the prime molecular targets for the chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals (Surh, 2008). Recently, it has been also shown that NF-κB and MAPKs have an important role in the pathogenesis of CP-induced HC in rats (Kiuchi et al., 2009; Tripathi and Jena, 2010; Song et al., 2014).
Garlic oil contains more than 20 organosulfur compounds, which can be considered as a dietary anticancer component. Among these compounds, diallyl disulfide (DADS), a major component of the secondary metabolites derived from garlic, has been well documented as a potent compound in prevention of cancer, genotoxicity, nephrotoxicity, urotoxicity, and hepatotoxicity (Nakagawa et al., 2001; Guyonnet et al., 2002; Pedraza-Chaverrí et al., 2003; Fukao et al., 2004; Kim et al., 2014; Lee et al., 2014). Accroding to our previous studies, DADS treatment results in increased antioxidant capacity and induction of antioxidant enzyme activities such as scavenging of ROS (Kim et al., 2014; Lee et al., 2014). Previous in vitro studies have also shown that DADS as a potential inhibitor revealed anti-inflammatory effect by down-regulation of NF-κB and MAPKs signaling pathways (Park et al., 2012; You et al., 2013). Furthermore, previous studies indicate that suppression of MAPKs and inactivation of NF-κB may be a useful therapy for attenuating the inflammatory responses (Koranteng et al., 2004; Zhou et al., 2008; Checker et al., 2009).
DADS has been confirmed to have a protective effect against CP-induced urotoxicity and oxidative stress (Manesh and Kuttan, 2002). Recently, we have demonstrated that the protective effects of DADS against CP-induced bladder toxicity possibly involve mechanisms related to its ability to induce antioxidant activity and antiapoptotic effect by activating Nrf-2-antioxidant response element pathway and block metabolic activation of CP by inhibiting CYP2C11 and inducing CYP3A1 (Kim et al., 2014). However, the role of DADS influencing NF-κB and MAPKs to regulate proinflammatory cytokines during urotoxicity is obscure. The present study investigated the protective effects of DADS against CP-induced HC in male rats. In order to better understand the protection mechanism of DADS, potential effects of DADS on serum proinflammatory cytokines, protein expression of NF-κB, iNOS, COX-2, TNF-α, and MAPKs, oxidative damage, and urinary nitrite-nitrate (NOx), malondialdehyde (MDA), and 8-hydroxy-2’-deoxyguanosine (8-OHdG) were also assessed.
MATERIALS AND METHODS
Animals and environmental conditions
Male Sprague-Dawley rats aged 12 weeks were obtained from a specific pathogen-free colony at Samtako Co. (Osan, Republic of Korea) and used after one week of quarantine and acclimation. Two animals per cage were housed in a room maintained at a temperature of 23 ± 3°C and a relative humidity of 50 ± 10% with artificial lighting from 08:00 to 20:00 and with 13 to 18 air changes per hour. Commercial rodent chow (Samyang Feed, Wonju, Republic of Korea) sterilized by radiation and sterilized tap water were available ad libitum. The Institutional Animal Care and Use Committee of Chonnam National University approved the protocols for the animal study, and the animals were cared for in accordance with the Guidelines for Animal Experiments of Chonnam National University.
Test chemicals and treatment
CP was purchased from Sigma Aldrich Co. (St. Louis, MO, USA). DADS was purchased from Tokyo Kasei Chemical Co. (Tokyo, Japan). All other chemicals were of the highest grade commercially available. CP and DADS were dissolved in saline and corn oil, respectively, and were prepared immediately before treatment. The daily application volumes of CP (2 ml/kg body weight) and DADS (5 ml/kg body weight) were calculated in advance based on the most recently recorded body weight of the individual animal. DADS was gavaged to rats once daily for a period of 5 days at 100 mg/kg/day. One hour after the final DADS treatment, the rats were given a single intraperitoneal dose of CP (100 mg/kg/day). All of the animals were sacrificed 12 h after administration of CP.
Experimental groups and dose selection
A total of 24 healthy male rats were randomly assigned to four experimental groups as follows: (1) vehicle control, (2) CP, (3) CP&DADS, and (4) DADS (n = 6 per group). The dose of CP and DADS was based on earlier studies (Kim et al., 2014; Lee et al., 2014).
NO metabolites, 8-OHdG, and MDA in urine
Urine samples were collected in metabolic cages for 2 h just before killing and frozen at −80°C until analysis. MDA and NOx levels in urine were measured by an enzyme-linked immunosorbent assay (ELISA) kit (Cayman, Ann Arbor, MI, USA) according to manufacturer’s instructions. NOx and MDA levels were expressed as micromoles. Urinary 8-OHdG levels were determined by a sandwich ELISA kit (Cayman). The concentration of creatinine in the urine samples was determined using a creatinine kit purchased from Cayman chemicals. Data were shown as the ratio of 8-OHdG to creatinine (Inano and Onoda, 2002).
Necropsy and tissue preparation
After 12 h of HC induction, all male rats were euthanized by carbon dioxide inhalation for blood sample collection. Serum samples were collected by centrifugation at 1,600×g for 10 min and stored in the −80°C freezer before they were analyzed. After bleeding, the urinary bladders were removed. The bladders were cut into two equal pieces from dome to bottom. Half of the bladder was fixed for 24 h in 10% buffered form-aldehyde for histopathological evaluation while the remainder was stored at −80°C to measure bladder oxidative damage and Western blot analysis.
Cytokines assay
Highly specific quantitative sandwich ELISA kits for IL-1β, IL-6, and TNF-α were purchased from Thermo Fisher Scientific (Rockford, IL, USA).
Histopathological examination
The bladders were routinely processed, embedded in paraffin and sectioned at 4-μm thickness, deparaffinized, and rehydrated using standard techniques. The sections were stained with hematoxylin-eosin stain for microscopic examination. All sections were examined with a light microscope by a pathologist blinded to the sample treatments.
Determination of lipid peroxidation (LPO), glutathione (GSH), and antioxidant enzymes
The weighed frozen bladder was homogenized in a glass-Teflon homogenizer with 50 mM phosphate buffer (pH 7.4) to obtain 1:9 (w/v) whole homogenate. The homogenates were then centrifuged at 11,000×g for 15 min at 4°C to discard any cell debris, and the supernatant was used for the measurement of MDA, reduced GSH concentrations and activities of glutathione reductase (GR) and glutathione-S-transferase (GST). The concentration of MDA was assayed by monitoring thiobarbituric acid reactive substance formation by the method of Berton et al. (1998). GSH content was measured by the method of Moron et al. (1979). The activities of antioxidant enzymes including GST (Habig et al., 1984), and GR (Carlberg and Mannervik, 1986) were also determined according to previously documented procedures. Total protein concentrations were determined by Bradford’s method (1976), using bovine serum albumin as the standard.
Western blot analysis
The frozen bladder tissues were lysed with a RIPA lysis buffer (Cell Signaling Technology, Lexington, KY, USA), and were centrifuged at 12,000×g at 4°C for 10 min to obtain the cellular proteins in the supernatant. The supernatant of bladder tissues separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA), and blocked in blocking buffer (150 mM NaCl in 10 mM Tris, pH 7.5 containing 5% non-fat dry milk) for 1 h at room temperature. The membranes were incubated with primary antibodies for 18 h at 4°C, washed three times (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, and 0.1% Tween 20), incubated with horse-radish peroxidase-conjugated secondary antibodies (1:5,000, Thermo Fisher Scientific) for 1 h at room temperature, washed three times, and then detected with enhanced chemiluminescence method (Supersignal West Pico, Pierce, USA). Antibodies against β-actin, NF-κB, iNOS, COX-2, TNF-α, ERK 1/2, p-ERK 1/2, p38, p-p38, JNK, and p-JNK (1:1,000) were purchased from Abcam (Cambridge, MA, USA) and Cell Signaling Technology. The protein concentration was determined by BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
Statistical analyses
The results are expressed as mean ± SD, and all statistical comparisons were made by means of one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Differences with a p-value of 0.05 or lower were considered to be statistically significant.
RESULTS
Effects of DADS on bladder histopathology
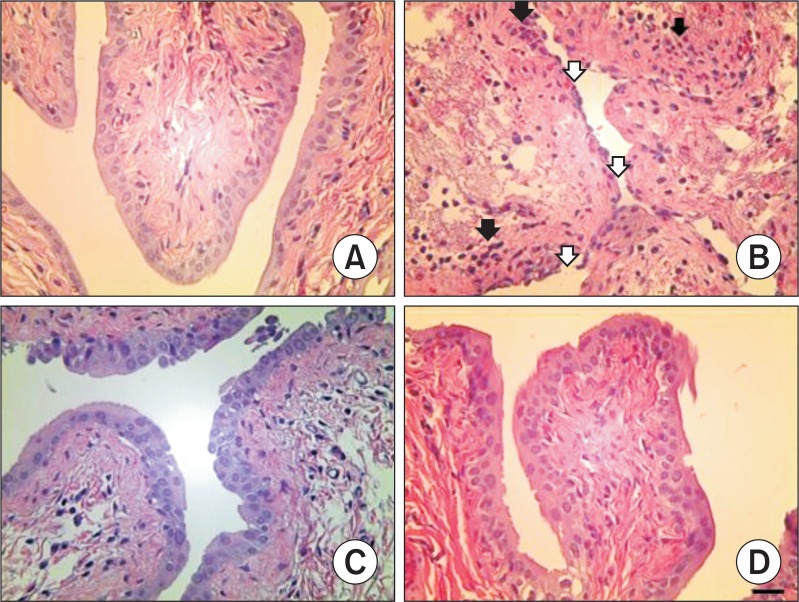
The results of histopathological examination are shown in Fig. 1. The control and DADS groups presented bladders with normal architecture (Fig. 1A, D). In the CP group, however, bladder tissues showed inflammatory cells infiltration and epithelial ulceration (Fig. 1B). Although these findings were also observed in the CP&DADS group (Fig. 1C), the incidence and severity of histopathological lesions decreased compared with those in the CP group.
Fig. 1.
Representative photographs of bladder sections treated with CP and/or DADS. Bladder from vehicle control (A) and DADS treated (D) rats showing normal appearance. However, bladder from a CP treated rat (B) showing inflammatory cells infiltration (black arrow) and epithelial ulceration (white arrow). Bladder from a CP&DADS treated rat (C) showing mild inflammatory cells infiltration. H&E stain. Bar=40 μm.
Effects of DADS on serum cytokines
The results of the serum cytokines are presented in Fig. 2. The levels of IL-1β, IL-6, and TNF-α in the CP group increased significantly compared with those in the control group. In contrast, the levels of IL-1β and IL-6 in the CP&DADS group decreased significantly compared with those in the CP group. The TNF-α level in the CP&DADS group was also slightly lower than that in the CP group but the difference was not significant.
Fig. 2.
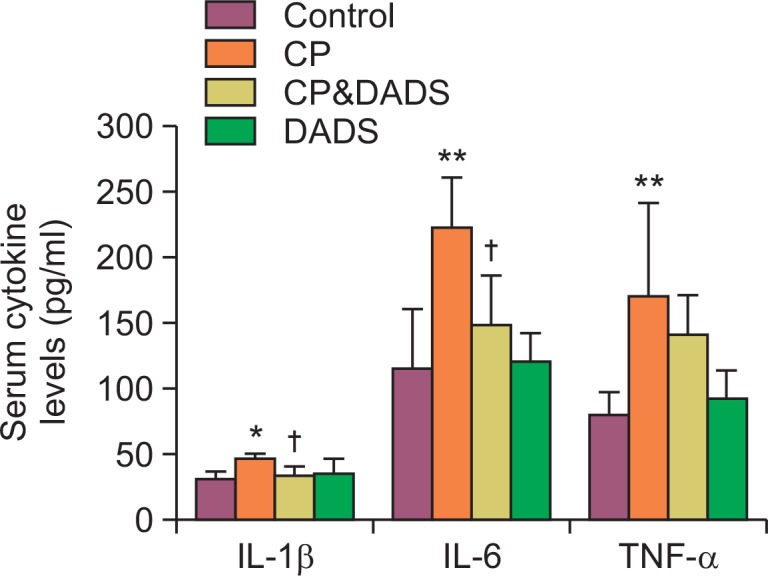
Effects of DADS on CP-induced changes in serum IL-1β, IL-6, and TNF-α levels. Statistical analysis was performed using one-way ANOVA followed by the Tukey’s multiple comparison test. Data are expressed as means ± SD (n=6). *p<0.05 compared with the control group. **p<0.01 compared with the control group. †p<0.05 compared with the CP group.
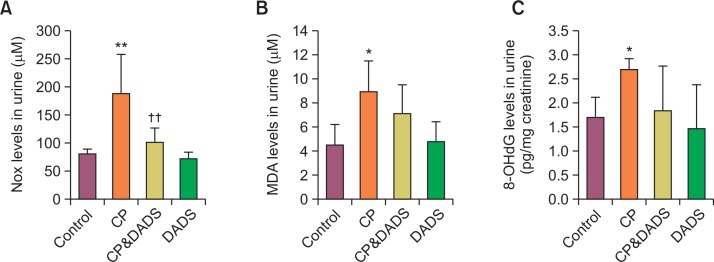
Effects of DADS on urinary NO metabolites, 8-OHdG, and MDA levels
Fig. 3A shows the effects of DADS on CP-induced changes in urinary NOx levels. The CP treatment caused a significant increase in the urinary NOx level when compared with that in the control group. In contrast, the urinary NOx level in the CP&DADS group decreased significantly compared with that in the CP group. To evaluate the extent of oxidative stress in the rats, urinary MDA (Fig. 3B), a sensitive indicator of LPO, and urinary 8-OHdG (Fig. 3C), a sensitive indicator of oxidative DNA damage, were determined. The CP treatment caused significant increase in the urinary MDA and 8-OHdG levels when compared with those in the control group. Although no significant differences were observed between the groups, the urinary MDA and 8-OHdG levels in the CP&DADS group were lower than those in the CP group.
Fig. 3.
Effects of DADS on CP-induced changes in urine NOx (A), MDA (B), and 8-OHdG (C) levels. Statistical analysis was performed using one-way ANOVA followed by the Tukey’s multiple comparison test. Data are expressed as means ± SD (n=6). *p<0.05 compared with the control group. **p<0.01 compared with the control group. ††p<0.01 compared with the CP group.
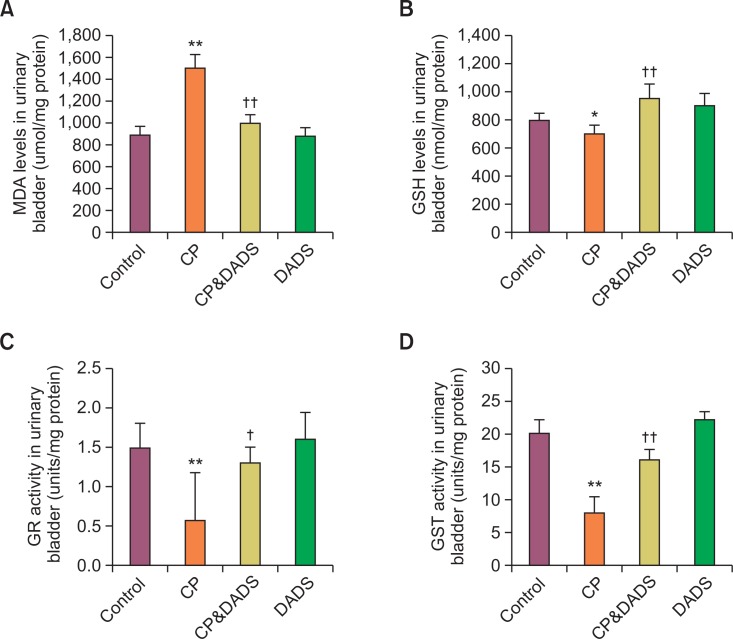
Effects of DADS on oxidative damage in urinary bladder
The MDA and GSH concentrations and the antioxidant enzymes activities in bladder tissues are presented in Fig. 4. The concentration of MDA increased significantly and GSH content and GR and GST activities decreased significantly in the CP group when compared with those in the control group. In contrast, the concentration of MDA in the CP&DADS group decreased significantly compared with that in the CP group, whereas the GSH content and GR and GST activities increased significantly.
Fig. 4.
Antioxidant enzymes, GSH, and LPO in the urinary bladder of male rats treated with CP and/or DADS. Statistical analysis was performed using one-way ANOVA followed by the Tukey’s multiple comparison test. Data are expressed as means ± SD (n=6). *p<0.05 compared with the control group. **p<0.01 compared with the control group. †p<0.05 compared with the CP group. ††p<0.01 compared with the CP group.
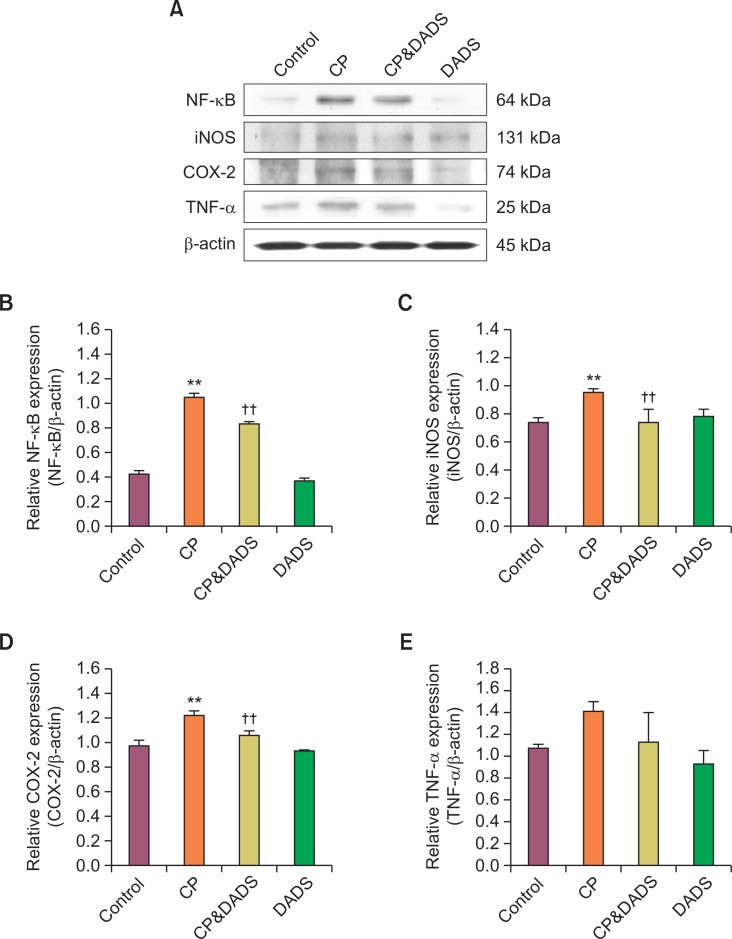
Effects of DADS on NF-κB, iNOS, COX-2, and TNF-α protein expression
The protein expression of NF-κB, iNOS, COX-2, and TNF-α is shown in Fig. 5. The expression levels of NF-κB, iNOS, and COX-2 in the CP group increased significantly compared with those in the control group. The TNF-α expression in the CP group was also slightly higher than that in the control group but the difference was not significant between the groups. In contrast, the expression levels of NF-κB, iNOS, and COX-2 in the CP&DADS group decreased significantly compared with those in the CP group. Although no significant difference was observed between the groups, the expression level of TNF-α in the CP&DADS group was lower than that in the CP group.
Fig. 5.
(A) Western blot analysis of NF-κB, iNOS, COX-2, and TNF-α expression in the urinary bladder of male rats treated with CP and/or DADS. Detection of β-actin expression was used a loading control. The bar graphs show quantitative relative levels of NF-κB (B), iNOS (C), COX-2 (D), and TNF-α (E) protein expression for vehicle, CP, CP&DADS, and DADS-treated rats. Values are presented as means ± SD (n=3). **p<0.01 compared with the control group. ††p<0.01 compared with the CP group.
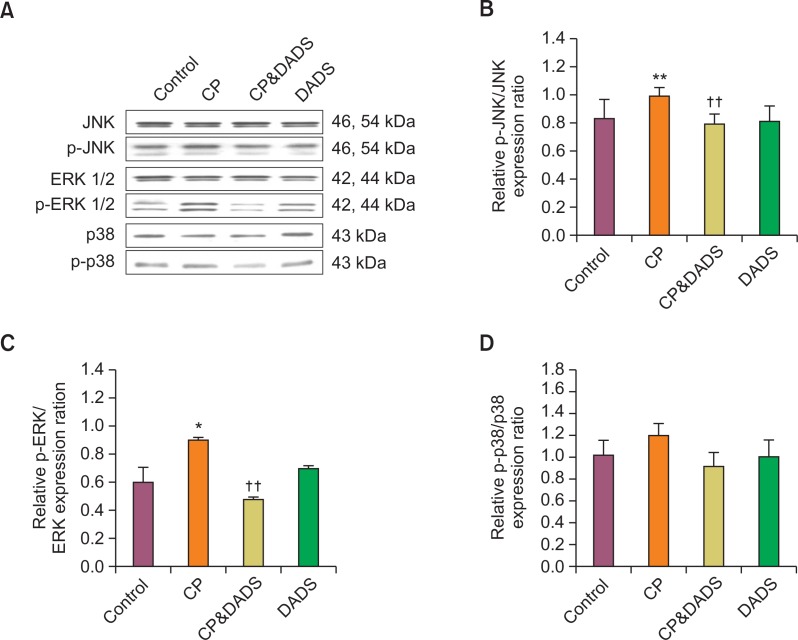
Effects of DADS on MAPKs protein expression
The protein expression of p-JNK, p-ERK, and p-p38 is shown in Fig. 6. The protein expression levels of p-ERK and p-JNK in the CP group increased significantly compared with those in the control group, but only slightly increased p-p38. However, the protein expression levels of p-ERK and p-JNK in the CP&DADS group decreased significantly compared with those in the CP group.
Fig. 6.
(A) Western blot analysis of p-JNK, p-ERK, and p-p38 expression in the urinary bladder of male rats treated with CP and/or DADS. The bar graphs show quantitative relative levels of p-JNK (B), p-ERK (C), and p-p38 (D) protein expression for vehicle, CP, CP&DADS, and DADS-treated rats. Values are presented as means ± SD (n=3). *p<0.05 compared with the control group. **p<0.01 compared with the control group. ††p<0.01 compared with the CP group.
DISCUSSION
It has been reported that DADS has a number of beneficial effects against various pathological conditions caused by oxidative stress and inflammation (Pedraza-Chaverrí et al., 2003; Chiang et al., 2006; Kim et al., 2014; Lee et al., 2014). The results obtained in this study showed that DADS prevents CP-induced HC and that the protective effects of DADS may be due to its ability to regulate proinflammatory cytokines production by inhibition of NF-κB and MAPKs expressions, and its potent anti-oxidative capability through reduction of oxidative DNA damage in the bladder.
The side effect of HC was not directly induced by CP, but its urinary metabolite, acrolein, indeed caused the urotoxicity (Kurowski and Wagner, 1997). Acrolein is the most reactive of the α,β-unsaturated aldehydes and can deplete the concentration of GSH (Huang et al., 2013). It has been demonstrated that CP treatment results in the production of ROS, which cause oxidative stress to urinary bladder (Bhatia et al., 2006; Tripathi and Jena, 2010; Kim et al., 2014). In the present study, CP treatment produced high levels of oxidative damage, as evidenced by a significant elevation in bladder MDA concentration and a significant decrease in GSH content, as well as GR and GST activities, which suggest a role for oxidative stress in CP induced HC. However, DADS caused a significant decrease in the MDA concentration and efficiently improved the CP-induced depletion of GSH concentration and suppression of GR and GST activities in the urinary bladder. Previous studies have also demonstrated that DADS treatment results in increased GSH content and modulation of enzymic and non-enzymic antioxidant activities (Kim et al., 2014; Lee et al., 2014). This apparent ameliorative effect might be due to the ability of DADS to protect against LPO and oxidative stress induced by acrolein.
8-OHdG is produced by the oxidative damage of DNA by ROS and reactive nitrogen species and serves as an established marker of oxidative stress (Beckman and Ames, 1997; Hamada et al., 2007). Hydroxylation of guanosine occurs in response to both normal metabolic processes and a variety of environmental factors. Increased levels of 8-OHdG are associated with the aging process as well as with a number of pathological conditions including cancer, diabetes, radiation, and hypertension (Inano and Onoda, 2002; Hamada et al., 2007; Shen et al., 2007). Moreover, urinary MDA and 8-OHdG levels indicate the extent of LPO, thereby serving as an index of oxidative stress (Inano and Onoda, 2002; Kang et al., 2013). As expected, CP treatment resulted in an increase in urinary MDA and 8-OHdG levels. However, DADS prevented increase in urinary MDA and 8-OHdG levels induced by CP. These changes were accompanied by a reduction in oxidative damage in the urinary bladder.
Nitric oxide (NO) is a highly reactive free radical which has emerged as a potent biological mediator (Martínez-Ruiz et al., 2011). Increasing evidence indicates that NO is involved in acute and chronic inflammation (Korhonen et al., 2005). NO is synthesized from L-arginine, with the formation of stoichiometric amounts of L-citrulline, by three NO synthase (NOS) isoforms: endothelial NOS, neuronal NOS, and iNOS. It is reported that cytokines and NO produced by iNOS were involved in CP-induced HC (Korkmaz et al., 2003; Oter et al., 2004). NF-κB stimulates the expression of iNOS, with an increase in NO formation (Szabó and Billiar, 1999). The COX-2 expression and subsequent production of prostaglandin were closely related to the generation of NO radicals (Pang and Hoult, 1997; Posadas et al., 2000). Therefore, agents that inhibit NF-κB, resulting in decreased iNOS and COX-2 expressions and NO generation, may have beneficial therapeutic effects in the treatment of inflammatory diseases. In this study, we hypothesized that blocking NF-κB would be an effective approach to prevent CP-induced HC. As expected, CP caused a significant increase in NF-κB, iNOS, and COX-2 expression levels and, suggesting that activation of NF-κB, iNOS, and COX-2 by CP might play a role in CP-induced HC. However, DADS pretreatment inhibited CP-induced NF-κB, iNOS, and COX-2 expressions accompanied by a reduction in urine NOx levels. These results suggest that the effects of DADS on the production of NO are at least partially mediated by suppression of NF-κB signaling. Previous studies also showed that DADS inhibited iNOS and COX-2 expressions and NO production (Chiang et al., 2006; You et al., 2013).
CP is a potent activator of the MAPK pathway, which is the other major extracellular signal transduction pathway. Once activated, MAPKs modulate the functional responses of cells through phosphorylation of transcription factors and activation of other kinases (Zhang and Dong, 2007). An in vitro study reported that acrolein-induced Chinese hamster ovary cell apoptosis is ERK and p38 dependent (Tanel and Averill-Bates, 2007). Evidences also confirmed that CP-induced cystitis leads to ERK activation in urinary bladder (Corrow and Vizzard, 2007). Chung et al. (2010) found that nerve growth factor induced bladder inflammation and cystitis with the activation of JNK and ERK but not p38 MAPK. In the present study, CP simultaneously activated JNK and ERK but not p38 MAPK in urinary bladder. However, DADS inhibited CP-stimulated phosphorylation of ERK and JNK. A previous study reported that DADS pretreatment markedly inhibited phosphorylation of MAPKs and production of downstream proinflammatory cytokine in lipopolysaccharide (LPS)-stimulated BV2 microglia (Park et al., 2012). Therefore, these results suggest that the antiinflammatory effects were due to inhibition of the ERK and JNK signaling pathway.
Cytokines, a large group of soluble extracellular proteins or glycoproteins, are key intercellular regulators and mobilizers and they are now seen to be crucial to innate and adaptive inflammatory responses. Modulation of cytokine secretion may offer novel approaches in the treatment of a variety of diseases. One strategy in the modulation of cytokine expression may be through the use of herbal medicines (Spelman et al., 2006). Recent studies have demonstrated that CP-induced HC is not only due to the direct contact of acrolein with the bladder mucosa, but also involves proinflammatory cytokines such as TNF-α, IL-6, and IL-1β (Dantas et al., 2010; Hamsa and Kuttan, 2012). Proinflammatory cytokines promote inflammation, leukocyte infiltration, granuloma formation, and tissue fibrosis, and are thought to be initiators of cytokine related inflammation states by stimulating cytokine production. In the present study, the elevated levels of IL-1β, IL-6, and TNF-α, the principal cytokines that mediates acute inflammation after CP administration, were effectively ameliorated by DADS, indicating their protective effect against CP-induced inflammation. A previous study showed an inhibitory effect of DADS on the production of TNF-α and IL-1β in endotoxin-stimulated whole blood culture (Keiss et al., 2003). Additionally, the decreased levels of TNF-α and IL-1β by DADS treatment were previously observed in LPS-activated BV2 microglia (Park et al., 2012). Recently we have also demonstrated that DADS pretreatment results in decreased IL-6 and IL-1β levels against carbon tetrachloride-induced hepatotoxicity in rats (Lee et al., 2014).
In summary, our data show that DADS inhibits CP-induced urine NOx production by suppressing iNOS and COX-2 protein expressions in the urinary bladder. DADS also inhibits the production of proinflammatory cytokines by down-regulation of NF-κB and MAPKs pathways. These uroprotective effects are mediated by the antioxidative action through inhibition of LPO and increase of antioxidant enzymes activity, which is accompanied by reduction of urine MDA and 8-OHdG. Therefore, DADS could be used as an attractive and characteristic probe for studying inflammation and oxidative damage.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2010835). This work was also supported by a grant from the KRIBB Research Initiative program. The animal experiment in this study was supported by the Animal Medical Institute of Chonnam National University.
REFERENCES
- Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–19666. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- Berton TR, Conti CJ, Mitchell DL, Aldaz CM, Lubet RA, Fischer SM. The effect of vitamin E acetate on ultraviolet-induced mouse skin carcinogenesis. Mol Carcinog. 1998;23:175–184. doi: 10.1002/(SICI)1098-2744(199811)23:3<175::AID-MC6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bhatia K, Kaur M, Atif F, Ali M, Rehman H, Rahman S, Raisuddin S. Aqueous extract of Trigonella foenum-grae- cum L. ameliorates additive urotoxicity of buthionine sulfoximine and cyclophosphamide in mice. Food Chem Toxicol. 2006;44:1744–1750. doi: 10.1016/j.fct.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. Reduction of 2,4,6-trinitrobenzenesulfonate by glutathione reductase and the effect of NADP+ on the electron transfer. J Biol Chem. 1986;261:1629–1635. [PubMed] [Google Scholar]
- Checker R, Sharma D, Sandur SK, Khanam S, Poduval TB. Anti-inflammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. Int Immunopharmacol. 2009;9:949–958. doi: 10.1016/j.intimp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Chiang YH, Jen LN, Su HY, Lii CK, Sheen LY, Liu CT. Effects of garlic oil and two of its major organosulfur compounds, diallyl disulfide and diallyl trisulfide, on intestinal damage in rats injected with endotoxin. Toxicol Appl Pharmacol. 2006;213:46–54. doi: 10.1016/j.taap.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Chung CW, Zhang QL, Qiao LY. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J Biol Chem. 2010;285:4206–4212. doi: 10.1074/jbc.M109.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrow KA, Vizzard MA. Phosphorylation of extra-cellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R125–134. doi: 10.1152/ajpregu.00857.2006. [DOI] [PubMed] [Google Scholar]
- Cuesta S, Kireev R, Forman K, García C, Escames G, Ariznavarreta C, Vara E, Tresguerres JA. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8) Exp Gerontol. 2010;45:950–956. doi: 10.1016/j.exger.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Dantas AC, Batista-Júnior FF, Macedo LF, Mendes MN, Azevedo IM, Medeiros AC. Protective effect of simvastatin in the cyclophosphamide-induced hemorrhagic cystitis in rats. Acta Cir Bras. 2010;25:43–46. doi: 10.1590/S0102-86502010000100011. [DOI] [PubMed] [Google Scholar]
- Fukao T, Hosono T, Misawa S, Seki T, Ariga T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem Toxicol. 2004;42:743–749. doi: 10.1016/j.fct.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Mechanisms of protection against aflatoxin B1 genotoxicity in rats treated by organosulfur compounds from garlic. Carcinogenesis. 2002;23:1335–1341. doi: 10.1093/carcin/23.8.1335. [DOI] [PubMed] [Google Scholar]
- Habig WH, Jakoby WB, Guthenberg C, Mannervik B, Vander Jagt DL. 2-Propylthiouracil does not replace glutathione for the glutathione transferases. J Biol Chem. 1984;259:7409–7410. [PubMed] [Google Scholar]
- Hamada Y, Miyata S, Nii-Kono T, Kitazawa R, Kitazawa S, Higo S, Fukunaga M, Ueyama S, Nakamura H, Yodoi J, Fukagawa M, Kasuga M. Overexpression of thioredoxin1 in transgenic mice suppresses development of diabetic nephropathy. Nephrol Dial Transplant. 2007;22:1547–1557. doi: 10.1093/ndt/gfm099. [DOI] [PubMed] [Google Scholar]
- Hamsa TP, Kuttan G. Tinospora cordifolia ameliorates urotoxic effect of cyclophosphamide by modulating GSH and cytokine levels. Exp Toxicol Pathol. 2012;64:307–314. doi: 10.1016/j.etp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hommes DW, Peppelenbosch MP, van Deventer SJ. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52:144–151. doi: 10.1136/gut.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Jin M, Pi R, Zhang J, Chen M, Ouyang Y, Liu A, Chao X, Liu P, Liu J, Ramassamy C, Qin J. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci Lett. 2013;535:146–151. doi: 10.1016/j.neulet.2012.12.051. [DOI] [PubMed] [Google Scholar]
- Inano H, Onoda M. Radioprotective action of curcumin extracted from Curcuma longa LINN: inhibitory effect on formation of urinary 8-hydroxy-2’-deoxyguanosine, tumorigenesis, but not mortality, induced by gamma-ray irradiation. Int J Radiat Oncol Biol Phys. 2002;53:735–743. doi: 10.1016/S0360-3016(02)02794-3. [DOI] [PubMed] [Google Scholar]
- Jurado JM, Sénchez A, Pajares B, Pérez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–586. doi: 10.1007/s12094-008-0254-7. [DOI] [PubMed] [Google Scholar]
- Kang S, Kim S, Park J, Kim HJ, Lee J, Choi G, Choi S, Kim S, Kim SY, Moon HB, Kim S, Kho YL, Choi K. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 2013;461–2:214–221. doi: 10.1016/j.scitotenv.2013.04.097. [DOI] [PubMed] [Google Scholar]
- Keiss HP, Dirsch VM, Hartung T, Haffner T, Trueman L, Auger J, Kahane R, Vollmar AM. Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr. 2003;133:2171–2175. doi: 10.1093/jn/133.7.2171. [DOI] [PubMed] [Google Scholar]
- Kim HG, Yoon DH, Lee WH, Han SK, Shrestha B, Kim CH, Lim MH, Chang W, Lim S, Choi S, Song WO, Sung JM, Hwang KC, Kim TW. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee IC, Baek HS, Shin IS, Moon C, Bae CS, Kim SH, Kim JC, Kim HC. Mechanism for the protective effect of diallyl disulfide against cyclophosphamide acute urotoxicity in rats. Food Chem Toxicol. 2014;64:110–118. doi: 10.1016/j.fct.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Kiuchi H, Takao T, Yamamoto K, Nakayama J, Miyagawa Y, Tsujimura A, Nonomura N, Okuyama A. Sesquiterpene lactone parthenolide ameliorates bladder inflammation and bladder overactivity in cyclophosphamide induced rat cystitis model by inhibiting nuclear factor-kappaB phosphorylation. J Urol. 2009;18:2339–2348. doi: 10.1016/j.juro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Koranteng RD, Swindle EJ, Davis BJ, Dearman RJ, Kimber I, Flanagan BF, Coleman JW. Differential regulation of mast cell cytokines by both dexamethasone and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580. Clin Exp Immunol. 2004;137:81–87. doi: 10.1111/j.1365-2249.2004.02510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Oter S, Deveci S, Ozgurtas T, Topal T, Sadir S, Bilgic H. Involvement of nitric oxide and hyperbaric oxygen in the pathogenesis of cyclophosphamide induced hemorrhagic cystitis in rats. J Urol. 2003;170:2498–2502. doi: 10.1097/01.ju.0000085593.31396.d8. [DOI] [PubMed] [Google Scholar]
- Kurowski V, Wagner T. Urinary excretion of ifosfamide, 4-hydroxyifosfamide, 3- and 2-dechloroethylifosfamide, mesna, and dimesna in patients on fractionated intravenous ifosfamide and concomitant mesna therapy. Cancer Chemother Pharmacol. 1997;39:431–439. doi: 10.1007/s002800050594. [DOI] [PubMed] [Google Scholar]
- Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174–185. doi: 10.1016/j.fct.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Manesh C, Kuttan G. Alleviation of cyclophosphamide-induced urotoxicity by naturally occurring sulphur compounds. J Exp Clin Cancer Res. 2002;21:509–517. [PubMed] [Google Scholar]
- Martínez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, Tsubura A. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;22:891–897. doi: 10.1093/carcin/22.6.891. [DOI] [PubMed] [Google Scholar]
- Oter S, Korkmaz A, Oztas E, Yildirim I, Topal T, Bilgic H. Inducible nitric oxide synthase inhibition in cyclophosphamide induced hemorrhagic cystitis in rats. Urol Res. 2004;32:185–189. doi: 10.1007/s00240-003-0398-y. [DOI] [PubMed] [Google Scholar]
- Pang L, Hoult JR. Repression of inducible nitric oxide synthase and cyclooxygenase-2 by prostaglandin E2 and other cyclic AMP stimulants in J774 macrophages. Biochem Pharmacol. 1997;53:493–500. doi: 10.1016/S0006-2952(96)00737-X. [DOI] [PubMed] [Google Scholar]
- Park HY, Kim ND, Kim GY, Hwang HJ, Kim BW, Kim WJ, Choi YH. Inhibitory effects of diallyl disulfide on the production of inflammatory mediators and cytokines in lipopolysaccharide-activated BV2 microglia. Toxicol Appl Pharmacol. 2012;262:177–184. doi: 10.1016/j.taap.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Pedraza-Chaverrí J, González-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003;473:71–78. doi: 10.1016/S0014-2999(03)01948-4. [DOI] [PubMed] [Google Scholar]
- Posadas I, Terencio MC, Guillén I, Ferrándiz ML, Coloma J, Payá M, Alcaraz MJ. Co-regulation between cyclooxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:98–106. doi: 10.1007/s002109900150. [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Deininger P, Hunt JD, Zhao H. 8-Hydroxy-2′-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007;109:574–580. doi: 10.1002/cncr.22417. [DOI] [PubMed] [Google Scholar]
- Song J, Liu L, Li L, Liu J, Song E, Song Y. Protective effects of lipoic acid and mesna on cyclophosphamide-induced haemorrhagic cystitis in mice. Cell Biochem Funct. 2014;32:125–132. doi: 10.1002/cbf.2978. [DOI] [PubMed] [Google Scholar]
- Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006;11:128–150. [PubMed] [Google Scholar]
- Surh YJ. NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr. 2008;17(S1):269–272. [PubMed] [Google Scholar]
- Szabó C, Billiar TR. Novel roles of nitric oxide in hemorrhagic shock. Shock. 1999;12:1–9. doi: 10.1097/00024382-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Tanel A, Averill-Bates DA. P38 and ERK mitogen-activated protein kinases mediate acrolein-induced apoptosis in Chinese hamster ovary cells. Cell Signal. 2007;19:968–977. doi: 10.1016/j.cellsig.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Tripathi DN, Jena GB. Effect of melatonin on the expression of Nrf2 and NF-kappaB during cyclophosphamide-induced urinary bladder injury in rat. J Pineal Res. 2010;48:324–331. doi: 10.1111/j.1600-079X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- You S, Nakanishi E, Kuwata H, Chen J, Nakasone Y, He X, He J, Liu X, Zhang S, Zhang B, Hou DX. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol Nutr Food Res. 2013;57:2049–2060. doi: 10.1002/mnfr.201200843. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong C. Regulatory mechanisms of mitogen-activated kinase signaling. Cell Mol Life Sci. 2007;64:2771–2789. doi: 10.1007/s00018-007-7012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K, Kang SS, Zou LB, Kim YS. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586:340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]