Abstract
The olfactory mucosa (OM) is a unique source of regenerative neural tissue that is readily obtainable from living human subjects and thus affords opportunities for the study of psychiatric illnesses. OM tissues can be used, either as ex vivo OM tissue or in vitro OM-derived neural cells, to explore parameters that have been difficult to assess in the brain of living individuals with psychiatric illness. As OM tissues are distinct from brain tissues, an understanding of the neurobiology of the OM is needed to relate findings in these tissues to those of the brain as well as to design and interpret ex vivo or in vitro OM studies. To that end, we discuss the molecular, cellular and functional characteristics of cell types within the olfactory mucosa, describe the organization of the OM and highlight its role in the olfactory neurocircuitry. In addition, we discuss various approaches to in vitro culture of OM-derived cells and their characterization, focusing on the extent to which they reflect the in vivo neurobiology of the OM. Finally, we review studies of ex vivo OM tissues and in vitro OM-derived cells from individuals with psychiatric, neurodegenerative and neurodevelopmental disorders. In particular, we discuss the concordance of this work with postmortem brain studies and highlight possible future approaches, which may offer distinct strengths in comparison to in vitro paradigms based on genomic reprogramming.
Introduction
A critical component of neuropsychiatric research is the delineation of neurobiological abnormalities in patients' brains. Although decades of postmortem studies have yielded vital insights, the lack of access to living patients' brain tissues has long been a major hurdle in the field. Recently, several in vitro paradigms have emerged, such as induced pluripotent stem cell (iPSC)1 and induced neuronal2 cell technologies, which offer unique and unprecedented opportunities to reprogram patients' cells into developing neurons and glial cells.
This review focuses on another paradigm with a similar purpose and with distinct strengths; the olfactory mucosa (OM) tissue approach. The OM harbors neurons and glial cells residing in the nasal cavity and is readily accessible via biopsy. Neural tissues without genomic reprogramming can be captured via olfactory biopsy. OM tissues offer ex vivo and in vitro neuronal cells that may more closely reflect in vivo neural characteristics of the donors. OM cells also have regenerative potential, which permits them to propagate in vitro, yielding neuronal or glial cells in sufficient quantity for various assays.
Another unique advantage of this paradigm arises from the fact that the OM is the peripheral component of the olfactory circuitry, connecting olfactory sensory neurons (OSNs) to the olfactory bulb, the neurons of which then connect to the olfactory cortex. As such, neurobiological alterations in patients' limbic regions may affect or be reflected in the olfactory circuitry, including neural cells in the OM. Indeed, Alzheimer's disease,3, 4 schizophrenia5, 6, 7, 8, 9 and mood and anxiety disorders (review10) are associated with olfactory dysfunction, and OM derived from these patients exhibit cellular and molecular alterations.11, 12, 13, 14, 15, 16, 17, 18, 19, 20
Recently, a growing number of groups have examined OM biopsies in these disorders11, 12, 17, 21, 22, 23 and others in which olfactory dysfunction is yet to be characterized as a key phenotype.14, 24, 25, 26 An important question is to what degree and/or in what ways these OM-derived cells reflect the pathophysiologic mechanisms in patients' brains. Given that the OM is a specialized neural tissue containing a regenerative neuroepithelium whose identity is distinct from brain cells, alterations in the OM cells can best be appreciated in the context of the neurobiological characteristics specific to the OM tissue. The goal of this paper, therefore, is to consider how the OM paradigm can contribute to pathophysiologic understanding of neuropsychiatric illnesses by reviewing the neurobiological context in which these OM cells arise in vivo and in vitro.
Cytoarchitecture and molecular characteristics of the OM
A distinctive characteristic of the OM is that it contains a regenerative neuroepithelium. In this neural tissue, cells continually slough off and are replenished by newborn cells including neurons and other cell types. Multipotent cells of the OM must continuously produce replacement OSNs and other cell types in the adult mammalian OM.27, 28 This continual process of renewal from multipotent cells to differentiated neurons offers a unique opportunity to capture the processes of neurodevelopment in human studies.
Although the OM is located external to the brain, OSN axons extend through the lamina propria (LP) and then the cribiform plate to the brain where they synapse onto neurons of the olfactory bulb. These olfactory bulb neurons then project to primary olfactory regions, including the piriform and entorhinal cortices and the amygdala.29 Cells within the projection regions then synapse onto cells in other neuropsychiatric disease-related regions, including the hippocampus, prefrontal cortex and hypothalamus, thus enabling olfactory information to influence emotional, cognitive and visceral functions.10, 29
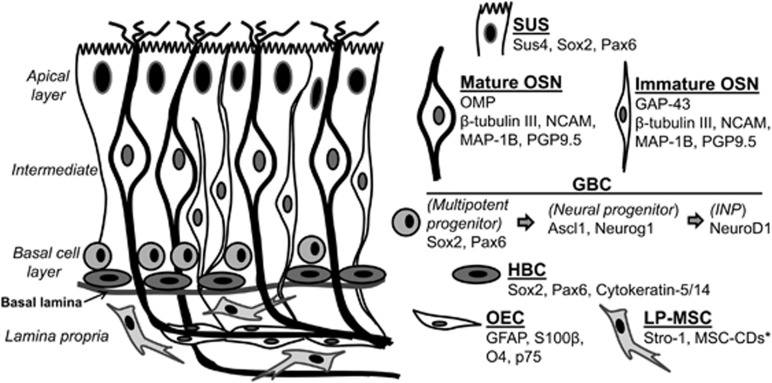
The current knowledge of the cytoarchitecture and molecular profiles of the OM stems largely from rodent studies. The human olfactory neuroepithelium (ON) is thought to recapitulate histologic and molecular characteristics of the rodent counterpart, though the precise degree of similarity remains under investigation. The ON exists as a pseudostratified epithelium in adult mammals, comprising three primary layers; a basal layer, an intermediate region and an apical layer (as depicted in Figure 1). The apical layer is exposed to the nasal cavity and contains glial-like supporting cells called sustentacular cells. The intermediate region contains layers of immature and mature OSNs and exhibits a distinct laminar organization in the rodent ON (Figure 1), with the immature OSN layer residing closer to the basal lamina. In the human ON, however, immature and mature OSNs are more dispersed throughout, suggesting a less pronounced laminar organization of OSNs than observed in the rodent ON.30, 31
Figure 1.
Cytoarchitecture and molecular characteristics of the olfactory mucosa (OM). The multilaminar organization of the rodent OM is depicted on the left, and core molecular markers that define and distinguish mammalian OM cell types in vivo are listed on the right. Most of the studies identifying these markers have been reviewed elsewhere.32, 33, 34, 35, 36 *Markers that distinguish lamina propria mesenchymal stem cells (LP-MSCs) have not been studied to the extent of markers for other OM cell types, but see Tome et al.,37 Lindsay et al.38 and Delorme et al.39 for MSC-specific cluster of differentiation (CD) expression in these cells. GBC, globose basal cell; HBC, horizontal basal cell; INP, intermediate neural progenitor; OEC, olfactory ensheathing cell; OSN, olfactory sensory neuron; SUS, sustentacular cell.
Deep to the intermediate region lies the basal layer that contains globose and horizontal basal cells (GBCs and HBCs, respectively), and is separated from the underlying LP by the basal lamina. As shown in the mouse ON, GBCs and HBCs are the stem cells that initiate the OSN lineage by proliferating into transit-amplifying progenitors that give rise to immediate neuronal precursors (INPs), which differentiate into immature and ultimately mature OSNs.32 Several rodent studies have delineated the expression of transcription factors in ON basal cells to define the lineage of ON cells.33, 34 These defining factors and associated cell types are listed in Figure 1. Depending upon the niche, both HBCs and GBCs comprise stem cell populations in the rodent ON that can be both neuro-competent and multipotent.28, 40
In the mouse ON, the basal layer exhibits a distinct organization. HBCs form a single layer next to the basal lamina, exhibit a flattened morphology and express cytokeratin-5 and -14, whereas GBCs exhibit a round morphology, do not express cytokeratins and reside in a single layer superficial to the HBCs (Figure 1).41, 42 Similarities between rodent and human ON basal cells have been observed,30, 31, 43 yet the laminar organization and morphologies of the different basal cell types (flattened versus rounded) appear to be less clearly defined in human ON.30, 31 In addition, molecular expression patterns of these basal cells may differ between species, as robust expression of the low affinity nerve growth factor p75NGFR has been observed in first- and second-layer basal cells in human ON, but not in adult rodent ON.13, 30, 44, 45 These basal cells, whether analogous to rodent HBCs, GBCs or both, are thought to comprise the presumptive neural precursor population in human ON.
Juxtaposed to the basal cell layer, separated by the basal lamina, is the LP. Rodent studies have shown this to be a neural crest-derived ectomesenchymal tissue, which contains multipotent mesenchymal stem cells (LP-MSCs), olfactory ensheathing cells (OECs), axons originating from the OSNs and the acynus of Bowman glands.37, 46, 47 Studies of LP-MSCs in rodents and humans have shown that these cells are able to generate multiple cell phenotypes, including, but not limited to, OECs and neural lineages, both during development and in response to regenerative cues.35, 37, 38, 46, 47, 48 The glial potential of OECs has been extensively studied in the context of spinal cord transplantation and has been reviewed elsewhere.49, 50 OECs represent a highly specialized, likely heterogeneous glial cell population that share morphological and neurochemical features with Schwann cells and astrocytes, although they differ from both. Their neurochemical composition highlights their functional heterogeneity, as they express sets of proteins shared with astrocytes (for example, glial fibrillary acidic protein (GFAP) and s100β), oligodendrocytes and oligodendrocyte precursors (O4, NG2), CNS radial glia (basic lipid binding protein, BLBP), as well as developmentally important proteins CD 44, β1 integrin, P200 and Notch 3.36
Use of ex vivo olfactory tissue in the study of neuropsychiatric and neurodevelopmental diseases
As a regenerative neuroepithelium containing a range of morphologically and molecularly distinct neuronal and glial cells, the ex vivo ON represents a useful tool for examining cell type-specific biological changes in neuropsychiatric illness. Compared with all other in vitro neuronal models or blood cell studies, this cell type-specific resolution is a unique feature of this paradigm. As such, the ON has been utilized as a platform for histologic assessment, investigation of intracellular signaling and gene expression profiling.
Using a histologic approach to study neuronal differentiation, Arnold et al.13 conducted immunohistochemical assessment of the ON using markers for specific stages of neuronal differentiation in ex vivo tissue from schizophrenic patients and controls. In this study, basal cells, immature and mature neurons were marked with antibodies for p75NGFR, GAP-43 and OMP, and densities of immunoreactivity for these markers were used as indices for specific stages of differentiation. Compared with controls, patients exhibited decreased density of p75-labeled basal cells, a higher density of GAP-43-labeled immature OSNs and an increased ratio of immature to OMP-labeled mature OSNs. Together these findings led to the postulate that neuronal lineage may be disrupted in the ON and by extension in the CNS of patients with schizophrenia.13
In a similar paradigm, Pantazopoulos et al.20 examined chondroitin sulfate proteoglycans, prominent components of the extracellular matrix, in ex vivo ON from schizophrenic patients compared with controls. These extracellular matrix proteins are critical for cellular differentiation and migration, and are postulated as a possible mechanism for the altered neuronal lineage as observed in by Arnold et al. In that study, the decreased chondroitin sulfate proteoglycan density reported in mature OSNs in schizophrenia is consistent with previous findings of reduced proteoglycans in multiple brain regions of postmortem brains of schizophrenic patients.20, 51 Thus, some of the neurobiological characteristics observed in the ON can be extrapolated to those of the brain.
Histologic changes in the ON that are specific to particular neuropsychiatric illnesses may hold promise as potential biomarkers. In the postmortem ON of patients with Alzheimer disease, Arnold et al. found higher frequency and abundance of amyloid-β and paired helical filament-tau pathologies in ON derived from Alzheimer patients compared with controls,11 essentially mirroring previous observations in postmortem brains of patients (reviewed in Hardy and Selkoe52). If these and other findings described above are extended to ON biopsy tissues of living patients, and are correlated with clinical severity or diagnostic subtypes, they could serve as cellular biomarkers of psychiatric illnesses.
The ON can be utilized for gene expression profiling of neural cells with cell type-specific resolution. McCurdy et al.14 used microarray analyses in ex vivo tissues to demonstrate altered expression of genes relating to cell cycle and neurogenesis in schizophrenic patients, findings that may be consistent with neurodevelopmental dysregulation. More recently, Mor et al.21 used laser capture microdissection in ex vivo tissues to isolate mature OSNs, and observed increased miRNA expression of MiR-382 in schizophrenic patients versus controls. Notably, similar dysregulation in MiR-382 has been observed in the postmortem dorsolateral prefrontal cortex,53 again supporting the notion that certain aspects of brain pathology may be represented in the ON.
Ex vivo ON tissues can also be examined for their electrophysiological properties. OSNs can be dissociated from biopsy tissues, readily identified by their morphology and examined using calcium imaging, voltage sensitive dye imaging and other electrophysiological measures. Indeed, Hahn et al.19 assessed odorant induced calcium signaling in OSNs derived from medication-free bipolar disorder patients and their matched controls, and demonstrated decreased intracellular calcium signaling in bipolar disorder patients. Given that dysregulated calcium signaling has been consistently reported in bipolar disorder patients in peripheral blood and brain,54, 55, 56 ON derived from bipolar patients may be useful for furthering understanding of the molecular pathologies of the disorder.
Ex vivo ON tissues also provide a unique paradigm for the study of neurodevelopmental disorders, given the lifelong regeneration of ON and the presence of OSNs at all the stages of development. One such developmental disorder where ex vivo ON has been studied is Rett syndrome, which results in profound intellectual disability and has been associated with deficits in neuronal maturation. Ronnett et al.26 reported fewer mature OSNs, increased immature OSNs and abnormal morphology of mature OSNs in Rett syndrome patients of various ages compared with age-matched controls. These observations support the notion that ON is a viable tool for studying neurodevelopmental aspects of diseases characterized by abnormal neuronal development.
In vitro use of cultured OM-derived cells in the study of neuropsychiatric illnesses
A second approach to the use of the OM in psychiatric research, which complements the study of ex vivo OM tissues, is the in vitro culture of OM-derived cells. Due to the regenerative nature of the OM, OM-derived cells can be readily propagated and are increasingly yielding insights into the cellular and molecular underpinnings of neuropsychiatric illness.
Properties of adult human OM-derived cells in vitro
When developing in vitro models for neuropsychiatric illnesses, it is critical to consider the specific characteristics of cell types arising in culture, which are determined by the tissue compartment from which they originate and conditions in which they are grown. In diverse variations of these factors, multiple approaches to the culture of OM-derived cells have been developed including the organotypic explants, dissociated cultures and neurospheres (see Box 1 for specifics of culture paradigms). Common to all these paradigms is that they seek to utilize regenerative characteristics of the ON and its underlying LP as neural tissues, within which progenitors differentiate into neurons, sustentacular cells and OECs.57, 58, 59
Box 1. In vitro culture paradigms of adult human olfactory mucosa (OM)-derived cells.
Human OM is harvested from the nasal septum or turbinates typically by biopsy, or also by exfoliation. Cells can be subsequently propagated from the olfactory neuroepithelium (ON),57 its underlying lamina propria (LP)60 or from tissue containing both regions.30 Protocols have been established to enhance the neural stem cell properties of OM cells through neurosphere57, 61 and monolayer culture.62
(A) Organotypic-monolayer cultures: Non-dissociated adult human ON biopsy tissues, containing a portion of underlying LP, can be utilized for propagating OM cells in monolayer cultures in serum-containing media on uncoated or fibronectin-coated plates30, 60, 62, 63 or on a Matrigel substrate.22, 64 In cultures on fibronectin-coated plates, epithelial cells first grow out from biopsy tissue to form sheets, over which grow round, more rapidly proliferating cells after 24–48 h.30, 60
(B) Neurosphere cultures: Multiple groups have reported that cells collected from the human ON itself (with or without some underlying LP) can be cultured in neurospheres from dissociated biopsy tissue. In serum-containing media, when adult human ON tissue (without underlying LP) is first dissociated, cells with the bipolar morphology of neurons, along with presumptive olfactory ensheathing cells, are present.61 Such cells soon disappear in serum-containing media, and are replaced by a proliferating population, which form spheres and can be cultured for extended periods.61, 65, 66, 67 Alternatively, adult human OM-derived cells can be dissociated and propagated on poly-L-lysine-coated plates in defined medium with EGF and FGF2 to form ‘primary neurospheres', before being plated onto uncoated or fibronectin-coated plates and maintained in serum-containing medium in monolayer culture.16, 57
Additional approaches that have been employed in rodent studies include culture of adult rat ON-derived cells on a feeder layer of newborn rat glial cells,68 and co-culture of ON-derived cells with a 3T3 cell feeder layer at the air–liquid culture interface.69
The experimental utility of cultured OM cells as a model for neuropsychiatric illness is, in part, related to the extent to which cultured cells recapitulate the characteristics of in vivo OM cells or of donors' neurobiological makeup. To define neuronal precursors, immature neurons or OSNs, a rapidly increasing database on the expression of molecular markers in cell types of each differentiation stage can be used as a guide. As described in Figure 1, human and rodent ON cells at different stages of neuronal differentiation in vivo are characterized by expression of molecular markers such as Cytokeratin-5, Sox2, Pax6, Ascl1, Neurog1, NeuroD1, Gap43 and OMP. Neuronal precursors can be defined as proliferative cells whose progeny ultimately become neuronal cells. Immature neurons and OSNs do not share this proliferative capacity, and OSNs are further defined functionally by their capacity to respond to odorants.70, 71
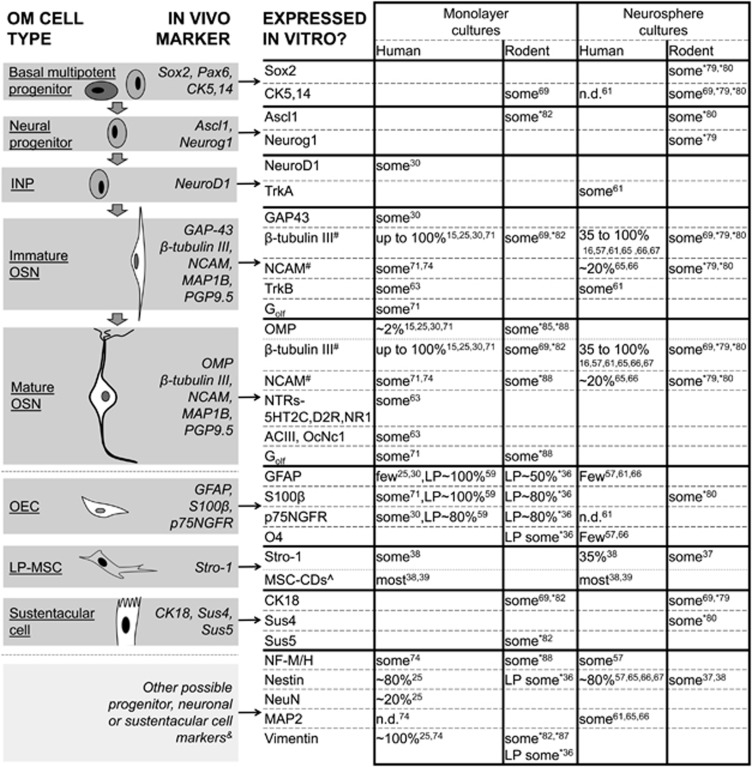
Numerous markers have been used to characterize OM-derived cells in vitro (Figure 2). While some markers, such as NeuroD1 and NCAM, are selectively expressed in neuronal cells at varying stages of differentiation in vivo, the selectivity or appropriateness of other markers is less clear. For example, β-tubulin III and vimentin are expressed not only in olfactory neurons in vivo, but also in other cell types,72, 73 thus limiting their utility as sole markers for neurons in vitro despite their widespread expression. While nestin has been recognized as a neural stem cell/progenitor marker in the mammalian brain, its expression in adult human ON in vivo has been demonstrated exclusively in sustentacular cells as opposed to basal cells of neural lineage.45 LP-MSCs, however, do express nestin in vitro, suggesting that the use of nestin immunoreactivity as a stem cell marker applies specifically to LP-derived neural stem cells.37, 38, 39
Figure 2.
Expression of putative molecular markers of stem cells, neural progenitors, neurons and glia by various olfactory mucosa (OM) cell types, both in adult OM in vivo and in cells generated from adult OM tissues in vitro. Studies of OM explant tissues or dissociated cells immediately ex vivo are not included. Percentage values are approximate, and refer to the proportion of cells in culture which express each marker. Values are only shown for markers displaying consistency between studies, and only for cells grown in serum-containing media. *, studies using neonatally-derived rodent or fetally-derived human ON culture cells; &, markers whose significance in OM culture cells is uncertain due to a discordance between human and rodent (for example, p75NGFR), low/uncertain levels of expression in adult ON in vivo (NF-M/H, Nestin, NeuN, MAP2) or a possible lack of cell type specificity (p75NGFR, Vimentin); #, markers whose expression increase when cells are cultured in base (unsupplemented defined) media rather than serum-containing media; ^, mesenchymal stem cell cluster of differentiation (MSC-CD) markers, used to confirm cell type, vary among studies. CK, cytokeratin; INP, intermediate neural progenitor; LP, lamina propria (indicates cultures derived solely from LP, without ON); LP-MSC, lamina propria mesenchymal stem cell; ND, not detected; NTR, neurotransmitter receptor; OEC, olfactory ensheathing cell; ON, olfactory neuroepithelium; OSN, olfactory sensory neuron.
Adult human OM-derived cells, originating from non-dissociated biopsy tissue and cultured in a monolayer in serum-containing media, are capable of expressing mRNA transcripts or proteins characteristic of a number of OM cells types in vivo (Figure 2). These include NeuroD1, Gap43, TrkB, NCAM, β-tubulin III, OMP, OcNc1 and OR3A1.25, 30, 61, 63, 66, 74 Markers of sustentacular and glial cells, such as Sus4, S100 and GFAP, and mesenchymal stem cells (Stro-1) have also been reported to be expressed in human OM culture cells (Figure 2).30, 36, 37, 38, 49, 66 Some ON-derived cells are also capable of progressing from replication (indicated by BrdU incorporation) to terminal differentiation (OMP expression) in culture.30 At a functional level, some human ON cells cultured under these conditions demonstrate the odorant responsiveness specific to OSNs, with ~5.5% of cells in culture responding to helional, a ligand for the OR3A1 odorant receptor,63 and up to 10% of OMP-positive cells responding to a mixture of other odorants.71 Receptors for the key neurotransmitters serotonin (5-HT2 receptors), dopamine (D2 receptor) and glutamate (NMDA receptors), which have been identified in cultured ON cells, also display functional activity.63 Benitez-King and colleagues collected nasal exfoliates24, 25 and reported that the cells harvested this way and propagated in monolayer culture expressed with varying frequencies a number of potential cell type-specific markers (Figure 2) and expressed action potentials.25
Neurosphere-forming cells cultivated from either the ON alone, or from both the ON and LP, express markers such as NCAM, β-tubulin III, TrkA/B, nestin and Map2 (Figure 2).16, 57, 61, 65, 66, 67 Overall, the expression of ON neuronal lineage markers, and of other putative neuronal markers, suggests that many OM-derived culture cells may be of a neural lineage. To date, although multiple OM-derived cell culture paradigms have been used in various studies, the relative expression of cell type-specific markers by cells cultured in these paradigms has not been directly compared. When indirectly compared, some similarities between paradigms exist in the expression of multiple markers, such as NCAM and nestin (Figure 2), suggesting a degree of consistency among paradigms employing serum-containing media. However, differences also exist, such as in β–tubulin III, which is expressed in 35% of ON-derived cells in the study of Matigian et al.,16 in the majority of cells in multiple other studies57, 61, 67 and in ~100% of ON-derived cells in the study of Benitez-King et al.25 Overall, further work to delineate the degree of consistency between culture paradigms, including characterization of cellular heterogeneity and marker expression, is needed.
The adult human olfactory LP alone can also be used to culture progenitors of ectomesenchymal origin, which are particularly suitable for the in vitro propagation of presumptive OECs.38, 75 When cultured in serum-free media supplemented with NT3, almost all adult human LP-derived proliferating cells express OEC markers S100 and GFAP, ~80% express p75NGFR, whereas none express HNK1 (a marker for Schwann cells).59, 75 These cells hold promise for autologous transplantation in the treatment of spinal cord injury.50, 59, 76
In vitro culture of OM-derived cells from rodents and fetal humans
The OM paradigm has been employed using in vitro cells from rodents, shedding light on the stem cell potential of OM-derived cells. For example, proliferation of neuronal precursors and successful genesis of mature OMP-positive OSNs from adult rat ON-derived cells in culture has been reported.68 When adult rodent ON-derived cells are co-cultured with a 3T3 cell feeder layer at the air–liquid culture interface, cells with the molecular phenotypes of GBCs, HBCs, sustentacular cells and neurons can be generated, which can successfully reintegrate into the ON after transplantation.69 Monolayer culture of rat p75NGFR-expressing OM-derived cells, which were purified by fluorescence-activated cell sorting, has been used for characterization of putative OECs in vitro.77 Cells from the mouse olfactory LP, when cultured in a monolayer, demonstrate the same OEC phenotype and cell type-specific marker expression as human LP-derived cells.36 When cultured in spheres, mouse olfactory LP-derived cells have the capacity to integrate into lesioned hippocampus in vivo, differentiate into neurons and restore learning and memory.78
Numerous studies have used OM-derived cells from pre-natal/neonatal rodents79, 80, 81, 82, 83, 84, 85, 86, 87, 88 and fetal humans,89, 90 in part because such cells can be propagated more readily. This is observed under controlled neurosphere assay conditions, in which ON-derived neurospheres are generated with progressively lower efficiencies from E13.5 mice, P5 mice and adult mice.35 Importantly, neurospheres derived from E13.5 and adult animals contain cells with different morphologies, which express different cell type-specific markers and proliferate at different rates.91 As a result, the extent to which findings in OM-derived cells from neonatal and early postnatal animals can be extrapolated to adults may be limited.
The effects of culture environment on neuronal identity of OM-derived cells
The properties of OM-derived cells, such as their proliferation, morphology and expression of cell type-specific markers, can be heavily influenced by the in vitro environment, particularly culture medium57, 60, 61, 65, 66 (Table 1). In culture, BDNF (brain-derived neurotrophic factor), NT3 and NT5 promote ON-derived cell survival,92 FGF2, FGF8, EGF and TGF-α (transforming growth factor-alpha) have been used to stimulate progenitor proliferation,82, 93, 94 and serum deprivation, Db-cAMP, IGF-I (insulin-like growth factor-I), retinoic acid, forskolin and sonic hedgehog have been used to drive human ON culture cells toward a neuronal phenotype.25, 95, 96 Proliferation of human LP-derived cells with an OEC phenotype is promoted by NT3, BDNF or NGF.75 Human LP-derived cells can be driven toward a neuronal phenotype with a cocktail of retinoic acid, forskolin and sonic hedgehog,97 or dexamethasone, insulin, 3-isobutyl-1-methylxanthine, indomethacin and ethanol,98 and toward a osteoblast phenotype with a cocktail of dexamethasone, L-ascorbic acid-2-phosphate, and β-glycerophosphate98 or NaH2PO4.39 Other additional factors, many of which are relevant to the control of neurogenesis and differentiation in vivo, have also been shown to modulate OM-derived cell proliferation, morphology and expression of cell type-specific markers in vitro (Table 1).
Table 1. In vivo and in vitro factors influencing the proliferation, differentiation and survival of cells within, or derived from, the olfactory mucosa (OM).
| Factor | Cell type impacted in vivo | Effect observed in vivo | Effect on OM-, ON- or LP-derived cells in vitro |
|---|---|---|---|
| TGF-α | Stem cell | Stimulates proliferation and progression of rodent HBCs99 | Stimulates ON-derived cell (HBC) proliferation93 |
| EGF | Stem cell | Stimulates proliferation of rodent HBCs99 | Stimulates ON-derived cell (HBC) proliferation93 |
| ΔNp63 (p63) | Stem cell | Required for rodent HBC genesis and differentiation100 | — |
| Sox2 | Stem cell | Increases Ascl1 expression and differentiation of rodent GBC/HBCs into neural progenitors94 | — |
| Meis1 | Stem cell | Suppresses Ascl1 expression and stem cell differentiation94 | — |
| Foxg1 | Stem cell | Promotes differentiation of rodent Sox2-positive stem cells into neural progenitors101 | — |
| Retinoic acid | Stem cell | Stimulates basal cell proliferation and/or differentiation after lesion102 | Promotes ON-derived cell (HBC) differentiation95 and LP-derived neuronal differentiation37 |
| Uncx | Early neural progenitor | Promotes proliferation of neural progenitors and survival of OSNs103 | — |
| FGF2 | Early neural progenitor | Enhances neural progenitor proliferation104 | Stimulates OM-derived cell proliferation37, 83, 90, 105 |
| FGF8 | Early neural progenitor | Promotes neurogenesis and progenitor proliferation106 by enhancing Sox2 and repressing Meis1 expression94 | — |
| Ascl1 | Early neural progenitor | Required for maturation of early neural progenitors into INPs107, 108 | — |
| Six1 | Early neural progenitor | Required for maturation of early neural progenitors into INPs109 | — |
| Ngn1 | Early neural progenitor | Required for maturation of neural progenitors into INPs108 | — |
| BMP2,4,7 | Early neural progenitor, immature OSN | At lower concentrations, promotes survival of newly formed OSNs (BMP4 only),110 at higher concentrations inhibit neurogenesis by targeting Ascl1 for proteolysis111 | — |
| FST | Early neural progenitor | Promotes progenitor proliferation by antagonizing GDF11 and ACTβB112, 113 | — |
| ACTβB (activin) | Early neural progenitor | Inhibits progenitor proliferation by promoting proteolysis113 | — |
| Hes1 | Early neural progenitor | Suppresses Ascl1-mediated neural progenitor maturation114, 115 | — |
| Hes5 (with Hes1) | Early neural progenitor | Suppresses Ngn1-mediated neural progenitor maturation114, 115 | — |
| MMP2, MTI-MMP | Early neural progenitor, INP | Downregulated following bulbectomy in rodent GBCs116 | — |
| TIMP | Early neural progenitor, INP | Upregulated following bulbectomy in rodent GBCs116 | — |
| GDF11 | INP | Inhibits proliferation of INPs by inducing reversible cell cycle arrest112, 113 | — |
| Runx1 | INP | Stimulates INP proliferation117 | Stimulates ON-derived cell proliferation117 |
| NPY | INP | Promotes, and is required for, INP proliferation and maturation118, 119, 120, 121 | Increases OM-derived cell proliferation via the Y1 receptor and PKC-dependent ERK1/2 phosphorylation119, 121 |
| Peptide YY | INP | Regulates INP differentiation118, 119 | Increases OM-derived cell proliferation118, 119 |
| Atf5 | Immature OSN | Promotes survival and maturation of immature OSNs122 | — |
| Emxl2 | Immature OSN | Promotes survival and maturation of immature OSNs123 | — |
| Lhx2 | Immature OSN | Promotes survival and maturation of immature OSNs124, 125 | — |
| ZFP423/OAZ | Immature OSN | Inhibits maturation of immature OSNs126 | — |
| LIF1 | Immature OSN | Promotes survival and inhibits maturation of immature OSNs127, 128 | Increases OM- and ON-derived cell proliferation,93, 129 decreases apoptosis, inhibits differentiation127, 128 |
| BDNF | Multiple cell types | May provide trophic support to maturing ON cells130, 131, 132 | Promotes LP-derived glial cell proliferation75 and neuronal differentiation37 |
| NT3,4 | Multiple cell types | May provide trophic support to maturing ON cells131 | (NT3) promotes LP-derived glial cell proliferation75 |
| NGF | Multiple cell types | Expressed in neuronal ON layers, may provide trophic support to maturing ON cells131 | Promotes LP-derived glial cell proliferation75 |
| PACAP | — | — | Promotes proliferation of OM-derived cells133 |
| IGF-I | — | — | Decreases OM-derived cell proliferation and promotes cell differentiation96 |
| Nitric oxide | — | — | Inhibits ON-derived cell proliferation and promotes differentiation134 |
| Serum deprivation | — | — | Decreases ON-derived cell proliferation and increases neuronal differentiation66, 82 |
| Db-cAMP | — | — | Promotes ON-derived cell differentiation25, 61 |
| Forskolin | — | — | Promotes ON-derived cell differentiation95 |
| Sonic hedgehog | — | — | Promotes ON-derived cell (HBC) differentiation95 |
Abbreviations: BDNF, brain-derived neurotrophic factor; GBC, globose basal cell; HBC, horizontal basal cell; IGF-I, insulin-like growth factor-I; INP, intermediate neuronal precursor; LP, lamina propria; NGF, nerve growth factor; ON, olfactory neuroepithelium; OSN, olfactory sensory neuron; TGF-α, transforming growth factor-alpha.
The use of OM-derived cells in psychiatry and neuroscience research
Historically, the tools to study neural function and mechanisms of drug action in humans at a cellular level have proved elusive. OM-derived cells have emerged as a promising platform for such investigations in a range of neuropsychiatric, neurodegenerative and developmental disorders.
The ability to investigate functional cellular characteristics represents a distinctive opportunity afforded by the OM-derived cell paradigm. The processes of cell proliferation, adhesion, motility, metabolism and apoptosis have been investigated by Mackay-Sim and colleagues, who showed that ON-derived cells from schizophrenia patients (but not bipolar disorder patients), when cultured in a monolayer from non-dissociated biopsy tissue, proliferate more rapidly than ON-derived cells from healthy controls.14, 15, 17 Such cells were also reported to adhere less effectively to the culture plate than those from controls, both during establishment of the initial explant15 and during subsequent monolayer culture.18 This abnormal adhesion was accompanied by increased cell motility, decreased vinculin-positive adhesion complexes and faster disassembly of focal adhesions in schizophrenia ON-derived cells than controls.18 Metabolic activity of ON-derived cells has been investigated in schizophrenia and Parkinson's disease, and was reduced in Parkinson's disease.16 Increased cell death has been observed in ON-derived cells from bipolar disorder patients (but not schizophrenia patients), relative to healthy controls,14 although a subsequent study reported increased caspase-3/7 activity in ON-derived cells from schizophrenia patients, suggesting increased apoptotic activity.16
Cellular processes governing microtubule formation and cytoskeletal structure have also been investigated in OM-derived cells from individuals with psychiatric illness. Dynamic structural processes have been monitored across time in ON-derived cells, and increased microtubule stability in ON-derived cells from individuals with schizophrenia than in ON-derived cells from controls has been reported.135 In addition, in a paradigm employing exfoliation and monolayer culture, ON-derived cells from schizophrenia patients were described by Benitez-King and colleagues to display abnormal microtubule organization, whereas ON-derived cells from bipolar disorder patients displayed decreased microtubule length and cytoplasmic β-tubulin III protein expression.24, 25 Decreased L-type voltage-activated calcium currents in the ON-derived cells from individuals with schizophrenia were also reported in this study.26 Overall, these studies suggest the potential utility of OM-derived cells for the investigation of cellular function in neuropsychiatric disorders. The reported findings in OM-derived cells implicate processes and pathways, which have been independently implicated in schizophrenia and bipolar disorder by postmortem studies, such as in neurogenesis,136, 137 neuronal migration137, 138 and apoptosis.139 However, they also hold the promise of revealing aspects of neural cell function in psychiatric illness that are difficult to investigate using traditional approaches.
The OM-derived cell culture approach also provides a powerful platform to explore mechanisms of drug action, and to target pharmacologically the molecular pathways of interest in psychiatric disorders. Studies have been performed investigating the effects of pharmacological agents on intracellular signaling, molecular expression and cell function in ON-derived cells derived from schizophrenia, bipolar disorder and major depressive disorder patients. Feron et al.140 demonstrated that dopamine increases apoptosis in control ON-derived cells, but decreases apoptosis in schizophrenia cells. Cellular effects of lithium, a first-line bipolar disorder treatment, have also been explored in ON-derived cells, revealing amelioration by lithium of ionic stress-induced apoptosis and TRPM2 overexpression.141 In major depressive disorder, there is evidence that ON-derived cells display decreased nuclear translocation of the glucocortocoid receptor, GR, in response to glucocorticoids relative to controls.142 This diminished receptor translocation was accompanied by greater cytoplasmic association of GR with the immunophillin FKBP51.142 These studies suggest the potential utility of ON-derived cells for understanding current treatments and developing novel pharmacological agents in psychiatry.
The experimental potential of OM-derived cells from psychiatric patients has also been highlighted by studies of subcellular molecular abnormalities. As in work with ex vivo ON tissue, many molecular and cellular studies using ON-derived cells have demonstrated a concordance with postmortem brain studies. ON-derived cells from Alzheimer's patients exhibited increased amyloid precursor protein levels,143 along with other protein alterations,23 consistent with changes in multiple brain regions in the illness.52 Dysregulation of gene expression in multiple cellular pathways, including cell cycle regulation,14, 17 oxidative stress response,16, 144 focal adhesion16 and axon guidance16 have been reported in ON-derived cells from schizophrenia patients, some of which may be meditated by altered histone H3 lysine 4 trimethylation.144 These findings are consistent with postmortem brain studies in schizophrenia showing aberrant histone H3 lysine 4 trimethylation within the GAD1 gene145 and dysregulated expression of immune genes.146, 147 Protein changes in oxidative stress and cell adhesion pathways have also been recently reported in a preliminary human iPSC study.148 As described earlier, increased expression of miR382 has been reported in ON-derived cells from individuals with schizophrenia,21 mirroring findings in ex vivo microdissected ON tissue21 and postmortem prefrontal cortex.53 In ON-derived cells from bipolar disorder patients, gene expression changes within the phosphatidylinositol signaling pathway14 have been reported. In Alzheimer's and Parkinson's diseases, OM-derived cells have also demonstrated altered gene and protein expression profiles, implicating mitochondrial function, oxidative stress and xenobiotic metabolism in these illnesses.16, 22 Finally, OM-derived cells have also yielded insights into molecular pathologies in developmental disorders. In an early study interpreted as suggesting blood–brain concordance of FMRP deficits in fragile X syndrome, decreased FMRP protein was observed in ON-derived cells from affected individuals.149 LP-MSCs from individuals with familial dysautonomia, which is caused by mutations within the IKBKAP gene, display lower IKBKAP mRNA expression, decreased IKAP/hELP1 protein, altered IKAP/hELP1 subcellular localization and dysregulation of genes involved in cell migration and cytoskeletal reorganization.97 Overall, these studies indicate the potential for OM-derived cells to shed light on molecular abnormalities associated with brain disorders, and the concordance of many OM-derived cell studies with postmortem findings highlights the potential for OM-derived cells to reflect pathophysiological mechanisms in the brain.
Conclusions
Advantages and challenges for olfactory paradigms in the study of neuropsychiatric illnesses
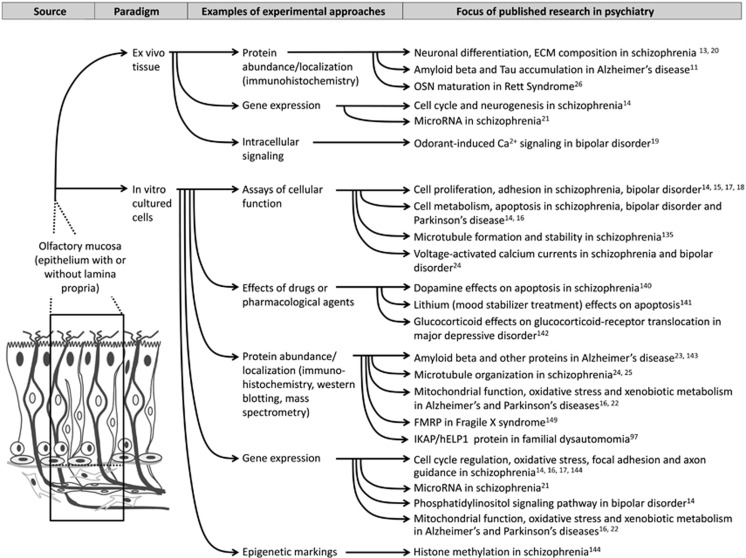
Olfactory mucosal biopsy studies afford a unique opportunity in neuropsychiatric research, offering ex vivo and in vitro neural cells from living individuals, which harbor biological characteristics that may be more relevant to neuropsychiatric disease than blood cells150 or skin fibroblasts. Figure 3 provides a summary of the previous and potential uses of OM-derived cells in psychiatric and neuroscience research, as described throughout this review.
Figure 3.
Examples of the use of ex vivo olfactory tissues and in vitro OM-derived cells in psychiatric research.
The OM model has a number of strengths. First, ex vivo OM tissues were previously exposed to the in vivo neurohormonal milieu and contain neurobiological signatures of the in vivo condition. As such, they can serve as a point of reference for in vivo and in vitro OM findings, bridging the gap between these two approaches. Second, OM biopsies, though limited by the quantity of tissue available, can be safely obtained from the same subjects multiple times (monthly, up to three consecutive times in our human subjects protocols) and can be fitted with longitudinal clinical study designs. In this approach, OM biopsies are obtained in specific phases of the illness, while patients' clinical conditions are carefully characterized. Interpretation of neurobiological parameters in the context of clinical changes is a rare opportunity in neuropsychiatric research. Examples of such use include but are not limited to the collection and examination of OM biopsies from (1) individuals at risk for schizophrenia and unaffected relatives before and after the onset of illness, (2) mood disorder patients before, during and after disease episode (such as in depressive disorders or bipolar disorder), (3) any neurodevelopmental or psychiatric disease before, during or after treatment. Finally, one promising aspect of using ex vivo OM tissues in psychiatric illness involves their relation with the broader olfactory neurocircuitry: olfactory mucosa→olfactory bulb→olfactory cortex. Disease-associated alterations within the ON may not occur in isolation, and may reflect brain abnormalities within the broader olfactory neurocircuitry. Indeed, numerous structural and functional decrements associated with neuropsychiatric diseases are reported in many of these brain regions. Olfactory circuitry can now be interrogated by psychophysical testing, electroolfactogram and evoked potentials. Combining these measures with molecular and cellular measures derived from OM biopsy tissues can help us to link molecules to cellular changes and then to circuit activity.
An important consideration in using ex vivo tissues is that the human ON is patchy and thus some biopsy tissues may not contain OSNs. Approximately 70 to 80% of ON biopsy tissues obtained from nasal septum or middle turbinate yield olfactory receptor neurons when dissociated immediately from the biopsy (unpublished observations). This is an important consideration, however, particularly for histologic examination of OE tissues. As the neuroepithelial patches are intermixed with respiratory epithelium, quantitative assessment of protein immunoreactivity or mRNA needs to be conducted by delimiting neuroepithelial versus respiratory patches in histologic sections. Under in vitro conditions, however, such limitations do not appear to figure greatly into the overall cellular composition of resultant culture cells. Nonetheless, quantitative assessment of cell type-specific proteins, whether neuronal, glial or epithelial, should be a guide for including cell lines for between-group comparison studies.
As noted in the preceding sections, the in vitro ON paradigm is particularly informative in studies of active cellular processes and pathways, where pharmacologic manipulations are to be tested or where larger numbers of cells from an individual are required. Importantly, as these cells are of neuronal and glial lineages they better represent the molecular dysregulations of neuropsychiatric disease than those of other cell types used in in vitro studies, such as blood cells. Indeed gene expression profiles of cells derived from OM biopsy more closely resemble brain tissue, stem cells and neurons derived from stem cells than blood cells (see Horiuchi et al.150 for a comparison of gene expression profiles of brain tissue, OM cells, stem cells and iPSCs). While iPS and induced neuronal cell paradigms offer unparalleled opportunities to generate differentiated neurons in vitro from living individuals, there has been considerably less study of the capacity of OM cells to generate differentiated neurons. Nevertheless, OM cells have distinct advantages (Table 2). The timeline from OM biopsy to availability of proliferated precursor cells is ~4 weeks, whereas iPSC reprogramming protocol timelines are considerably longer, varying on the basis of source cells and methods. In addition, OM cells are readily proliferative and can be rapidly expanded over multiple passages. Thus OM studies have lower costs and require less time and labor (Table 2).
Table 2. General comparison of some characteristics of olfactory mucosa (OM)-derived, induced pluripotent (iPS) and induced neural (iN) stem cells relevant to the use of these cells in the study of psychiatric, neurodevelopmental and neurodegenerative disorders.
| Characteristic | Adult human OM-derived cells | Adult human iPS cells | Adult human iN cells |
|---|---|---|---|
| Ease of collection | Moderatea | High | High |
| Current cost to prepare cells for assay | Low–moderate | High | Moderate |
| Extent of in vitro modification | Little modification: only the influence of in vitro conditions | Substantial modification: reprogramming for pluripotency plus the influence of in vitro conditions | Substantial modification: reprogramming for somatic conversion plus the influence of in vitro conditions |
| Availability of ex vivo neural reference tissue | Yes | No | No |
| Proliferative capacity of cells used for assays | Moderate–high | Moderate | None |
| Capacity to be driven to neuronal differentiation | Varying between protocols | Robust, high efficiency | Robust, moderate efficiency |
| Cellular phenotype commonly used in assays | Proliferative putative neural progenitor cell | Differentiated neuron | Differentiated neuron |
| Action potential generation | To be further demonstrated25 | Yes | Yes |
Detailed discussion of iPS and iN cell paradigms is beyond the scope of this review, but these paradigms have been reviewed extensively elsewhere.1, 2 See text for details and references for OM-derived cell studies.
Collection of OM tissues is performed by a qualified otorhinolaryngologist using local anesthetic.
Most importantly, OM tissues are obtained as neural tissues (Table 2) and thus they do not require genomic reprogramming as with iPS or induced neuronal cells. A challenge in the iPS and induced neuronal paradigms is to determine to what degree phenotypes of these cells have been modified compared with those of brain cells. In the OM approach, however, neurobiological properties, such as epigenomic profiles, may be less modified than in the cells genetically reprogrammed. Given that the ex vivo tissues are available as the source of in vitro cells, the extent of modification could at least be evaluated in OM cells.
There are a number of challenges for researchers working with in vitro OM cells. Differences in protocols between research groups must be understood and controlled for, in particular during studies with OM-derived cells since such cells may be susceptible to variation in the means of cell harvest and the culture environment. To what degree OM-derived cells assume characteristics of differentiated neurons or glia is not entirely clear at present. As discussed earlier, for these olfactory paradigms to provide consistent insight into the pathophysiology and treatment of neuropsychiatric illnesses, additional characterization of OM-derived cells in different protocols is required to determine the characteristics of their molecular phenotypes and the similarities and differences between protocols. Ultimately, the key question surrounding the use of OM tissues and OM-derived cells in the study of psychiatric illness will be in what way findings from these studies can shed light on abnormalities in other important brain regions and neural circuits in psychiatric illness. This will involve the examination of olfactory-derived tissues and function at multiple levels of the olfactory circuit, as well as direct parallels between OM findings and brain pathology.
Acknowledgments
This work was supported by funding from K23MH079498 (KB-W), MH059852 and MH080193 (C-GH), T32MH019112-24 (SLW) and funding from the NHMRC (Australia) CJ Martin Fellowship APP1072878 (DS).
The authors declare no conflict of interest.
References
- Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet. 2011;20:R109–R115. doi: 10.1093/hmg/ddr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Ng YH, Pang ZP, Sudhof TC, Wernig M. Induced neuronal cells: how to make and define a neuron. Cell Stem Cell. 2011;9:517–525. doi: 10.1016/j.stem.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Martinez R, Salgado-Puga K, Pena-Ortega F. Amyloid beta inhibits olfactory bulb activity and the ability to smell. PLoS One. 2013;8:e75745. doi: 10.1371/journal.pone.0075745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MZ, Vicini-Chilovi B, Riva M, Zanetti M, Liberini P, Padovani A, et al. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer's disease. Arch Clin Neuropsychol. 2013;28:391–399. doi: 10.1093/arclin/act032. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2013;40:50–59. doi: 10.1093/schbul/sbt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Calkins ME, Bilker WB, Frishberg N, Borgmann-Winter K, et al. The effect of odor valence on olfactory performance in schizophrenia patients, unaffected relatives and at-risk youth. J Psychiatr Res. 2013;47:1636–1641. doi: 10.1016/j.jpsychires.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Goetz R, Keller A, Messinger JW, Bruder G, Goetz D, et al. Olfactory processing, sex effects and heterogeneity in schizophrenia. Schizophr Res. 2012;135:144–151. doi: 10.1016/j.schres.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Arnold SE, Moberg PJ. Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology. 2009;34:767–774. doi: 10.1038/npp.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, Oberbauer H, Scholtz AW, Wanko C, et al. Various bilateral olfactory deficits in male patients with schizophrenia. Schizophr Bull. 2005;31:155–165. doi: 10.1093/schbul/sbi018. [DOI] [PubMed] [Google Scholar]
- Buron E, Bulbena A. Olfaction in affective and anxiety disorders: a review of the literature. Psychopathology. 2013;46:63–74. doi: 10.1159/000338717. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67:462–469. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ. Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer's disease and schizophrenia. Ann N Y Acad Sci. 1998;855:762–775. doi: 10.1111/j.1749-6632.1998.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. doi: 10.1001/archpsyc.58.9.829. [DOI] [PubMed] [Google Scholar]
- McCurdy RD, Feron F, Perry C, Chant DC, McLean D, Matigian N, et al. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82:163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40:211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Matigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech. 2010;3:785–798. doi: 10.1242/dmm.005447. [DOI] [PubMed] [Google Scholar]
- Fan Y, Abrahamsen G, McGrath JJ, Mackay-Sim A. Altered cell cycle dynamics in schizophrenia. Biol Psychiatry. 2012;71:129–135. doi: 10.1016/j.biopsych.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Fan Y, Abrahamsen G, Mills R, Calderon CC, Tee JY, Leyton L, et al. Focal adhesion dynamics are altered in schizophrenia. Biol Psychiatry. 2013;74:418–426. doi: 10.1016/j.biopsych.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Gomez G, Restrepo D, Friedman E, Josiassen R, Pribitkin EA, et al. Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry. 2005;162:616–618. doi: 10.1176/appi.ajp.162.3.616. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Boyer-Boiteau A, Holbrook EH, Jang W, Hahn CG, Arnold SE, et al. Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia. Schizophr Res. 2013;150:366–372. doi: 10.1016/j.schres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor E, Kano S, Colantuoni C, Sawa A, Navon R, Shomron N. MicroRNA-382 expression is elevated in the olfactory neuroepithelium of schizophrenia patients. Neurobiol Dis. 2013;55:1–10. doi: 10.1016/j.nbd.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari HA, Ghanbari K, Harris PL, Jones PK, Kubat Z, Castellani RJ, et al. Oxidative damage in cultured human olfactory neurons from Alzheimer's disease patients. Aging Cell. 2004;3:41–44. doi: 10.1111/j.1474-9728.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- Johnson GS, Basaric-Keys J, Ghanbari HA, Lebovics RS, Lesch KP, Merril CR, et al. Protein alterations in olfactory neuroblasts from Alzheimer donors. Neurobiol Aging. 1994;15:675–680. doi: 10.1016/0197-4580(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Solis-Chagoyan H, Calixto E, Figueroa A, Montano LM, Berlanga C, Rodriguez-Verdugo MS, et al. Microtubule organization and L-type voltage-activated calcium current in olfactory neuronal cells obtained from patients with schizophrenia and bipolar disorder. Schizophr Res. 2013;143:384–389. doi: 10.1016/j.schres.2012.11.035. [DOI] [PubMed] [Google Scholar]
- Benitez-King G, Riquelme A, Ortiz-Lopez L, Berlanga C, Rodriguez-Verdugo MS, Romo F, et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J Neurosci Methods. 2011;201:35–45. doi: 10.1016/j.jneumeth.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Leopold D, Cai X, Hoffbuhr KC, Moses L, Hoffman EP, et al. Olfactory biopsies demonstrate a defect in neuronal development in Rett's syndrome. Ann Neurol. 2003;54:206–218. doi: 10.1002/ana.10633. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Han LY, Rawson NE, Mirza N, Borgmann-Winter K, Lenox RH, et al. In vivo and in vitro neurogenesis in human olfactory epithelium. J Comp Neurol. 2005;483:154–163. doi: 10.1002/cne.20424. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Bonnin A, Crocker C, Kawauchi S, Murray RC, Shou J, et al. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech. 2002;58:176–188. doi: 10.1002/jemt.10147. [DOI] [PubMed] [Google Scholar]
- Guo Z, Packard A, Krolewski RC, Harris MT, Manglapus GL, Schwob JE. Expression of pax6 and sox2 in adult olfactory epithelium. J Comp Neurol. 2010;518:4395–4418. doi: 10.1002/cne.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A, Giel-Moloney M, Leiter A, Schwob JE. Progenitor cell capacity of NeuroD1-expressing globose basal cells in the mouse olfactory epithelium. J Comp Neurol. 2011;519:3580–3596. doi: 10.1002/cne.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007;38:581–599. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Tome M, Lindsay SL, Riddell JS, Barnett SC. Identification of nonepithelial multipotent cells in the embryonic olfactory mucosa. Stem Cells. 2009;27:2196–2208. doi: 10.1002/stem.130. [DOI] [PubMed] [Google Scholar]
- Lindsay SL, Johnstone SA, Mountford JC, Sheikh S, Allan DB, Clark L, et al. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia. 2013;61:368–382. doi: 10.1002/glia.22440. [DOI] [PubMed] [Google Scholar]
- Delorme B, Nivet E, Gaillard J, Haupl T, Ringe J, Deveze A, et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2010;19:853–866. doi: 10.1089/scd.2009.0267. [DOI] [PubMed] [Google Scholar]
- Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Prasol MS, Estrada J, Baudhuin A, Vranizan K, Choi YG, et al. p63 regulates olfactory stem cell self-renewal and differentiation. Neuron. 2011;72:748–759. doi: 10.1016/j.neuron.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickland H, Westrum LE, Kott JN, Patterson SL, Bothwell MA. Nerve growth factor receptor expression in the young and adult rat olfactory system. Brain Res. 1991;565:269–279. doi: 10.1016/0006-8993(91)91659-o. [DOI] [PubMed] [Google Scholar]
- Minovi A, Witt M, Prescher A, Gudziol V, Dazert S, Hatt H, et al. Expression and distribution of the intermediate filament protein nestin and other stem cell related molecules in the human olfactory epithelium. Histol Histopathol. 2010;25:177–187. doi: 10.14670/HH-25.177. [DOI] [PubMed] [Google Scholar]
- Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31:6915–6927. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Shibata S, Fukuda K, Sato M, Satoh E, Nagoshi N, et al. The dual origin of the peripheral olfactory system: placode and neural crest. Mol Brain. 2011;4:34. doi: 10.1186/1756-6606-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, et al. Neural crest origin of olfactory ensheathing glia. Proc Natl Acad Sci USA. 2010;107:21040–21045. doi: 10.1073/pnas.1012248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair—what can it achieve. Nat Clin Pract Neurol. 2007;3:152–161. doi: 10.1038/ncpneuro0447. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, St, John JA. Olfactory ensheathing cells from the nose: clinical application in human spinal cord injuries. Exp Neurol. 2011;229:174–180. doi: 10.1016/j.expneurol.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A, et al. High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am J Psychiatry. 1997;154:976–982. doi: 10.1176/ajp.154.7.976. [DOI] [PubMed] [Google Scholar]
- Dubovsky SL, Christiano J, Daniell LC, Franks RD, Murphy J, Adler L, et al. Increased platelet intracellular calcium concentration in patients with bipolar affective disorders. Arch Gen Psychiatry. 1989;46:632–638. doi: 10.1001/archpsyc.1989.01810070058010. [DOI] [PubMed] [Google Scholar]
- Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic PS, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA. 2003;100:313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W, Feron F, Wetzig A, Cameron N, Splatt K, Bellette B, et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- Murrell W, Sanford E, Anderberg L, Cavanagh B, Mackay-Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009;9:585–594. doi: 10.1016/j.spinee.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Girard SD, Mackay-Sim A. Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol. 2013;1059:107–114. doi: 10.1007/978-1-62703-574-3_10. [DOI] [PubMed] [Google Scholar]
- Roisen FJ, Klueber KM, Lu CL, Hatcher LM, Dozier A, Shields CB, et al. Adult human olfactory stem cells. Brain Res. 2001;890:11–22. doi: 10.1016/s0006-8993(00)03016-x. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, McGrath JJ, Mackay-Sim A. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg. 1998;124:861–866. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- Borgmann-Winter KE, Rawson NE, Wang HY, Wang H, Macdonald ML, Ozdener MH, et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience. 2009;158:642–653. doi: 10.1016/j.neuroscience.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Sunderland T, Zheng BB, Resau J, Dufy B, Barker J, et al. Continuous culture of neuronal cells from adult human olfactory epithelium. J Mol Neurosci. 1992;3:137–146. doi: 10.1007/BF02919405. [DOI] [PubMed] [Google Scholar]
- Othman M, Lu C, Klueber K, Winstead W, Roisen F. Clonal analysis of adult human olfactory neurosphere forming cells. Biotech Histochem. 2005;80:189–200. doi: 10.1080/10520290500469777. [DOI] [PubMed] [Google Scholar]
- Zhang X, Klueber KM, Guo Z, Lu C, Roisen FJ. Adult human olfactory neural progenitors cultured in defined medium. Exp Neurol. 2004;186:112–123. doi: 10.1016/j.expneurol.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Winstead W, Marshall CT, Lu CL, Klueber KM, Roisen FJ. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol. 2005;19:83–90. [PubMed] [Google Scholar]
- Grill RJ, Pixley SK. In vitro generation of adult rat olfactory sensory neurons and regulation of maturation by coculture with CNS tissues. J Neurosci. 1997;17:3120–3127. doi: 10.1523/JNEUROSCI.17-09-03120.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Lambropoulos J, Woo JK, Peluso CE, Schwob JE. Maintaining epitheliopoietic potency when culturing olfactory progenitors. Exp Neurol. 2008;214:25–36. doi: 10.1016/j.expneurol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Gomez G, Rawson NE, Hahn CG, Michaels R, Restrepo D. Characteristics of odorant elicited calcium changes in cultured human olfactory neurons. J Neurosci Res. 2000;62:737–749. doi: 10.1002/1097-4547(20001201)62:5<737::AID-JNR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Jouhilahti EM, Peltonen S, Peltonen J. Class III beta-tubulin is a component of the mitotic spindle in multiple cell types. J Histochem Cytochem. 2008;56:1113–1119. doi: 10.1369/jhc.2008.952002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Farber NB, Gottlieb DI. Neurons of the olfactory epithelium in adult rats contain vimentin. J Neurosci. 1986;6:208–217. doi: 10.1523/JNEUROSCI.06-01-00208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Basaric-Keys J, Li Y, Lester DS, Lebovics RS, Lesch KP, et al. Human olfactory neuroepithelial cells: tyrosine phosphorylation and process extension are increased by the combination of IL-1beta, IL-6, NGF, and bFGF. Exp Neurol. 1996;142:179–194. doi: 10.1006/exnr.1996.0189. [DOI] [PubMed] [Google Scholar]
- Bianco JI, Perry C, Harkin DG, Mackay-Sim A, Feron F. Neurotrophin 3 promotes purification and proliferation of olfactory ensheathing cells from human nose. Glia. 2004;45:111–123. doi: 10.1002/glia.10298. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahed A, Rowland JW, McDonald T, Boyd JG, Doucette R, Kawaja MD. Olfactory ensheathing cells express smooth muscle alpha-actin in vitro and in vivo. J Comp Neurol. 2007;503:209–223. doi: 10.1002/cne.21385. [DOI] [PubMed] [Google Scholar]
- Nivet E, Vignes M, Girard SD, Pierrisnard C, Baril N, Deveze A, et al. Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Invest. 2011;121:2808–2820. doi: 10.1172/JCI44489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski RC, Jang W, Schwob JE. The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo. Exp Neurol. 2011;229:308–323. doi: 10.1016/j.expneurol.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud P, He X, Zhao C, Ibanez C, Raha-Chowdhury R, Caldwell MA, et al. Contrasting effects of basic fibroblast growth factor and epidermal growth factor on mouse neonatal olfactory mucosa cells. Eur J Neurosci. 2007;26:3345–3357. doi: 10.1111/j.1460-9568.2007.05950.x. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995;6:363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Wolozin BL, Schwob JE. FGF2 suppresses neuronogenesis of a cell line derived from rat olfactory epithelium. J Neurobiol. 1997;33:411–428. [PubMed] [Google Scholar]
- MacDonald KP, Murrell WG, Bartlett PF, Bushell GR, Mackay-Sim A. FGF2 promotes neuronal differentiation in explant cultures of adult and embryonic mouse olfactory epithelium. J Neurosci Res. 1996;44:27–39. doi: 10.1002/(SICI)1097-4547(19960401)44:1<27::AID-JNR4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McEntire JK, Pixley SK. Olfactory receptor neurons in partially purified epithelial cell cultures: comparison of techniques for partial purification and identification of insulin as an important survival factor. Chem Senses. 2000;25:93–101. doi: 10.1093/chemse/25.1.93. [DOI] [PubMed] [Google Scholar]
- Pixley SK. Characterization of olfactory receptor neurons and other cell types in dissociated rat olfactory cell cultures. Int J Dev Neurosci. 1996;14:823–839. doi: 10.1016/s0736-5748(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim SY, Nam S, Ronnett GV, Han HS, Moon C, et al. Direct measurement of extracellular electrical signals from mammalian olfactory sensory neurons in planar triode devices. Analyst. 2012;137:2047–2053. doi: 10.1039/c2an16205a. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Hester LD, Snyder SH. Primary culture of neonatal rat olfactory neurons. J Neurosci. 1991;11:1243–1255. doi: 10.1523/JNEUROSCI.11-05-01243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AM, Manis PB, Reed RR, Ronnett GV. Olfactory receptor neurons exist as distinct subclasses of immature and mature cells in primary culture. Neuroscience. 1999;93:1301–1312. doi: 10.1016/s0306-4522(99)00193-1. [DOI] [PubMed] [Google Scholar]
- Vannelli GB, Ensoli F, Zonefrati R, Kubota Y, Arcangeli A, Becchetti A, et al. Neuroblast long-term cell cultures from human fetal olfactory epithelium respond to odors. J Neurosci. 1995;15:4382–4394. doi: 10.1523/JNEUROSCI.15-06-04382.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli F, Fiorelli V, Vannelli B, Barni T, De Cristofaro M, Ensoli B, et al. Basic fibroblast growth factor supports human olfactory neurogenesis by autocrine/paracrine mechanisms. Neuroscience. 1998;86:881–893. doi: 10.1016/s0306-4522(98)00104-3. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. A novel embryonic nestin-expressing radial glia-like progenitor gives rise to zonally restricted olfactory and vomeronasal neurons. J Neurosci. 2008;28:4271–4282. doi: 10.1523/JNEUROSCI.5566-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb JD, Mumm JS, Calof AL. Apoptosis in the neuronal lineage of the mouse olfactory epithelium: regulation in vivo and in vitro. Dev Biol. 1995;172:307–323. doi: 10.1006/dbio.1995.0025. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker ES, Lehtinen MK, Maynard T, Zirlinger M, Dulac C, Rawson N, et al. Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development. 2010;137:2471–2481. doi: 10.1242/dev.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Klueber KM, Guo Z, Cai J, Lu C, Winstead WI, et al. Induction of neuronal differentiation of adult human olfactory neuroepithelial-derived progenitors. Brain Res. 2006;1073-1074:109–119. doi: 10.1016/j.brainres.2005.12.059. [DOI] [PubMed] [Google Scholar]
- McCurdy RD, Feron F, McGrath JJ, Mackay-Sim A. Regulation of adult olfactory neurogenesis by insulin-like growth factor-I. Eur J Neurosci. 2005;22:1581–1588. doi: 10.1111/j.1460-9568.2005.04355.x. [DOI] [PubMed] [Google Scholar]
- Boone N, Loriod B, Bergon A, Sbai O, Formisano-Treziny C, Gabert J, et al. Olfactory stem cells, a new cellular model for studying molecular mechanisms underlying familial dysautonomia. PLoS One. 2010;5:e15590. doi: 10.1371/journal.pone.0015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner JF, Hauser S, Widera D, Muller J, Qunneis F, Zander C, et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur Cell Mater. 2011;22:403–419. doi: 10.22203/ecm.v022a30. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Narla RK, Little S, Hyde JF, Getchell ML. Horizontal basal cell proliferation in the olfactory epithelium of transforming growth factor-alpha transgenic mice. Cell Tissue Res. 2000;299:185–192. doi: 10.1007/s004419900149. [DOI] [PubMed] [Google Scholar]
- Packard A, Schnittke N, Romano RA, Sinha S, Schwob JE. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J Neurosci. 2011;31:8748–8759. doi: 10.1523/JNEUROSCI.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Santos R, Kim J, Hollenbeck PL, Murray RC, Calof AL. The role of foxg1 in the development of neural stem cells of the olfactory epithelium. Ann N Y Acad Sci. 2009;1170:21–27. doi: 10.1111/j.1749-6632.2009.04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso CE, Jang W, Drager UC, Schwob JE. Differential expression of components of the retinoic acid signaling pathway in the adult mouse olfactory epithelium. J Comp Neurol. 2012;520:3707–3726. doi: 10.1002/cne.23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammeta N, Hardin DL, McClintock TS. Uncx regulates proliferation of neural progenitor cells and neuronal survival in the olfactory epithelium. Mol Cell Neurosci. 2010;45:398–407. doi: 10.1016/j.mcn.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, et al. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL. Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron. 1994;13:1083–1097. doi: 10.1016/0896-6273(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, et al. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kageyama R, Suzuki Y, Kawakami K. Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int J Dev Biol. 2010;54:1453–1464. doi: 10.1387/ijdb.093041ki. [DOI] [PubMed] [Google Scholar]
- Shou J, Murray RC, Rim PC, Calof AL. Opposing effects of bone morphogenetic proteins on neuron production and survival in the olfactory receptor neuron lineage. Development. 2000;127:5403–5413. doi: 10.1242/dev.127.24.5403. [DOI] [PubMed] [Google Scholar]
- Shou J, Rim PC, Calof AL. BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor. Nat Neurosci. 1999;2:339–345. doi: 10.1038/7251. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, et al. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Gokoffski KK, Wu HH, Beites CL, Kim J, Kim EJ, Matzuk MM, et al. Activin and GDF11 collaborate in feedback control of neuroepithelial stem cell proliferation and fate. Development. 2011;138:4131–4142. doi: 10.1242/dev.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Tsukatani T, Fillmore HL, Hamilton HR, Holbrook EH, Costanzo RM. Matrix metalloproteinase expression in the olfactory epithelium. Neuroreport. 2003;14:1135–1140. doi: 10.1097/01.wnr.0000075306.76650.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault FM, Nuthall HN, Dong Z, Lo R, Barnabe-Heider F, Miller FD, et al. Role for Runx1 in the proliferation and neuronal differentiation of selected progenitor cells in the mammalian nervous system. J Neurosci. 2005;25:2050–2061. doi: 10.1523/JNEUROSCI.5108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KL, Hort YJ, Herzog H, Shine J. Neuropeptide Y and peptide YY have distinct roles in adult mouse olfactory neurogenesis. J Neurosci Res. 2012;90:1126–1135. doi: 10.1002/jnr.23008. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Karl T, Hort Y, Duffy L, Shine J, Herzog H. Y1 receptors are critical for the proliferation of adult mouse precursor cells in the olfactory neuroepithelium. J Neurochem. 2008;105:641–652. doi: 10.1111/j.1471-4159.2007.05188.x. [DOI] [PubMed] [Google Scholar]
- Jia C, Hegg CC. NPY mediates ATP-induced neuroproliferation in adult mouse olfactory epithelium. Neurobiol Dis. 2010;38:405–413. doi: 10.1016/j.nbd.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Ou J, Zhu LJ, Green MR. Transcription factor ATF5 is required for terminal differentiation and survival of olfactory sensory neurons. Proc Natl Acad Sci USA. 2012;109:18589–18594. doi: 10.1073/pnas.1210479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JC, Bose SC, Stromberg AJ, McClintock TS. Emx2 stimulates odorant receptor gene expression. Chem Senses. 2008;33:825–837. doi: 10.1093/chemse/bjn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota J, Mombaerts P. The LIM-homeodomain protein Lhx2 is required for complete development of mouse olfactory sensory neurons. Proc Natl Acad Sci USA. 2004;101:8751–8755. doi: 10.1073/pnas.0400940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterud A, Alenius M, Carlsson L, Bohm S. The Lim homeobox gene Lhx2 is required for olfactory sensory neuron identity. Development. 2004;131:5319–5326. doi: 10.1242/dev.01416. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Reed RR. Zfp423/OAZ participates in a developmental switch during olfactory neurogenesis. Neuron. 2007;54:547–557. doi: 10.1016/j.neuron.2007.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Liu BQ, Kim SY, Kim EJ, Park YJ, Yoo JY, et al. Leukemia inhibitory factor promotes olfactory sensory neuronal survival via phosphoinositide 3-kinase pathway activation and Bcl-2. J Neurosci Res. 2009;87:1098–1106. doi: 10.1002/jnr.21919. [DOI] [PubMed] [Google Scholar]
- Moon C, Yoo JY, Matarazzo V, Sung YK, Kim EJ, Ronnett GV. Leukemia inhibitory factor inhibits neuronal terminal differentiation through STAT3 activation. Proc Natl Acad Sci USA. 2002;99:9015–9020. doi: 10.1073/pnas.132131699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Yoshida T. Promotion of neurogenesis in mouse olfactory neuronal progenitor cells by leukemia inhibitory factor in vitro. Neurosci Lett. 1997;225:165–168. doi: 10.1016/s0304-3940(97)00216-4. [DOI] [PubMed] [Google Scholar]
- Buckland ME, Cunningham AM. Alterations in expression of the neurotrophic factors glial cell line-derived neurotrophic factor, ciliary neurotrophic factor and brain-derived neurotrophic factor, in the target-deprived olfactory neuroepithelium. Neuroscience. 1999;90:333–347. doi: 10.1016/s0306-4522(98)00270-x. [DOI] [PubMed] [Google Scholar]
- Feron F, Bianco J, Ferguson I, Mackay-Sim A. Neurotrophin expression in the adult olfactory epithelium. Brain Res. 2008;1196:13–21. doi: 10.1016/j.brainres.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Clevenger AC, Salcedo E, Jones KR, Restrepo D. BDNF promoter-mediated beta-galactosidase expression in the olfactory epithelium and bulb. Chem Senses. 2008;33:531–539. doi: 10.1093/chemse/bjn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, May V, Eipper BA, Ronnett GV. Pituitary adenylyl cyclase-activating peptides and alpha-amidation in olfactory neurogenesis and neuronal survival in vitro. J Neurosci. 2001;21:4625–4636. doi: 10.1523/JNEUROSCI.21-13-04625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulz L, Astorga G, Bellette B, Iturriaga R, Mackay-Sim A, Bacigalupo J. Nitric oxide regulates neurogenesis in adult olfactory epithelium in vitro. Nitric Oxide. 2009;20:238–252. doi: 10.1016/j.niox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Brown AS, Borgmann-Winter K, Hahn CG, Role L, Talmage D, Gur R, et al. Increased stability of microtubules in cultured olfactory neuroepithelial cells from individuals with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;48:252–258. doi: 10.1016/j.pnpbp.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, et al. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry. 2012;72:725–733. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Feron F, Vincent A, Mackay-Sim A. Dopamine promotes differentiation of olfactory neuron in vitro. Brain Res. 1999;845:252–259. doi: 10.1016/s0006-8993(99)01959-9. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lei Z, Lu C, Roisen FJ, El-Mallakh RS. Effect of ionic stress on apoptosis and the expression of TRPM2 in human olfactory neuroepithelial-derived progenitors. World J Biol Psychiatry. 2010;11:972–984. doi: 10.3109/15622975.2010.507784. [DOI] [PubMed] [Google Scholar]
- Borgmann-Winter K, Jefferson S, Sinclair D, Ray R, Willard S, Willis B, et al. Glucocorticoid receptor translocation is decreased in olfactory neuroepithelial cells of patients with depressionSubmitted.
- Wolozin B, Lesch P, Lebovics R, Sunderland T. Olfactory neuroblasts from Alzheimer donors: studies on APP processing and cell regulation. Biol Psychiatry. 1993;34:824–838. doi: 10.1016/0006-3223(93)90051-e. [DOI] [PubMed] [Google Scholar]
- Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2013;18:740–742. doi: 10.1038/mp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry. 2014;4:e365. doi: 10.1038/tp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2014. [DOI] [PMC free article] [PubMed]
- Abrams MT, Kaufmann WE, Rousseau F, Oostra BA, Wolozin B, Taylor CV, et al. FMR1 gene expression in olfactory neuroblasts from two males with fragile X syndrome. Am J Med Genet. 1999;82:25–30. doi: 10.1002/(sici)1096-8628(19990101)82:1<25::aid-ajmg5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Horiuchi Y, Kano S, Ishizuka K, Cascella NG, Ishii S, Talbot CC, Jr, et al. Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: utility and limitation of the surrogate tissues in research for brain disorders. Neurosci Res. 2013;77:247–250. doi: 10.1016/j.neures.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]