Abstract
We tested the hypothesis that micro-computed tomography (micro-CT) analysis provides a better quantitative readout of the therapeutic potential of methotrexate (MTX) for treating collagen-induced arthritis (CIA) in rats and compared to conventional histopathological examination. Rats were divided into three groups: Group 1 (G1) was treated with 0.9% saline, whereas groups 2 (G2) and 3 (G3) were boosted with type II collagen at days 0 and 7. Following the first collagen immunization, rats in G1 and G2 were treated with 0.9% saline and those in G3 were treated with 1.5 mg/kg MTX from day 14 to 28. All rats were sacrificed on day 28, at which point and all hind knee joints were analyzed by micro-CT and histopathological examination. Micro-CT analyses showed that bone volume and trabecular number were significantly decreased in G2 and G3 compared to G1 (p<0.01), as was percent bone volume (p<0.05 and p<0.01, respectively). However, bone surface/bone volume was significantly increased in G2 and G3 compared to G1 (p<0.05 and p<0.01, respectively). Trabecular separation was significantly increased in G3 compared to G1 (p<0.05). Histopathological examination showed that knee joints of rats in G2 and G3 showed severe joint destruction with inflammatory cell infiltration. However, cartilage destruction was slightly reduced in G3 compared to G2. Taken together, these results suggest that MTX treatment reduced cartilage destruction in rats with CIA, and micro-CT analyses made it possible to quantify arthritic bony lesion.
Keywords: Arthritis, Collagen, Micro-computed tomography, Histopathology
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease caused by immune reactivity that results in joint destruction and chronic pain (Scott et al., 2010). RA therapeutics cause a reduction in joint inflammation, recover erosive damage, and improve the patient’s quality of life (Scott, 2012).
Autoimmune arthritis is induced by immunization with an emulsion of complete Freund’s adjuvant and type II collagen (Brand et al., 2007). Immunization of rodents with type II collagen induces a high incidence of collagen-induced arthritis (CIA) (Myers et al., 1997), that involves macrophages (Maruotti et al., 2007) and neutrophils, as well as CD4+ T cells and CD8+ T cells (Bevaart et al., 2010; Carvalheiro et al., 2013). Because the rat CIA model shares many similarities with that of human, this model is useful for addressing the effects of compounds in the late, chronic stage of arthritis (Bevaart et al., 2010).
Oral administration of methotrexate (MTX) is widely used method of treating arthritis (Weinblatt et al., 1991; Weinblatt et al., 1992). MTX treatment is able to slow the rate of joint destruction through its inhibitory effects on the cascade of events initiated by inflammatory cytokines and subsequent joint-destructive enzymes (Perkins et al., 1998; Mello et al., 2000), and may improve bone mass and turnover in the arthritic rat, in which several cytokines affecting bone cells are involved (Segawa et al., 1997). However, quantitative assays of the therapeutic potential of MTX have not been fully assessed.
To assess arthritis in joint lesions, the combination of advanced imaging with three-dimensional systems and intravital animal models may provide more informative and disease-relevant platforms (Conway et al., 2014). Furthermore, by enabling longitudinal studies, imaging of living animals allows continuous, dynamic, and sometimes nearly instantaneous identification and quantification of disease progression (Koba et al., 2013). Such imaging techniques, including micro-computed tomography (micro-CT), are advantageous for taking anatomical images of animals in high resolution (Schambach et al., 2010).
We tested the hypothesis that micro-CT analysis provided a better quantitative readout of the therapeutic potential of MTX in a rat CIA model and compared to conventional histopathological examination.
MATERIALS AND METHODS
Collagen-induced arthritis
Twenty-four female Wistar rats (8 weeks old) were obtained from Orient Bio (Kapyung, Korea) and acclimated for 7 days prior to the study. Rats were maintained in a temperature-controlled environment (22 ± 3°C), 55 ± 5% relative humidity under a 12-h light/dark cycle. Rats were fed a rodent diet and filtered water ad libitum.
Rats were randomly divided into three groups. Rats in group 1 (G1) were treated with 0.9% saline and those in groups 2 (G2) and 3 (G3) were boosted with type II collagen at days 0 and 7 to induce arthritis. Briefly, bovine type II collagen (Chondrex, Redmond, WA, USA) was dissolved in 0.01 M acetic acid overnight at 4°C. Then the mixture was emulsified in an equal volume of incomplete Freund’s adjuvant (Chondrex). Rats in G2 and G3 were immunized intradermally at the tail base with 0.1 mL of emulsion containing 100 μg type II collagen. From day 14 to 28, rats in G1 and G2 were treated orally with 0.9% saline, and those in G3 were treated orally with 1.5 mg/kg MTX. All rats were sacrificed on day 28 after the first collagen immunization, and all hind knee joints were fixed in 10% neutral phosphate-buffered formalin. Then, micro-CT and histopathological analyses were performed. This study was approved by the animal experiment committee of Namseoul University based on the Animal Protection Act.
Micro-CT analysis
Quantitative analysis of hind knee joints was performed using a micro-CT system (Skyscan 1172, Bruker, Kontich, Belgium). Specimens were scanned using micro-CT with an X-ray source of 40 kV/250 μV, pixel size 23 μm, and a 0.5 mm aluminum filter. After scanning, cross-sectional slices were reconstructed and each scan result was reconstructed using the 0–0.14 threshold values to distinguish bone from air.
Three-dimensional analysis was performed using CTAn software (Bruker). The fraction of bone volume, percent bone volume, trabecular number, trabecular thickness, bone surface/bone volume and trabecular separation were performed using the built-in software. In addition, osteophytes within each contiguous coronal image section were manually outlined and their volumes were calculated using CTvol software (Bruker).
Histopathological observation
Hind knee joints were fixed in 10% phosphate-buffered formalin for 24 h and decalcified in 14% EDTA-glycerol for 14 days at room temperature. Samples were routinely processed and embedded in paraffin, and 4 μm sections were stained with hematoxylin and eosin (H&E) for histopathological examination. For cartilage staining, safranin O-fast green was used on hind knee joints. Histopathological analysis of joints was performed to examine infiltration of inflammatory cells, cartilage degradation, bone destruction and synovial proliferation.
Immunohistochemical analysis of MMP-13
The avidin-biotin complex method was used to stain matrix metalloproteinase (MMP) in 4-μm joint sections. Sections were dewaxed with xylene and hydrated through a graded ethanol series, and incubated in hyaluronase (H3506, Sigma Chemical Co., St Louis, MO, USA) for 30 min. Then the sections were sequentially treated with 0.3% hydrogen peroxide, blocking serum, and anti-MMP-13 antibody (ab39012; 1:100; Abcam, Cambridge, MA, USA). Then they were washed with Tris-buffered saline containing Tween 20® (TBS-T) and subjected to ABC-peroxidase procedures (ABC Kit; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blocking serum, instead of primary antibody, was used as a negative control.
Immune complexes were visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB; D5637; Sigma-Aldrich, St. Louis, MO, USA) as the chromogen. Sections were counter-stained with Mayer’s hematoxylin to facilitate their examination under a light microscope.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). All data were analyzed using Dunnett’s multiple comparison test following one-way analysis of variance (ANOVA) and Student’s t-test, and were expressed as the mean ± standard deviation (SD). p-values <0.05 were considered statistically significant.
RESULTS
Micro-CT analysis
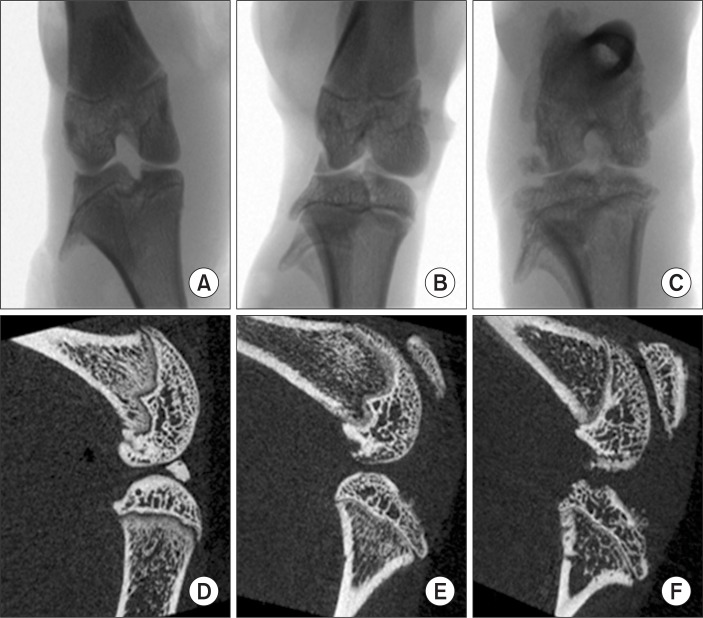
X-ray, coronal and sagittal micro-CT images showed a normal joint appearance was shown in G1 rats and severe joint destruction was observed in G2 animals. Moreover, this joint destruction was also observed in G3 rats, although no difference in arthritic lesions was obvious in G3 compared to G2 (Fig. 1).
Fig. 1.
X-ray, coronal and sagittal micro-CT image of hind knee joint in rats treated with collagen. Note the X-ray (A–C), sagittal images (D–F) for G1, G2 and G3, respectively. Note the normal appearance of joints for G1 animals and severe joint destruction in G2. Moreover, this joint destruction remained in G3 and there were no differences of lesions in G3 compared to G2.
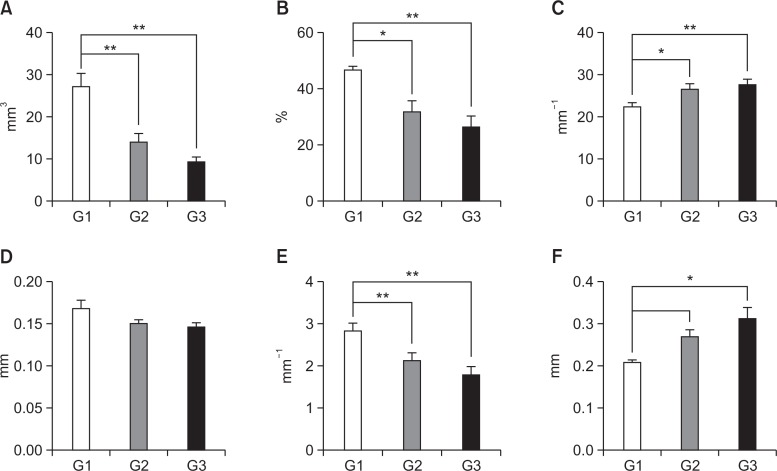
Micro-CT analysis revealed several altered bone parameters (Fig. 2). Bone volume (p<0.01) and percent bone volume (p<0.05, p<0.01, respectively) was significantly decreased in G2 and G3 rats compared to G1. However, bone surface/ bone volume was significantly increased in G2 and G3 rats compared to G1 (p<0.05 and p<0.01, respectively). Trabecular thickness was not significantly different in G2 and G3 rats compared to G1. Trabecular number was significantly decreased in G2 and G3 rats compared to G1 (p<0.01). Trabecular separation was significantly increased in G3 animals compared to G1 (p<0.05). However, none of the previous parameters were significantly different in G3 rats compared to G2.
Fig. 2.
Micro-CT analyses parameters of tibial trabecular bone in the control (G1), CIA +saline (G2) and CIA+MTX (G3). (A) bone volume, (B) percent bone volume, (C) bone surface/bone volume, (D) trabecular thickness, (E) trabecular number, and (F) trabecular separation. *,**Significantly different from G1 (p<0.05, p<0.01, respectively); Values represents shown as mean ± standard deviation.
Histopathological observation
No inflammation or tissue destruction was observed in G1. However, in G2 rats, knee joints showed severe joint destruction with inflammatory cell infiltration, and bone and enlarged cavities filled with synovial fibroblasts and inflammatory cells and these changes were also present in G3 animals. However, cartilage destruction was decreased in G3 rats compared to G2 (Fig. 3).
Fig. 3.
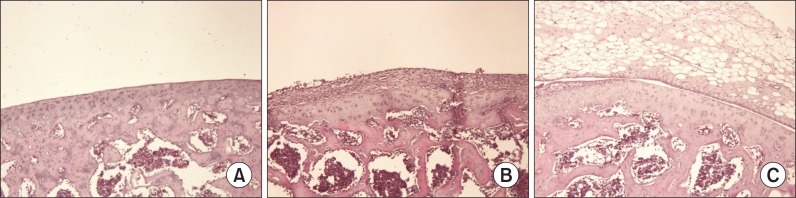
Histopathological examination of hind knee joint of female rats. Note the normal microscopic structure of the joint of control rats (A). However, cartilage destruction was remarkable in those treated with collagen in G2 (B). However, cartilage destruction was reduced in G3 animals (C). H&E staining of paraffin embedded sections from hind joints Magnification, ×200.
According to safranin O-fast green staining, normal cartilage structures of the joints were noted as red color in G1 animals. However, cartilage staining was remarkable reduced in G2. Although cartilage destruction was present in the joint surface, in G3 animals, safranin O-fast green staining was present around chondrocytes (Fig. 4).
Fig. 4.
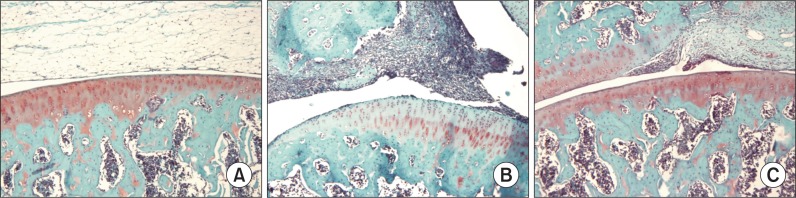
Safranin O-fast green staining of hind knee joint of female rats treated with collagen. Note the normal cartilage staining of the joint (red) in G1 animals (A), which is remarkably reduced in G2 rats (B). Also note that, in G3 animals, there was cartilage staining in joints of rats (C). Magnification, ×200.
Immunohistochemical analysis of MMP-13
With respect to MMP-13, there was no immunostaining in the joints of G1 animals. In G2 rats, MMP-13 was remarkably increased in the joint surface. However, MMP-13 in joint surface was decreased in G3 rats compared to G2 (Fig. 5).
Fig. 5.

Immunohistochemistry of MMP-13 in hind knee joint of female rats treated with collagen. Note the normal cartilage staining of the joint in G1 rats (A). However, MMP-13 staining was remarkably increased in the joint of G2 rats (B) and weakly stained in G3 (C). Magnification, ×200.
DISCUSSION
Micro-CT analysis provided several quantitative parameters for evaluating MTX treatment of RA. It showed that bone volume, percent bone volume, and trabecular number were significantly decreased, and bone surface/bone volume was increased in CIA lesions compared to the control. These results suggest that bony parameters are altered following collagen treatment. However, MTX treatment did not result in therapeutic advantages at these bony parameters. The main therapeutic action of methotrexate was related to the inhibitory effects on the cascade of events initiated by inflammatory cytokines and subsequent joint-destructive enzymes. It was reported that trabecular thickness values was significantly increased in the MTX-treated groups compared to that of the Mycobacterium-induced arthritic control group, however another bony parameters of MTX-treated groups were not significant difference compared to arthritic control group (Segawa et al., 1997). These data were similar to our results. Even though there were difference of animal species, animal model of arthritis and analysis instruments, we think the therapeutic potential of MTX was minimal in bony parameters.
To evaluate disease-specific regional variables of interest, such as bone volume fraction and joint space, a consistent and reliable spatial sampling method was developed. In general, micro-CT has an advantage for bone detection over other methods. To quantify arthritis progression, micro-CT was utilized in this study. Micro-CT can measure joint space narrowing and reveal trabecular bone structure and osteophyte formation (Koba et al., 2013). As there were high individual variation of osteophyte numbers in CIA rats, it did not show the therapeutic potential of MTX in this study (data not shown).
Because micro-CT analysis somewhat limited for cartilage detection, H&E and safranin O-fast green staining were utilized. Normal cartilage structures were present in joints in control rats. However, cartilage staining was remarkably reduced in joints from rats treated with collagen. In G3 rats, H&E and safranin O-fast green staining showed that MTX treatment caused a slight reduction in cartilage destruction.
Histopathological examination also showed that knee joints of CIA rats exhibited severe joint destruction with inflammatory cell infiltration. As histopathologic examination provides qualitative or semi-quantitative information, there are some limitations for the quantification of lesions. In this study, MTX treatment did reduce cartilage destruction compared to CIA, although it did not result in therapeutic advantages in bone parameters
Because MMP, collagenases, and gelatinases are involved in joint destruction in arthritis (Ishikawa et al., 2005), it is possible to assess the breakdown products of joint matrix components (Otero and Goldring, 2007). In the present study, MMP-13 immunostaining was remarkably increased in G2 animals. However, MMP-13 was reduced in the joint surface of G3 rats compared to G2. The MMPs are believed to play a central role in the process of timely breakdown of extracellular matrix for embryonic development, morphogenesis, reproduction, and tissue resorption and remodeling (Nagase and Woessner, 1999). Among the MMPs, MMP-13 is expressed by chondrocytes and synovial cells in arthritis and is thought to play a critical role in cartilage destruction (Takaishi et al., 2008). We found that MMP-13 expression was increased in G2 animals and MTX treatment reduced its expression by immunohistochemical method. Further studies are warranted to investigate the inhibitory mechanism of MMP-13 by MTX treatment.
While histopathological examination is a valuable method used to evaluate therapeutics (Gerwin et al., 2010), it may limit the quantitative information, especially in cartilage. For simultaneous analyses of cartilage and bone parameters, new high technologies, such as optical tomography, fluorescence molecular tomographic imaging and near infrared absorption spectra could be useful. The specific use of optical tomography in arthritis allows accurate three-dimensional imaging and quantification within the picomolar range (Peterson et al., 2010), and a bone-specific polymeric probe provides early diagnosis of arthritis and visualization of arthritis progression, which could be used for early diagnosis of RA and to monitor drug and surgical therapies in individual cases (Ryu et al., 2011). Furthermore, a near infrared spectroscopic probing technique could be used to detect, qualify and quantify changes in arthritis (Afara et al., 2013), and making it possible to visualize the tissue of living animals combined with immunohistochemistry using corresponding intravital microscopy images (Ritsma et al., 2013). Further studies are warranted to evaluate the therapeutic potential of pharmaceuticals using micro-CT and fluorescence imaging system simultaneously.
Overall, our results show that MTX treatment caused a reduction in cartilage destruction in rats with CIA, and micro-CT analysis made it possible to quantify bony lesions of arthritis. New technologies associated with histopathological examination should be applied to evaluate cartilage involved in arthritis lesions.
Acknowledgments
We would like to thank Dr. Won Kil Lee for his data analyses and Ms. Joo Hye Sim and Kyung A Kwak for their technical assistance. This study was supported by a grant of O-song Medical Innovation Foundation R&D project (HI13C0998) of Ministry of Health and Welfare in South Korea.
Footnotes
CONFLICT OF INTEREST
Authors declare that there are no conflicts of interest.
REFERENCES
- Afara IO, Prasadam I, Crawford R, Xiao Y, Oloyede A. Near infrared (NIR) absorption spectra correlates with subchondral bone micro-CT parameters in osteoarthritic rat models. Bone. 2013;53:350–357. doi: 10.1016/j.bone.2012.12.042. [DOI] [PubMed] [Google Scholar]
- Bevaart L, Vervoordeldonk MJ, Tak PP. Evaluation of therapeutic targets in animal models of arthritis: how does it relate to rheumatoid arthritis? Arthritis Rheum. 2010;62:2192–2205. doi: 10.1002/art.27503. [DOI] [PubMed] [Google Scholar]
- Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- Carvalheiro H, da Silva JA, Souto-Carneiro MM. Potential roles for CD8(+) T cells in rheumatoid arthritis. Autoimmun Rev. 2013;12:401–409. doi: 10.1016/j.autrev.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Conway JR, Carragher NO, Timpson P. Developments in preclinical cancer imaging: innovating the discovery of therapeutics. Nat. Rev. Cancer. 2014;14:314–328. doi: 10.1038/nrc3724. [DOI] [PubMed] [Google Scholar]
- Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Nishigaki F, Miyata S, Hirayama Y, Minoura K, Imanishi J, Neya M, Mizutani T, Imamura Y, Naritomi Y, Murai H, Ohkubo Y, Kagayama A, Mutoh S. Prevention of progressive joint destruction in collagen-induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR255031. Br J Pharmacol. 2005;144:133–143. doi: 10.1038/sj.bjp.0706054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba W, Jelicks LA, Fine EJ. MicroPET/SPECT/CT imaging of small animal models of disease. Am J Pathol. 2013;182:319–324. doi: 10.1016/j.ajpath.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Macrophages in rheumatoid arthritis. Histol Histopathol. 2007;22:581–586. doi: 10.14670/HH-22.581. [DOI] [PubMed] [Google Scholar]
- Mello SB, Barros DM, Silva AS, Laurindo IM, Novaes GS. Methotrexate as a preferential cyclooxygenase 2 inhibitor in whole blood of patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;39:533–536. doi: 10.1093/rheumatology/39.5.533. [DOI] [PubMed] [Google Scholar]
- Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/S0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Otero M, Goldring MB. Cells of the synovium in rheumatoid arthritis. Chondrocytes. Arthritis Res Ther. 2007;9:220. doi: 10.1186/ar2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DJ, St Clair EW, Misukonis MA, Weinberg JB. Reduction of NOS2 overexpression in rheumatoid arthritis patients treated with anti-tumor necrosis factor alpha monoclonal antibody (cA2) Arthritis Rheum. 1998;41:2205–2210. doi: 10.1002/1529-0131(199812)41:12<2205::AID-ART16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Labranche TP, Vasquez KO, Kossodo S, Melton M, Rader R, Listello JT, Abrams MA, Misko TP. Optical tomographic imaging discriminates between disease-modifying anti-rheumatic drug (DMARD) and non-DMARD efficacy in collagen antibody-induced arthritis. Arthritis Res Ther. 2010;12:R105. doi: 10.1186/ar3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsma L, Vrisekoop N, van Rheenen J. In vivo imaging and histochemistry are combined in the cryosection labelling and intravital microscopy technique. Nat Commun. 2013;4:2366. doi: 10.1038/ncomms3366. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Lee A, Chu JU, Koo H, Ko CY, Kim HS, Yoon SY, Kim BS, Choi K, Kwon IC, Kim K, Youn I. Early diagnosis of arthritis in mice with collagen-induced arthritis, using a fluorogenic matrix metalloproteinase 3-specific polymeric probe. Arthritis Rheum. 2011;63:3824–3832. doi: 10.1002/art.30628. [DOI] [PubMed] [Google Scholar]
- Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA. Application of micro-CT in small animal imaging. Methods. 2010;50:2–13. doi: 10.1016/j.ymeth.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Scott DL. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 2012;91:30–43. doi: 10.1038/clpt.2011.278. [DOI] [PubMed] [Google Scholar]
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- Segawa Y, Yamaura M, Aota S, Omata T, Tuzuike N, Itokazu Y, Oka H, Tamaki H, Nakamura T. Methotrexate maintains bone mass by preventing both a decrease in bone formation and an increase in bone resorption in adjuvant-induced arthritic rats. Bone. 1997;20:457–464. doi: 10.1016/S8756-3282(97)00023-9. [DOI] [PubMed] [Google Scholar]
- Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kaplan H, Germain BF, Merriman RC, Solomon SD, Wall B, Anderson L, Block S, Small R, Wolfe F, et al. Methotrexate in rheumatoid arthritis: effects on disease activity in a multicenter prospective study. J Rheumatol. 1991;18:334–338. [PubMed] [Google Scholar]
- Weinblatt ME, Weissman BN, Holdsworth DE, Fraser PA, Maier AL, Falchuk KR, Coblyn JS. Long-term prospective study of methotrexate in the treatment of rheumatoid arthritis. 84-month update. Arthritis Rheum. 1992;35:129–137. doi: 10.1002/art.1780350202. [DOI] [PubMed] [Google Scholar]