Abstract
Injectable extended-release naltrexone (XRNTX) presents an effective therapeutic strategy for opioid addiction, however its utility could be hampered by poor adherence. To gain a better insight into this phenomenon, we utilized blood oxygenation level-dependent functional magnetic resonance imaging (fMRI) in conjunction with a validated cue-induced craving procedure to examine neural correlates of XRNTX adherence. We operationalized treatment adherence as the number of monthly XRNTX injections (range: 0–3) administered to a group of fully detoxified heroin-dependent subjects (n=32). Additional outcomes included urine toxicology screening and self-reported tobacco use. The presented heroin-related visual cues reliably elicited heroin craving in all tested subjects. Nine, five, three and 15 of the participants, respectively, received zero, one, two and three XRNTX injections, predicted by the individual baseline fMRI signal change in response to the cues in the medial prefrontal cortex, a brain region involved in inhibitory self-control and emotional appraisal. The incidence of opioid-positive urines during the XRNTX therapy was low and remained about half the pre-treatment rate after the XRNTX ended. During the treatment, cigarette smoking behaviors followed patterns of opioid use, while cocaine consumption was increased with reductions in opioid use. The present data support the hypothesis that medial prefrontal cortex functions are involved in adherence to opioid antagonist therapy. A potential role of concurrent non-opioid addictive substances consumption during the XRNTX pharmacotherapy warrants further investigation. Our findings set the stage for further bio-behavioral investigations of the mechanisms of relapse prevention in opioid dependence.
Introduction
Heroin addiction is a resurgent public health problem in the United States driven by the enhanced purity of the drug, its relatively low cost and consequent availability as a cheap substitute for opioid painkillers, the use of which has reached epidemic proportions.1, 2 Over half a million people in the United States are addicted to heroin.3, 4 Many of them die from overdose, partially explaining the fourfold increase in mortality rates attributed to opioid drugs use over the past two decades,2, 5 let alone steadily rising crime behaviors6 and medical system usage, with decreased productivity and breakup of the family and societal ties observed in this population. These behaviors also make effective treatment delivery difficult. For example, only ~14% of those addicted to heroin in the United States receive opioid agonist replacement pharmacotherapy using methadone or buprenorphine.7 Since treatment with the above agonist agents can be accompanied by serious medical complications, clinicians and researchers alike have been seeking alternative pharmacological strategies that would share their beneficial features, without the cognitive, metabolic, endocrine or cardiac side effects.8, 9 Treatment with the opioid antagonists naltrexone is devoid of such side effects and is pharmacologically analogous to abstinence. However, non-adherence is a contributing factor to at least 50% of overall therapeutic failures10 and significantly more so in the oral naltrexone treatment of opioid dependence, limiting its overall therapeutic effectiveness.11, 12 Injectable extended-release naltrexone (XRNTX; Vivitrol) has been developed to overcome this obstacle. XRNTX is highly effective at producing extended pharmacological abstinence from opioids, even if patients continue to crave or use opioids.13 During active XRNTX treatment, craving and opioid use does not result in immediate psychopharmacological effects. Therefore, adherence to treatment is critical to the overall therapeutic outcomes of XRNTX.10 While controlled trials comparing agonist and antagonist therapies are still underway (http://clinicaltrials.gov/show/NCT02032433), available data indicate that there are significant individual differences in treatment adherence.14, 15, 16, 17 Therefore, continued research on XRNTX adherence mechanisms in individual patients is critical for the development of personalized abstinence and relapse prevention strategies. One challenge to the investigation of adherence is the operationalization of key terms. Treatment adherence is a complex construct encompassing factors related to the patient (for example, adequacy of information about the illness, the medicine and therapeutic alternatives), the drug (for example, adverse effects, ease of administration and costs) and the healthcare system (for example, clarity of communication, complexity of regimen, therapeutic alliances and access to care).10
We operationalized adherence to XRNTX as the number of injections out of the maximum available, received by participants. Although this is only one of several ways in which treatment adherence might be defined, this approach enjoys several notable advantages: an unequivocal, measurable definition, independence from underreporting due to denial and/or cognitive deficits secondary to drug use, and a resistance to potential gaps in urine toxicology screening.18, 19, 20, 21, 22 The effects of pharmacological opioid blockade induced by XRNTX are nearly uniform, making adherence to the monthly injections a key determinant of its overall clinical efficacy. In the present study, we identified the neural correlates of adherence to XRNTX injections (range 0–3) during a 4-month clinical trial in fully detoxified patients with opioid (that is, heroin) dependence. To probe the brain regions implicated in heroin addiction, we used a validated heroin cues-induced craving procedure in conjunction with blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI). Notably, our previous study in heroin-dependent subjects reported significant increases in XRNTX-induced fMRI signal in the medial prefrontal cortex (mPFC) in response to the same visual heroin cues.23 These results could be construed as a moderation of cortical limbic activity primed by conditioned cues24 or as inhibitory control over the motivational salience attributed to the drug reward.25, 26 This may be why, in treatment-seeking smokers, mPFC response to individually tailored anti-tobacco messages predicted therapeutic outcomes.27 With these considerations in mind, we hypothesized that pre-treatment mPFC response to heroin-related stimuli will predict the number of XRNTX injections accepted by the participants.
Materials and methods
Subjects
Thirty-two opioid-dependent individuals using intravenous heroin as the drug of choice (mean age±s.d.=29.19±7.5 years, education 13.2±1.9 years, 15 female, 28 Caucasian, 2 African American and 2 Asian, all right-handed) were recruited through local advertising. Each gave written informed consent to participate in the University of Pennsylvania Institutional Review Board-approved study.
A DSM-IV-TR diagnosis of opioid dependence was established using the best estimate format, on the basis of all available sources of information, including history, clinical interview, the Structured Clinical Interview for DSM-IV28 and the Addiction Severity Index (ASI) 5th Edition.29 The average ASI Drug Composite Score was 0.29±0.11 (range 0–1.00). The subjects intravenously used an average of 6.7±4.3 (range of 1.3–15.0) ‘bags' (0.1–1.5 gm) of heroin per day during the 90 days before the enrollment. Handedness was assessed with the Edinburgh Handedness Inventory.30
Inclusion criteria were: (1) 18–55 years of age; (2) DSM-IV-TR diagnosis of opioid dependence; (3) active opioid use, confirmed by urine toxicology screen and self-reported monthly intravenous heroin use for more than 2 weeks in the past 3 months; (4) urine toxicology screen negative for opioids after detoxification; (5) good physical health as determined by history and physical examination, by screening blood work-up and by urinalysis.
Exclusion criteria were: (1) current chronic medical illnesses; (2) current use of potentially confounding medications such as anti-dopaminergic agents, anti-depressants, anticonvulsants, mood stabilizers and beta-blockers; (3) current DSM-IV-TR Axis I psychiatric disorders with the exception of opioid and nicotine dependence, non-dependent cocaine abuse and depressive disorders; (4) lifetime history of concurrent intravenous cocaine and heroin (speedball) administration; (5) pregnancy or breastfeeding; (6) history of clinically significant head trauma; (7) contraindications for XRNTX treatment including medical conditions requiring opioid analgesics, for example, chronic pain or planned surgery, obesity, elevated liver enzymes (>3 times upper limit of normal), failure to complete opioid detoxification; and (8) contraindications for MRI, such as indwelling magnetically active foreign bodies and phobia to enclosed spaces.
Enzyme-linked immunosorbent assay urine drug screens (UDSs, Redwood Toxicology Laboratory, Santa Rosa, CA, USA) were used for qualitative testing for morphine, oxycontin, methadone, buprenorphine, cocaine, amphetamine, methamphetamine, benzodiazepines, marijuana and phencyclidine.
Study medicine
To be eligible for the XRNTX injection, participants were required to have a UDS negative for opioids. A challenge with 0.6 mg of naloxone HCL intravenously, was performed for all subjects to ascertain the pharmacodynamic completeness of their detoxification. Eligible participants could receive up to three monthly injections of XRNTX (manufactured by Alkermes, Cambridge, MA, USA). In this formulation, 380 mg of naltrexone is gradually released from dissolvable polymer microspheres. XRNTX suspension was administered intramuscularly into the deep gluteal muscle via a custom-honed needle provided for this purpose by the manufacturer. Buttocks were alternated at monthly injection. Injection sites were monitored for 45 minutes after XRNTX administration for localized reactions and followed up by phone daily for 3 days post injection and then at each weekly visit.
As part of the consent procedure, participants were briefed about the loss of pharmacological effects of opioids resulting from the XRNTX treatment, and the dangers of attempting to overcome the opiate receptor blockade with higher than usual opioid doses.31, 32
Assessments timeline
The UDSs were conducted on a weekly basis. Given our prior report on post-detoxification naltrexone-induced diminution of smoking,33 the weekly Timeline Followback questionnaire, which assessed the number of cigarettes smoked per day (CPD) was collected. In addition, the Beck Depression Inventory was assessed weekly. Also, plasma concentrations of naltrexone and 6-beta-naltrexol (an active metabolite) were measured 13±7 days after the first injection, 22±13 days after the second injection and 21±5 days after the third injection with established liquid chromatography and tandem mass spectrometry techniques used in our prior study.23, 34
MRI sessions were conducted after detoxification and before the first XRNTX injection (Pre-XRNTX) and ~6 weeks (37±4 days) after the third injection (Post-XRNTX). During the second and third months of the study, continuation of care was discussed with the participants and they were given referrals to treatment providers in the community.
fMRI cue reactivity task
Two comparable sets of previously reported cue reactivity tasks23, 35 were presented in two MRI sessions and counterbalanced across participants. Each stimuli set comprised 48 heroin-related and 48 neutral images. The former included images of heroin injection, preparation and paraphernalia (Cityvision, Boston, MA, USA). The neutral images, depicting household objects and chores, were graphically and contextually matched to the heroin-related stimuli.23 All the images had a uniform black background and none contained human faces. Stimuli were separated by a variable interval (0–18 s) during which a crosshair was displayed. Presentation software (Neurobehavioral Systems, San Francisco, CA, USA) was used to present the stimuli in a random, event-related fashion. Stimuli were rear-projected to the center of the visual field through a mirror mounted on the scanner head coil. Task duration was 10.6 min. Subjects were asked to rate their craving for heroin on a scale of 0 (not at all) to 9 (extremely), before and after the cue reactivity task.35 Post-session craving was managed clinically by debriefing and ‘talk down' until craving fully subsided. Conventional craving handling strategies (for example, finding alternative activities, ‘going with' the craving, avoiding cues and reaching out to loved ones and exercising) were thoroughly reviewed and discussed with all the participants.36
Imaging data acquisition
A Siemens Tim Trio 3T (Siemens USA, Malvern, PA, USA) system and 32-channel head coil were used for the MRI imaging. BOLD fMRI37, 38 was performed with a whole-brain, single-shot gradient-echo echoplanar sequence with the following parameters: TR/TE=2000/30 ms, FOV=220 mm, matrix=64 × 64, slice thickness/gap=3.4/0 mm, 33 slices, effective voxel resolution of 3.4 × 3.4 × 3.4 mm. An oblique acquisition, oriented along the anterior–posterior commissure line allowed for coverage of the entire brain with the exception of the lower cerebellum and minimized susceptibility artifacts in the subcortical and prefrontal regions. Before BOLD fMRI, a 5-min MPRAGE T1-weighted image (TR/TE=1810/3.51 ms, FOV=250 mm, matrix=192 × 256, effective voxel resolution of 1 × 1 × 1 mm) was acquired for anatomic overlays of functional data and spatial normalization.39
Data analysis
Clinical variables
Statistical analyses were performed using the IBM Statistical Package of the Social Sciences (IBM SPSS version 19, Armonk, NY, USA). To compare the effect of heroin cue exposure on self-reported craving, before and after the XRNTX treatment, a 2 × 2 analysis of variance (ANOVA) was conducted with the self-reported craving data. The within-subject factors were treatment (that is, Pre-XRNTX vs Post-XRNTX) and cue (pre-cue exposure vs post-cue exposure). One-way repeated-measures ANOVA was conducted for naltrexone and 6-beta-naltrexol plasma concentrations, the number of CPD and Beck Depression Inventory scores during the treatment period. Urine toxicology results were summarized as a proportion of positive findings out of the total number of collected samples and analyzed using generalized estimating equation (GEE) logistic regression models for the rates of opioids and cocaine use across the periods defined by the XRNTX injection time points (that is, baseline, Pre-XRNTX, first XRNTX, second XRNTX, third XRNTX and Post-XRNTX). Further, given the frequency of non-dependent cocaine use in opioid dependence,40, 41, 42 a multivariate GEE logistic regression model was used to examine the between-substance (that is, opioids vs cocaine) differences in patterns of substance use across the periods. In addition, GEE logistic regression models were also applied to examine whether baseline ASI scores or education level would predict urine toxicology results. Poisson regression models were applied to examine whether baseline ASI scores or the educational level would predict the number of injections (that is, zero, one, two and three).
Imaging data
BOLD time series data were preprocessed and analyzed using standard procedures in fMRI Expert Analysis Tool (version 5.98) of FSL (FMRIB's Software Library). Single-subject preprocessing included non-brain removal using Brain Extraction Tool43, slice time correction, motion correction to the median image using MCFLIRT,44 high-pass temporal filtering (50 s), spatial smoothing using a Gaussian kernel (5 mm full-width at half-maximum, isotropic), and mean-based intensity normalization of all volumes using the same multiplicative factor. The median functional volume was co-registered to the anatomical T1-weighted structural volume and then transformed into standard anatomical space (Montreal Neurological Institute (MNI) T1 template) using FLIRT.44, 45 Transformation parameters were later applied to all statistical contrast maps for group-level analyses.
Subject-level statistical analyses were performed voxel-wise using FILM (FMRIB's Improved General Linear Model) with local autocorrelation correction.46 Two condition contrasts (drug cue > neutral, neutral > drug cue) were modeled using a canonical hemodynamic response function. Six rigid body motion correction parameters were included as nuisance covariates. Image analyses were completed for each individual in subject space and the resulting contrast maps of parameter estimates were spatially normalized as described above.
Voxelwise whole-brain analysis—A paired t-test was applied to compare the brain response to cues before and after XRNTX treatment (Pre-XRNTX vs Post-XRNTX). Resulting z (Gaussianized t) statistic maps of pre > post and post > pre were thresholded at z⩾2.3 and corrected for multiple comparison at P<0.05 using familywise error rate.47 This threshold is a common error-control method in small-sample neuroimaging studies in psychiatry.48, 49, 50, 51, 52, 53, 54 Anatomic assignment of clusters was based on the peak z-score within the cluster using the Talairach Daemon Database, and confirmed by visual inspection.
To investigate whether neural response to heroin cues at baseline could predict adherence to XRNTX treatment, the number of injections that each subject received (for example, zero, one, two or three injections) was entered as a covariate of interest for the drug cue > neutral contrast. The resulting positive and negative correlation maps were thresholded at z⩾2.3 and cluster corrected at P⩽0.05, as described above. Percent signal change was extracted from significant clusters for further statistical testing. To calculate the positive and negative predictive value of the brain response to drug cues for treatment adherence, we categorized zero and one injection as a negative outcome (that is, 0, N=14) and two or three injections as a positive outcome (that is, 1, N=18).
To explore the correlation between brain responses to drug cues and craving before XRNTX treatment, self-reported craving was entered as a covariate of interest for the drug cue > neutral contrast. The resulting positive and negative correlation maps were thresholded at z⩾2.3 cluster corrected at P⩽0.05 as described above. Then significant clusters associated with self-report craving were used as masks to extract percent signal changes from drug cue > neutral contrasts of Pre-XRNTX and Post-XRNTX sessions, respectively. A paired t-test was used to examine the differences in the brain response to drug cues before and after XRNTX treatment.
Results
Participants' attrition
Thirty-two individuals who completed detoxification and enrolled in the study underwent the Pre-XRNTX fMRI scan. Out of thirty-two enrolled participants, nine were excluded before the first injection, (that is, received zero injections). Five received one injection, three received two injections and fifteen participants received all three XRNTX injections offered. Reasons for attrition included relapse to opioids demonstrated by positive urine toxicology screen, a positive naloxone challenge test or a failure to comply with the study protocol and scheduled appointments. Overall, the study retention rate was 47%. In addition, imaging data from two participants were excluded owing to excessive (>2 s.d. from the mean) head motion, expressed in temporal signal-to-noise ratio and relative volume-to-volume displacements. Thus, neuroimaging data from thirty participants in the Pre-XRNTX scan, 14 of whom had a Post-XRNTX scan, were included in the final neuroimaging analyses.
Adverse events
One subject indicated that they experienced excessive pain after the first injection but continued to participate in the study. No other adverse events were reported or identified. Out of eight subjects who received less than three injections and more than zero injection, none reported injection site problems or other adverse events. Given the monitoring schedule, unrecognized injection site pain or irritation was unlikely. Six individuals dropped out of the study 3 to 4 weeks after their last injection and cited dropout reasons including: (1) fear of protracted withdrawal; (2) desire to get ‘high' and (3) family and social issues.
Clinical findings
The two-way repeated-measures ANOVA for the cue-induced heroin craving revealed significant main effects of cue (F(1,13)=34.47, P<0.0001) but not treatment (F(1,13)=1.12, P=0.31). Importantly, the significant interaction between these two factors (F(1,13)=4.55, P=0.05, Figure 1) indicated that the magnitude of cue-induced craving was reduced, in comparison to the baseline, after the last injection.
Figure 1.
Self-reported craving for heroin before and after cue exposure, during the Pre-XRNTX session (blue line) and Post-XRNTX session (red line). Error bars present s.e.m. X axis: before cue exposure rating (before) and after cue exposure rating (after). Y axis: heroin craving ratings (0–9). XRNTX, extended-release naltrexone.
A one-way repeated-measures ANOVA showed an overall XRNTX effect on Beck Depression Inventory scores (F(4,12)=2.97 P=0.03); post hoc tests indicated that Beck Depression Inventory scores significantly decreased after the first injection (P<0.05) and remained stable thereafter (first vs second injection P=0.39, second vs third injection P=0.50, third injection vs Post_XRNTX P=0.44). Average consumption of CPD declined after the first XRNTX injection from average of 15 to 12 CPD (F(4,12)=2.56 P<0.05). Post hoc tests also indicated that self-reported CPD was reduced after the first and second injections compared with baseline (baseline vs first injection P=0.028; baseline vs second injection P=0.032). Last, as expected a one-way repeated-measures ANOVA revealed overall effects of treatment on naltrexone (F(2,12)=6.11, P=0.003) and 6-beta-naltrexol (F(2,12)=4.54, P=0.012) plasma concentrations. Post hoc tests showed that naltrexone and 6-beta-naltrexol plasma concentrations were significantly lower but detectable at 5–6 weeks after the last injection, when XRNTX is no longer expected to be clinically effective (Table 1). Naltrexone plasma concentrations were stable during the treatment period (first vs second P=0.482, first vs third P=0.603, second vs third P=0.173), and so were 6-beta-naltrexol plasma concentration (first vs second P=0.281, first vs third P=0.555, second vs third P=0.078).
Table 1. Clinical measures at Pre-XRNTX, On-XRNTX (1st, 2nd and 3rd injection) and Post-XRNTX time points.
| BDI | CPD | Naltrexone | 6-β-naltrexol | |
|---|---|---|---|---|
| Pre-XRNTX | 13.91±2.09 | 14.98±3.01 | NA | NA |
| On-XRNTX | ||||
| 1st Injection | 9.67±2.22 | 12.11±2.66 | 2.21±0.26 | 8.05±2.21 |
| 2nd Injection | 8.33±2.42 | 11.20±2.41 | 1.84±0.39 | 5.61±1.05 |
| 3rd Injection | 8.17±2.61 | 11.62±1.89 | 2.44±0.54 | 9.07±2.40 |
| Post-XRNTX | 8.26±2.32 | 11.61±1.94 | 0.79±0.29 | 2.51±0.99 |
| F(df); P-value | F(4,12)=2.97 P=0.03 | F(4,12)=2.56 P=0.05 | F(3,12)=6.11 P=0.003 | F(3,12)=4.55 P=0.012 |
| Post hoc | Pre vs. 1st P=0.047 | Pre vs 1st P=0.028, pre vs 2nd P=0.032 | 1st vs post P=0.013 2nd vs post P=0.001 3rd vs post P=0.007 | 2nd vs post P=0.006 3rd vs post P=0.018 |
Abbreviation: BDI, Beck Depression Inventory; CPD, cigarettes per day; NA, not available; XRNTX, injectable extended-release naltrexone.
Throughout the study, a meaningful proportion of the urine toxicology screens (UDS) was positive for opioids (oxycontin, buprenorphine, methadone and morphine), cocaine or both. The GEE model for cocaine use showed no overall significant differences in rates of use across the periods (chi-square (4)=5.01, P=0.29); Pairwise comparisons of one period to the next showed a significant increase between the Pre-XRNTX injection period and the period covered by the first injection (chi-square (1)=4.71, P=0.03), with no differences between later pairs of periods (P>0.59). Second, the GEE model for opioid use showed a significant difference in rates of use over the entire study periods (chi-square (4)=14.24, P=0.007). Pairwise comparisons showed a significant increase in opioid use at the Post-XRNTX time point (that is, 5–6 weeks after the last injection, beyond the 4-week period of clinical effectiveness of a XRNTX injection; chi-square (1)=9.92, P=0.002). Last, the multivariate GEE model showed that the differences in patterns observed between the two substances were statistically significant (chi-square (4)=13.43, P=0.009). Direct within-periods comparisons showed cocaine use was significantly higher than opioid use during the month after the first and second injections (P=0.001 and P=0.009, respectively), but not in the periods before the first injection (P=0.31) or the month after the third injection (P=0.44). Significantly higher use of opioids than cocaine was observed at weeks 5 and 6 after the last (third) injection (P=0.005, Figure 2). At this time point, the pharmacological effects of XRNTX should have disappeared, with only trace levels of naltrexone and 6-beta-naltrexol remaining in their blood (Table 1).
Figure 2.
Changes of positive UDS in cocaine and opioid over the treatment period (that is, baseline, Pre-XRNTX, first (1st)-XRNTX, second (2nd)-XRNTX, third (3rd)-XRNTX and Post-XRNTX). UDS, urine drug screen; XRNTX, injectable extended-release naltrexone.
In addition, the GEE model showed that neither the ASI subscales nor educational level predicted opiate-positive UDS (medical condition: P=0.14; alcohol use history: P=0.71; drug use history: P=0.77; legal status: P=0.76; family and social support: P=0.27; psychiatric comorbidity: P=0.24; years of education: P=0.49). Drug use history score (P=0.05), but not other ASI scores, predicted cocaine-positive UDS (medical condition: P=0.70; alcohol use history: P=0.79; legal status: P=0.12; family and social support: P=0.64; psychiatric comorbidity: P=0.45), nor did level of education (P=0.61). A poisson regression model showed that higher drug use history predicted more missed injections at a marginal level (P=0.08), but not other ASI scores (medical condition: P=0.85; alcohol use history: P=0.45; legal status: P=0.51; family and social support: P=0.80; psychiatric comorbidity: P=0.34) nor did level of education (P=0.92).
Imaging results
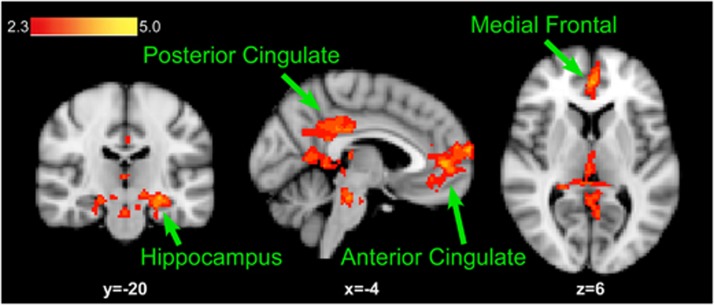
Before XRNTX treatment, brain response to drug (vs neutral) cues was observed in the parahippocampus, superior parietal lobule and the medial frontal, anterior cigulate and fusiform gyri (Figure 3, Table 2). A voxel-wise whole-brain paired t-test revealed that there was no significant difference in brain response to drug cues before and after the XRNTX treatment. Whole-brain correlation revealed that activation in the medial frontal and anterior cingulate gyrus clusters were positively correlated with treatment adherence, expressed as the number of XRNTX injections participants accepted (Figure 4, Table 3). To establish continuity with prior literature, we limited the mPFC to the medial frontal gyrus cluster and treated the anterior cingulate gyrus cluster separately.23-27 In addition, we found that the positive predictive value (PPV) of % BOLD signal change in the mPFC was 60% and the negative predictive value (NPV) was 100% when the activation threshold was −0.51% BOLD fMRI signal change, while the PPV was 100% and the NPV was 58.33% when the threshold was 0.12% BOLD signal change (Figure 5).
Figure 3.
Brain regions associated with response to drug cues before the XRNTX treatment. Statistical maps (red–yellow scale) are superimposed on the Montreal Neurological Institute (MNI) brain template and thresholded at z=2.3 (cluster corrected at P<0.05). XRNTX, injectable extended-release naltrexone.
Table 2. Locations and magnitude of brain responses to drug cues before the XRNTX treatment.
| Regiona | BAb | Size | Z-maxc | Xd | Y | Z |
|---|---|---|---|---|---|---|
| Drug > Neutral | ||||||
| Posterior cingulate | 29,30 | 3539 | 4.75 | −1 | −44 | 20 |
| Parahippocampus | 28,35 | 3.78 | −26 | −21 | −11 | |
| Superior parietal lobule | 7 | 3.74 | −37 | −66 | 44 | |
| Cingulate | 23,31 | 3.71 | −1 | −35 | 28 | |
| Anterior cingulate | 32 | 786 | 3.8 | −5 | 44 | 10 |
| Medial frontal gyrus | 10 | 3.65 | −5 | 63 | 22 | |
| Anterior cingulate | 32 | 3.58 | 14 | −64 | 38 | |
| Medial frontal gyrus | 10 | 3.25 | −5 | 50 | 5 | |
| Medial frontal gyrus | 9 | 3.02 | −5 | 53 | 21 | |
| Medial frontal gyrus | 10 | 3.01 | −5 | 57 | 16 | |
| Occipital gyrus | 19 | 470 | 3.34 | −32 | −66 | 8 |
| Fusiform gyrus | 19 | 3.19 | −49 | −68 | −11 | |
| Fusiform gyrus | 19 | 3.12 | −37 | −68 | −10 | |
| Middle occipital/ temporal gyrus | 37 | 3.06 | −47 | −63 | −8 | |
| Declive | 19 | 2.94 | −37 | −63 | −6 | |
| Lingual gyrus | 18,19 | 2.87 | −33 | −60 | 5 | |
Abbreviations: BOLD, blood-oxygen-level dependent; fMRI, functional magnetic resonance imaging; XRNTX, injectable extended-release naltrexone.
Locations of the clusters and the local maxima of BOLD fMRI signal change.
z⩾2.3 and (corrected) cluster significance P<0.05.
Brodmann's area.
Z-max values represent peak activation for the cluster.
Talairach (1988) coordinates.
Figure 4.
Medial prefrontal and anterior cingulate cortical response to drug cues was positively correlated with the number of XRNTX injections received. Statistical map (red–yellow scale) is displayed over the MNI brain template and thresholded at z=2.3 (cluster corrected at P<0.05). MNI, Montreal Neurological Institute; XRNTX, injectable extended-release naltrexone.
Table 3. Locations and magnitude of brain regions positively correlated with numbers of injections received.
| Regiona | BAb | Size | Z-maxc | Xd | Y | Z |
|---|---|---|---|---|---|---|
| Anterior cingulate gyrus | 24 | 748 | 3.67 | 10 | 30 | −2 |
| Anterior cingulate gyrus | 25 | 3.31 | 5 | 19 | −10 | |
| Anterior cingulate gyrus | 32 | 3.58 | 7 | 43 | −3 | |
| Superior frontal gyrus | 8 | 454 | 3.91 | −14 | 46 | 37 |
| Medial frontal gyrus | 9 | 3.69 | −16 | 51 | 32 |
Abbreviations: BOLD, blood-oxygen-level dependent; fMRI, functional magnetic resonance imaging.
Locations of the clusters and the local maxima of BOLD fMRI signal change.
z⩾2.3 and (corrected) cluster significance P<0.05.
Brodmann's area.
Z-max values represent peak activation for the cluster.
Talairach (1988) coordinates.
Figure 5.
Predictive value of % BOLD signal change in the mPFC for adherence to more than one XRNTX injection. PPV is 60% and NPV is 100% when the mPFC % signal change threshold is −0.51 (highlighted in grey), whereas PPV is 100% and NPV is 58% when the mPFC % signal change threshold is 0.12 (highlighted in orange). BOLD, blood-oxygen-level dependent; mPFC, medial prefrontal cortex; NPV, negative predictive value; PPV, positive predictive value; XRNTX, injectable extended-release naltrexone.
Whole-brain correlation revealed that activation in the precuneus (X=−4,Y=−68, Z=62) and middle temporal gyrus (X=−54,Y=−54, Z=−14) were positively correlated with self-report cravings before the XRNTX treatment. However, a paired t-test showed no significant differences before vs. after the XRNTX treatment in either precuneus (t=−0.17, P=0.87) or middle temporal gyrus (t=0.02, P=0.99) activation.
Discussion
Consistent with earlier studies,40, 55, 56 XRNTX therapy was well tolerated with no participant attrition attributed to side effects. On the contrary, patients who stayed in treatment were by and large abstinent from opioid use, as evident by the urine toxicology data (Figure 2). Furthermore, even 2 weeks after the expected cessation of the XRNTX pharmacological effect (i.e., 6 weeks after the third XRNTX injection), over 50% of the cohort remained abstinent from opioid drugs, and displayed diminished cue-induced craving response. Consistent with prior reports of overall quality of life improvement,57 participants showed a dramatic and sustained decline in depression measure (Table 1). A review of the relevant trials conducted over the past 35 years revealed that the retention rate on oral NTX therapy is generally <15% over 4 months,12, 58 whereas about 50% of our patients remained in treatment. Thus, our results suggest that XRNTX may offer superior outcomes, particularly in a subpopulation of heroin addicts displaying a specific pattern of brain responses to opioid-related cues (see below). On the other hand, the upward trend in the opioid-positive urine toxicology results observed near the end of the last 28-day inter-dose interval calls for enhanced psychosocial interventions during that period, as well as for potential supplementation with oral NTX similarly to the combined use of oral and depot antipsychotic agents.59
We previously reported the XRNTX-induced reduction in corticolimbic responsivity to heroin cues in patients with heroin dependence.23 Although there were important methodological similarities between the prior and the present study (for example, heroin-dependent participants and the neuroimaging procedure), there are also substantial differences; most notably the prior study used an experimental formulation and collected the follow-up fMRI scan under the effects of XRNTX, whereas the present study used an FDA-approved, pharmacodynamically distinct preparation and collected the follow-up scan 6 weeks after the last (third) injection, when the pharmacological XRNTX effects have waned. In sum, these independent findings extend our previously reported data by suggesting that the XRNTX efficacy generalizes to naturally fluctuating heroin addiction phenomena that are not confounded by criminal justice system involvement, prior incarcerations or more severe end-stage consequences that may impact cue-induced brain responses and outcomes.
Although several brain regions (i.e., parahippocampus, superior parietal lobule, mPFC, anterior cingulate and fusiform gyri) were engaged by heroin-related cues, only the mPFC and anterior cingulate activity predicted adherence to the 3-month-long course of XRNTX therapy, pointing to the heuristic value of our cue-induced brain activation procedure in tailoring and optimizing therapeutic interventions. In addition, when percent BOLD fMRI signal change was 0.12 in the mPFC, positive and negative predictive values were 100% and 58.33%, respectively (Figure 5). When percent BOLD fMRI signal change was −0.51 in the mPFC, positive and negative predictive values were 60% and 100%, respectively. A number of functional neuroimaging studies in individuals with substance use disorders provide evidence that hypofunctionality of the mPFC during protracted withdrawal is linked to a state of reward deficiency expressed by decreased drive for natural rewards.60 Moreover, a hypoactive mPFC may fail to properly inhibit amygdala61, 62, 63 and striatal64 hyper-reactivity to drug-related stimuli, giving rise to heightened craving65, 66 along with anxiety-laden conditioned drug withdrawal effects, that is, anti-reward state.67 Therefore present data are consistent with the proposition that heightened mPFC activity contributes, to both diminished craving and negative affective states and thus buffers against relapse. Accordingly, this activity could be also used to ascertain treatment adherence. However, as mPFC hyperactivity during drug-related cue exposure is also linked to craving and drug-seeking behavior, an alternative interpretation is that individuals with such activations stay in treatment because they are experiencing higher levels of distress in response to visual drug cues. Our study design does not allow us to determine which of the interrelated reward deficiency and anti-reward characteristics comes first. Nonetheless, these results underscore the need for follow-up studies involving serial (not just baseline) assessments of brain activity, probes of self-control25 and detailed measurements of emotional and motivational states throughout the course of the trial.
We observed changes in the concurrent use of cocaine during the XRNTX therapy. This observation adds support to the theory implicating opioidergic mechanisms in the psychostimulant mechanism of action24, 68 and renders ‘increased general health'56 an incomplete description of the XRNTX safety profile. Non-dependent cocaine abuse is quite common in opioid-dependent individuals42, 69, 70 and, given the role that the opioids had in preserving cardiovascular homeostasis during ischemia and stress,60 may increase cardiovascular morbidity and mortality.71, 72, 73, 74 Hence, patients with even seemingly mild cocaine problems should be counseled and targeted for earlier intervention and tighter monitoring, such as more frequent urine toxicology screens taking into consideration cocaine's short half-life.
The decrease in daily tobacco intake during XRNTX is in keeping with our prior report on oral NTX's efficacy for smoking cessation in opioid-dependent individuals.33 Prior studies generally do not support the efficacy of oral NTX for smoking cessation in non-opioid-dependent individuals.75 However, over 80% of opioid-dependent individuals are also nicotine dependent, making this population physiologically distinct.76, 77 Therefore, smoking reduction in opioid-dependent smokers may be explained by the endogenous opioid system involvement in nicotine reward and addiction,78 its dysregulation by chronic opioids,77, 79, 80 and potential normalization by the extended mu-opioid receptor blockade with XRNTX.77, 80 Moreover, the rewarding and reinforcing effects of opioids tend to sensitize over time.81 If such sensitization has a role in the course of heroin dependence, then cross-sensitization may also be demonstrated; i.e., delivery of a priming dose of an addictive drug after a long period of abstinence can re-establish drug consumption even if the drug used for priming is drawn from a different class than the initially abused substance. In that case, exposure to nicotine or cocaine after a long period of XRNTX-mediated abstinence might increase the vulnerability to relapse to heroin consumption and vice versa.82 Thus, better understanding of co-occurring drug consumption and its relation to heroin use could have important clinical and theoretical implications.
Several caveats should be considered when interpreting these data. The small sample size, the relatively brief duration of treatment, the timing of the Post-XRNTX fMRI scan and only two fMRI data time points render these findings preliminary and requiring replication, confirmation and extension. For example, lack of significant decrease in the brain fMRI response to heroin-related visual cues on the post-treatment scan could be due to insufficient duration of treatment, a return to pre-treatment baseline after treatment ended, or the modest size of the post-treatment sample. Hence a longer study with larger initial sample is needed to confirm these findings and to discern possible curative clinical and neurobiological effects. It should be noted that the number of XRNTX injections is only a general indicator of therapeutic adherence, and these data could have been enhanced with serial measurements of addiction severity. In turn, this would have clearly defined the relationship between XRNTX therapy and clinical outcomes. Last, the observed XRNTX effects may not necessarily be specific to the clinical outcomes arising in the context of XRNTX pharmacotherapy and could be generalized to the less expensive oral naltrexone preparation. Therefore, it would be revealing to perform a follow-up comprehensive assessment of both oral and parenteral anti-opioidergic agents.
In conclusion, in detoxified individuals addicted to heroin, pre-treatment medial prefrontal cortical response to heroin-related cues was associated with greater adherence to XRNTX. After the treatment ended, subjective craving response to heroin cues was reduced, but the brain response to these cues remained at pre-treatment levels. If confirmed in larger trials, these results may contribute to the identification of a clinical marker of therapeutic effects as well as of disease staging and assignment to the proper level of care. Further research is needed to confirm our initial, group-level findings, to address the mechanisms of XRNTX therapeutic action, to take into account psychosocial treatments and to inform empirically driven treatment-matching algorithms that would allow the provision of individualized care83 in opioid dependence.
Acknowledgments
This study was supported by the National Institute on Drug Abuse (NIDA) grants R01DA024553, R01DA033670 and R01DA036028, NIDA contract N01DA-9-7767A, Veterans Administration VISN4 Mental Illness Research, Education and Clinical Center (MIRECC) and the Center for Magnetic Resonance and Spectroscopy of the University of Pennsylvania. Study medication was donated by Alkermes, plc through an Investigator-Sponsored Study Agreement with DDL.
CPO'B served as a consultant to Alkermes plc and Tekmira Pharmaceuticals. The remaining authors declare no conflicts of interest.
References
- Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305:1346–1347. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370:2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Substance Abuse and Mental Health Services Administration: Rockville, MD, USA; 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- NIDA . Research Report Series. National Institutes of Health: Bethesda, MD, USA; 2014. Heroin. [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RK, Fletcher BW, Volkow ND. Treating drug abuse and addiction in the criminal justice system: improving public health and safety. JAMA. 2009;301:183–190. doi: 10.1001/jama.2008.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Drug Facts: Treatment Statistics 2011 ; http://www.drugabuse.gov/publications/drugfacts/treatment-statistics ; accessed on 20 December 2014.
- Yee A, Loh HS, Hisham Hashim HM, Ng CG. The prevalence of sexual dysfunction among male patients on methadone and buprenorphine treatments: a meta-analysis study. J Sex Med. 2014;11:22–32. doi: 10.1111/jsm.12352. [DOI] [PubMed] [Google Scholar]
- Ehret GB, Voide C, Gex-Fabry M, Chabert J, Shah D, Broers B, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: high frequency in hospitalized patients and risk factors. Arch Intern Med. 2006;166:1280–1287. doi: 10.1001/archinte.166.12.1280. [DOI] [PubMed] [Google Scholar]
- Brown MT, Bussell JK. Medication adherence: WHO cares. Mayo Clin Proc. 2011;86:304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72:1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, et al. Management of relapse in naltrexone maintenance for heroin dependence. Drug Alcohol Depend. 2007;91:289–292. doi: 10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Bisaga A, Mariani JJ, Glass A, Levin FR, Comer SD, et al. Naltrexone treatment for opioid dependence: does its effectiveness depend on testing the blockade. Drug Alcohol Depend. 2013;133:80–85. doi: 10.1016/j.drugalcdep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Altice FL, Herme M, Di Paola A. Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for alcohol dependent and hazardous drinking prisoners with HIV who are transitioning to the community. Contemp Clin Trials. 2014;37:209–218. doi: 10.1016/j.cct.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Mello D, Lonergan S, Bourgault C, O'Toole TP. Aversion to injection limits acceptability of extended-release naltrexone among homeless, alcohol-dependent patients. Subst Abus. 2013;34:94–96. doi: 10.1080/08897077.2012.763083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aklin WM, Severtson SG, Umbricht A, Fingerhood M, Bigelow GE, Lejuez CW, et al. Risk-taking propensity as a predictor of induction onto naltrexone treatment for opioid dependence. J Clin Psychiatry. 2012;73:e1056–e1061. doi: 10.4088/JCP.09m05807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszi G, Anton RF, O'Malley S, Swift R, Pettinati H, Couper D, et al. OPRM1 Asn40Asp predicts response to naltrexone treatment: a haplotype-based approach. Alcohol Clin Exp Res. 2009;33:383–393. doi: 10.1111/j.1530-0277.2008.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL, Singleton EG. Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction. 2000;95:S177–S187. doi: 10.1080/09652140050111753. [DOI] [PubMed] [Google Scholar]
- Jhingan HP, Jain R, Desai NG, Vaswani M, Tripathi BM, Pandey RM. Validity of self-report of recent opiate use in treatment setting. Indian J Med Sci. 2002;56:495–500. [PubMed] [Google Scholar]
- Chen WJ, Fang CC, Shyu RS, Lin KC. Underreporting of illicit drug use by patients at emergency departments as revealed by two-tiered urinalysis. Addict Behav. 2006;31:2304–2308. doi: 10.1016/j.addbeh.2006.02.015. [DOI] [PubMed] [Google Scholar]
- de Beaurepaire R, Lukasiewicz M, Beauverie P, Castera S, Dagorne O, Espaze R, et al. Comparison of self-reports and biological measures for alcohol, tobacco, and illicit drugs consumption in psychiatric inpatients. Eur Psychiatry. 2007;22:540–548. doi: 10.1016/j.eurpsy.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Preston KL. Nonreporting of cannabis use: predictors and relationship to treatment outcome in methadone maintained patients. Addict Behav. 2007;32:938–949. doi: 10.1016/j.addbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Loughead JW, Busch EL, Cornish J, et al. Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol. 2014;19:262–271. doi: 10.1111/j.1369-1600.2012.00462.x. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, et al. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.(ed). Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision American Psychiatric Association: Washington, DC, USA; 2002 [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ruan X, Chen T, Gudin J, Couch JP, Chiravuri S. Acute opioid withdrawal precipitated by ingestion of crushed embeda (morphine extended release with sequestered naltrexone): case report and the focused review of the literature. J Opioid Manag. 2010;6:300–303. doi: 10.5055/jom.2010.0028. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. J Pharmacol Exp Ther. 2011;336:488–495. doi: 10.1124/jpet.110.173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, D'Ambra MN, Krause S, Breiter H, Kane M, Morris R, et al. Ultrarapid opioid detoxification: effects on cardiopulmonary physiology, stress hormones and clinical outcomes. Drug Alcohol Depend. 2001;61:163–172. doi: 10.1016/s0376-8716(00)00139-3. [DOI] [PubMed] [Google Scholar]
- Slawson MH, Chen M, Moody D, Comer SD, Nuwayser ES, Fang WB, et al. Quantitative analysis of naltrexone and 6beta-naltrexol in human, rat, and rabbit plasma by liquid chromatography-electrospray ionization tandem mass spectrometry with application to the pharmacokinetics of Depotrex in rabbits. J Anal Toxicol. 2007;31:453–461. doi: 10.1093/jat/31.8.453. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, et al. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Ho A, Bahl A, Varma P, Kellogg S, Borg L, et al. Former heroin addicts with or without a history of cocaine dependence are more impulsive than controls. Drug Alcohol Depend. 2012;124:113–120. doi: 10.1016/j.drugalcdep.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremmani I, Pani PP, Mellini A, Pacini M, Marini G, Lovrecic M, et al. Alcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment program. J Addict Dis. 2007;26:61–70. doi: 10.1300/J069v26n01_08. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Zaca D, Nickerson JP, Deib G, Pillai JJ. Effectiveness of four different clinical fMRI paradigms for preoperative regional determination of language lateralization in patients with brain tumors. Neuroradiology. 2012;54:1015–1025. doi: 10.1007/s00234-012-1056-2. [DOI] [PubMed] [Google Scholar]
- Auer T, Schweizer R, Frahm J. An iterative two-threshold analysis for single-subject functional MRI of the human brain. Eur Radiol. 2011;21:2369–2387. doi: 10.1007/s00330-011-2184-5. [DOI] [PubMed] [Google Scholar]
- Suarez RO, Whalen S, Nelson AP, Tie Y, Meadows ME, Radmanesh A, et al. Threshold-independent functional MRI determination of language dominance: a validation study against clinical gold standards. Epilepsy Behav. 2009;16:288–297. doi: 10.1016/j.yebeh.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Detecting activation in fMRI data. Stat Methods Med Res. 2003;12:401–418. doi: 10.1191/0962280203sm340ra. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Busch EL, O'Brien CP, Elman I. Depot naltrexone decreases rewarding properties of sugar in patients with opioid dependence. Psychopharmacology (Berl) 2012;220:559–564. doi: 10.1007/s00213-011-2503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Gastfriend DR, Forman RF, Schweizer E, Pettinati HM. Long-term opioid blockade and hedonic response: preliminary data from two open-label extension studies with extended-release naltrexone. Am J Addict. 2011;20:106–112. doi: 10.1111/j.1521-0391.2010.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011. p. CD001333. [DOI] [PubMed]
- Barnes TR, Shingleton-Smith A, Paton C. Antipsychotic long-acting injections: prescribing practice in the UK. Br J Psychiatry Suppl. 2009;52:S37–S42. doi: 10.1192/bjp.195.52.s37. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Oeltgen PR, Lishmanov YB, Brown SA, Barzakh EI, Krylatov AV, et al. Activation of peripheral delta opioid receptors increases cardiac tolerance to arrhythmogenic effect of ischemia/reperfusion. Acad Emerg Med. 2014;21:31–39. doi: 10.1111/acem.12286. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagaspe P, Schwartz S, Vuilleumier P. Fear and stop: a role for the amygdala in motor inhibition by emotional signals. Neuroimage. 2011;55:1825–1835. doi: 10.1016/j.neuroimage.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, et al. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Hum Brain Mapp. 2006;27:652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, McDonald RJ. Context, emotion, and the strategic pursuit of goals: interactions among multiple brain systems controlling motivated behavior. Front Behav Neurosci. 2012;6:50. doi: 10.3389/fnbeh.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao LY, Copersino ML, Fang YX, Chen Y, Tian J, et al. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol. 2008;579:160–166. doi: 10.1016/j.ejphar.2007.09.042. [DOI] [PubMed] [Google Scholar]
- Quek LH, Chan GC, White A, Connor JP, Baker PJ, Saunders JB, et al. Concurrent and simultaneous polydrug use: latent class analysis of an Australian nationally representative sample of young adults. Front Public Health. 2013;1:61. doi: 10.3389/fpubh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Naryzhnaia NV, Tsibulnikov SY, Kolar F, Zhang Y, Wang H, et al. Role of endogenous opioid peptides in the infarct size-limiting effect of adaptation to chronic continuous hypoxia. Life Sci. 2013;93:373–379. doi: 10.1016/j.lfs.2013.07.018. [DOI] [PubMed] [Google Scholar]
- McCann B, Hunter R, McCann J. Cocaine/heroin induced rhabdomyolysis and ventricular fibrillation. Emerg Med J. 2002;19:264–265. doi: 10.1136/emj.19.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder JJ. Naloxone—for intoxications with intravenous heroin and heroin mixtures—harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34:409–416. doi: 10.3109/15563659609013811. [DOI] [PubMed] [Google Scholar]
- Merigian KS. Cocaine-induced ventricular arrhythmias and rapid atrial fibrillation temporally related to naloxone administration. Am J Emerg Med. 1993;11:96–97. doi: 10.1016/0735-6757(93)90074-l. [DOI] [PubMed] [Google Scholar]
- David SP, Chu IM, Lancaster T, Stead LF, Evins AE, Prochaska JJ. Systematic review and meta-analysis of opioid antagonists for smoking cessation. BMJ Open. 2014;4:e004393. doi: 10.1136/bmjopen-2013-004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli P, Wu LT, Peindl KS, Gorelick DA. Smoking and opioid detoxification: behavioral changes and response to treatment. Nicotine Tob Res. 2013. [DOI] [PMC free article] [PubMed]
- Zirakzadeh A, Shuman C, Stauter E, Hays JT, Ebbert JO. Cigarette smoking in methadone maintained patients: an up-to-date review. Curr Drug Abuse Rev. 2013;6:77–84. doi: 10.2174/1874473711306010009. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011;60:1209–1220. doi: 10.1016/j.neuropharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Wand GS, Kuwabara H, Xu X, Frost JJ, Wong DF, et al. Association of smoking with mu-opioid receptor availability before and during naltrexone blockade in alcohol-dependent subjects. Addict Biol. 2012;19:733–742. doi: 10.1111/adb.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusco B, Chiamulera C, Quaglio G, Moro L, Casari R, Amen G, et al. Tobacco addiction and smoking status in heroin addicts under methadone vs buprenorphine therapy. Int J Environ Res Public Health. 2012;9:932–942. doi: 10.3390/ijerph9030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip-Cammack KM, Reed B, Zhang Y, Ho A, Kreek MJ. Tolerance and sensitization to chronic escalating dose heroin following extended withdrawal in Fischer rats: possible role of mu-opioid receptors. Psychopharmacology (Berl) 2013;225:127–140. doi: 10.1007/s00213-012-2801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Kandel DB. Shattuck Lecture. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932–943. doi: 10.1056/NEJMsa1405092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]