Abstract
During adolescence, white matter microstructure undergoes an important stage of development. It is hypothesized that the alterations of brain connectivity that have a key role in autism spectrum conditions (ASCs) may interact with the development of white matter microstructure. This interaction may be present beyond the phenotype of autism in siblings of individuals with ASC, who are 10 to 20 times more likely to develop certain forms of ASC. We use diffusion tensor imaging to examine how white matter microstructure measurements correlate with age in typically developing individuals, and how this correlation differs in n=43 adolescents with ASC and their n=38 siblings. Correlations observed in n=40 typically developing individuals match developmental changes noted in previous longitudinal studies. In comparison, individuals with ASC display weaker negative correlation between age and mean diffusivity in a broad area centred in the right superior longitudinal fasciculus. These differences may be caused either by increased heterogeneity in ASC or by temporal alterations in the group's developmental pattern. Siblings of individuals with ASC also show diminished negative correlation between age and one component of mean diffusivity—second diffusion eigenvalue—in the right superior longitudinal fasciculus. As the observed differences match for location and correlation directionality in our comparison of typically developing individuals to those with ASC and their siblings, we propose that these alterations constitute a part of the endophenotype of autism.
Introduction
Adolescence is a period of intense increase in neural connectivity and growth of white matter (WM).1, 2, 3, 4, 5, 6, 7 The developmental maturation of WM microstructure is one of the most prominent intrinsic changes occurring in the adolescent brain.3, 4, 5, 6, 7, 8, 9, 10 Observations of both longitudinal and cross-sectional studies have consistently shown a continuous increase in fractional anisotropy (FA) and a gradual decrease in mean diffusivity (MD) in WM throughout this period.3, 4, 5, 6, 7, 8, 10, 11, 12 Both FA and MD represent different aspects of the diffusion tensor ellipsoid, which characterizes the average directionality of water movement observed in a voxel of a diffusion sensitive image. High FA represents high fibre coherence and directionality,3, 13 whereas low MD indicates fibre characteristics such as small axon calibre, high axon density or thick myelin sheath.3, 13, 14, 15 Therefore, high FA and low MD are associated with mature WM microstructure. The diffusion tensor ellipsoid is described by three mutually perpendicular diffusivity directions. The first (L1) is assumed to be parallel to the axonal direction of a fibre dominant in a voxel, and the second and the third (L2 and L3) are thought to be perpendicular to the axon.16 L2 carries additional information about the actual predominance of the water diffusion in the direction of L1 and about the possibility of crossing fibres in a voxel.17
Autism spectrum conditions (ASCs) arise during childhood18 and continue throughout adolescence.19 The presence of ASC during periods critical for brain growth such as early childhood and adolescence may lead to an interaction between ASC and neural development particularly in WM.20, 21, 22, 23 Indeed, WM disconnectivity in long-range fibres is one of the suggested pathophysiological mechanisms of ASC.24, 25, 26 Several studies have examined differences in WM microstructure between ASCs and typically developing children at different stages of their lives.7, 20, 27, 28, 29, 30, 31, 32, 33 When corrected for whole-brain multiple comparisons, younger children with ASC show increased maturation in WM microstructure in comparison with typically developing controls in the corpus callosum, cingulum, arcuate fasciculus, external and internal capsule.29, 34 Conversely, in later childhood and in adolescence, typically developing individuals display a more mature WM microstructure than the ASC group in the inferior and superior longitudinal fasciculi, internal and external capsule, uncinate fasciculus, cingulum, corona radiata and thalamic radiation.27, 28, 30 Similar trends were noted when region of interest analyses were applied.20, 31, 32 This indicates that the developmental pattern of WM microstructure may be affected by the presence of ASC. It also suggests that although at the beginning of puberty individuals with ASC show higher maturity in WM microstructure than typically developing controls, at the end of adolescence typically developing persons display more mature WM than the ASC group.35
ASC is partially heritable36, 37, 38, 39 and aggregates in families.40 Biological siblings of individuals with ASC are 10 to 20 times more likely to develop ASC than the general population.40, 41, 42, 43 First-degree relatives of individuals with ASC display more autistic traits, including communication and social difficulties40, 41, 44 in addition to rigid personality characteristics, interests and behaviour,45 compared with typically developing controls. This indicates that some unaffected family members of individuals with ASC inherit the associated genetic predisposition for ASC, and that they may have the broader autism endophenotype. An endophenotype is an internal characteristic present both in individuals with the condition and in their relatives, and has a genetic basis.46 It is an intermediate feature connecting genotype and behavioural symptoms.46
Variability in WM microstructure in adolescents and adults has strong genetic basis,47, 48 and the loci implicated in this variability (3q27 and 15q25)48 have also been linked to ASC susceptibility.49 In early and late childhood, individuals with ASC and their siblings show similar differences in WM microstructure in comparison with typically developing controls.28 How these differences relate to WM development during adolescence is yet to be determined. Our study aimed to test whether there is an interaction between diagnosis of ASC and age in WM microstructure in adolescents and thus determine age effects on the differences between the groups in terms of WM connectivity. We also aimed to test whether a similar interaction was observed in siblings of individuals with ASC.
Materials and methods
Participants
Our sample comprised 43 individuals with ASC, 38 unaffected full biological siblings of our ASC participants and 40 typically developing controls. Initially, diffusion tensor imaging (DTI) scans were obtained for 49 individuals with ASC and 40 siblings. However, six scans of participants with ASC and two scans of siblings were excluded from analysis due to motion-related artefacts and technical problems. The procedure is described in detail in the DTI analysis section. On the basis of parental report, all the siblings of individuals with ASC were their full biological siblings. The typically developing controls had no first- or second-degree relatives with ASC. The methods of reaching potential subjects and the recruitment process have been described previously.50
The participants with ASC were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), with either autistic disorder or Asperger's disorder. In addition, all participants with ASC scored above the cut-off for ‘autism' on the Autism Diagnostic Interview-Revised51 and for ‘autism spectrum' on the Autism Diagnostic Observation Schedule-Generic.52 All unaffected siblings and typically developing controls scored below the cut-off threshold (score of 15)53 on the Social Communication Questionnaire,54 a screening tool for autism.55 Autistic traits were quantified with the Autism Spectrum Quotient56 and Social Responsiveness Scale.57 No differences in autistic symptoms and traits were observed between typically developing controls and siblings of individuals with ASC (Table 1).
Table 1. Demographic and clinical characteristics of the study groups.
| ASC (N=43) | Siblings (N=38) | Controls (N=40) | Group differences | |
|---|---|---|---|---|
| Age (years) | 14.52±1.74 | 14.99±2.13 | 15.06±1.63 | Not significant |
| Gender (M/F) | 28/15 | 12/26 | 20/20 | No significant difference between controls and the other two groups Significant difference between ASC and siblings; P=0.002 |
| Wechsler Abbreviated Scale of Intelligence | 104.65±14.57 | 113.63±9.95 | 112.38±11.12 | No significant difference between siblings and controls Significant differences between: ASC < Siblings; P=0.003 ASC < Controls; P=0.012 |
| Social Communication Questionnaire | 25.03±5.5 | 1.6±2.19 | 2.2±2.33 | No significant difference between siblings and controls Significant differences between: ASC > Siblings; P<0.001 ASC > Controls; P<0.001 |
| Autism Spectrum Quotient | 38.59±6.72 | 8.61±5.63 | 9.1±5.59 | |
| Social Responsiveness Scale | 112.85±31.13 | 17.22±12.34 | 15.43±11.4 |
Abbreviations: ASC, autism spectrum condition; F, female; M, male. Interval measurements are presented as a group mean with s.d. The significance of differences between the groups means in every measurement was calculated with t-tests for each pair of the groups.
All participants were between 12 and 19 years of age and all groups were matched for age (Table 1). Both sexes were represented in all the three groups (Table 1). Typically developing individuals (20 males; 20 females) did not differ significantly in sex ratio from participants with ASC (28 males; 15 females) or from their siblings (12 males; 26 females). There was a difference in the sex ratio between the ASC and sibling groups. As individuals with ASC and their siblings were not directly compared, any difference due to their sex is of lesser importance to our conclusions. Furthermore, male siblings of individuals with ASC are at higher risk of also having an ASC diagnosis than female siblings.58 Intelligence quotient (IQ) was measured using the Wechsler Abbreviated Scale of Intelligence.50, 59 Only participants with an IQ over 70 were recruited. The mean IQ of each group was not lower than the average general population IQ.60 Participants with ASC had a significantly lower general IQ than the other two groups, whereas siblings and typically developing individuals did not differ in this regard (Table 1). Similar observations were seen in a previous study of cognitive abilities in siblings of children with ASC61 and in a study of WM maturity in adults with ASC.62 None of our participants had ever been medicated in relation to any psychiatric condition. The protocol was approved by the Cambridgeshire 1 Ethics Committee. All the participants and their parents have given written informed consent to participate in the study.50
DTI acquisition
We used 3T MRI Magnetom TrimTrio scanner (Siemens, Erlangen, Germany) at the Medical Research Council Cognition and Brain Sciences Unit, Cambridge, UK to acquire the data. Diffusion-weighted data were obtained in 64 directions plus a single B0 (non-diffusion weighted) acquisition. Forty-eight (48) 2.5-mm slices with 20% gap (3 mm per slice in total) were acquired per direction. The voxel size was non-isotropic 1.8 × 1.8 × 2.5 mm3 with a field of view 230 × 230 × 144 mm3, TR of 6600 ms, TE of 93 ms and bandwidth of 1396 Hz per pixel.
DTI analysis
Pre-processing and tensor fitting
We performed all steps of pre-processing using FMRIB Software Library (FSL).63 We visually inspected all the scans with FSLView. We removed from further analysis each scan with motion artefacts (‘ghosting' and severe signal loss)64 in over 10% of the diffusion weighted directions (seven images in total). Each slice in each diffusion direction was inspected individually and the direction was classified as compromised if three or more of its slices displayed a motion artefact. If three or more slices displayed motion artefact in the B0 image used as a baseline in calculation of the diffusion-weighted signal, the scan would have been excluded regardless of the quality of the remaining diffusion-weighted images; although in our sample, scans which displayed such signal loss in B0 also showed artefacts in more than 10% of the other images. Ultimately, scans of four participants with ASC and two siblings were removed accordingly. Two additional scans of ASC participants were removed due to low signal-to-noise ratio in the raw data, which caused them to be outliers in the number of voxels with negative MD and FA >1 (both physically implausible values). These two scans showed 8407 and 9357 voxels with negative MD as well as 3039 and 3934 voxels with FA >1 with the respective means in the remaining scans at 6397 and 2017 voxels. The low signal-to-noise ratio value in these two scans was confirmed by using B0 image and a region of interest outside the brain to calculate the signal-to-noise ratio, which was calculated as mean signal within the brain mask divided by the mean value of the noise in the region of interest outside the brain. The noise region of interest was defined manually and carefully checked to make sure it did not include skull, ghosting or other artefacts. This resulted in the ultimate sample sizes described in the Participants section.
We then corrected the data for stretches and shears produced by eddy currents and gross head motion with eddy current correction in FSL FMRIB's Diffusion Toolbox. Next, we removed all non-brain tissue from the images using Brain Extraction Tool FSL65 with a fractional intensity threshold of 0.2. The threshold estimating a larger brain outline was used to preserve all brain tissue and to account for variation in the participants' skull-size. Finally, we fit a diffusion tensor model to each voxel of the diffusion images with FSL FMRIB's Diffusion Toolbox DTIFit.66 Thus, we calculated FA, MD, L1, L2 and L3 maps for every participant.
Tract-based spatial statistics pre-processing
We performed all tract-based spatial statistics pre-processing in FSL.67 All the initial stages of tract-based spatial statistics pre-processing were performed on FA maps. First, we aligned the FA maps of all the participants to one another to identify the brain that was most representative for our sample. This approach was used to account for difference in brain proportions between our participants (children and adolescents) and an adult-derived default image used in FSL to register participants to standard space (FMRIB58_FA). The subject with the least registration warping required to align to all other subjects68 was selected as a target for registration to the standard space. The target was then affine-aligned to 1 × 1 × 1 mm3 MNI152 space. Next, the scans of other subjects underwent nonlinear transformation to the target and affine-alignment to standard space.69, 70 Then, the mean FA file was produced and thinned to create WM mean FA skeleton representing centres of fibres common to the entire sample.67 Subsequently, the 0.2 threshold was applied to produce mean FA skeleton mask representing the most prominent and consistent tracts of subjects' brains, which were later used in voxel-wise statistics.67 Finally, each participant's FA and non-FA (MD, L1, L2, L3) maps were projected onto the FA-derived skeleton.67 The resulting data were fed into intra and inter-group voxel-wise cross-subject statistics.
Second level analysis
First, we checked whether typically developing individuals showed patterns known to be associated with WM development by virtue of a correlation between their age and either their FA or MD, the two main WM connectivity indices with the most reliably documented developmental patterns. Then, we tested whether the neural phenotype and endophenotype of autism were characterized by alterations of typical correlations between WM measurements and age. Thus, we performed non-parametric t-tests comparing individuals with ASC and their siblings to typically developing participants28 in the correlation between age and either FA and MD and thereby tested group by age interaction for the two pairs. To further verify that the observed differences were not due to variations in IQ, we excluded six participants with the lowest IQ from the ASC group and thus equalized it among the study groups. Subsequently, we repeated comparisons between individuals with ASC and typically developing participants. Then, we compared the two pairs of groups in the correlation between age and L1, L2 and L3 to test which of the component vectors accounted most for the difference observed in the main diffusivity indices. Finally, those of the inter-group comparisons having significant results were repeated, this time controlling for sex as a confounding variable to test whether this changed the results. To perform all these comparisons, we used FSL General Linear Model tool where in one design we modelled both means and correlation with age for all the groups and FSL non-parametric randomize with 5000 permutations.71 Threshold-Free Cluster Enhancement with default parameters (height 2; extent 1; connectivity 26; P<0.05) was used to perform the whole-brain family-wise error correction for multiple comparisons in the obtained results.72 We defined significant results with a P<0.05 (threshold-free cluster enhancement corrected). JHU ICBM-DTI-81 White-Matter Labels and JHU White-Matter Tractography Atlas73 were used to determine in which structures the significant results were localized.
Results
FA and MD correlations with age in typically developing individuals
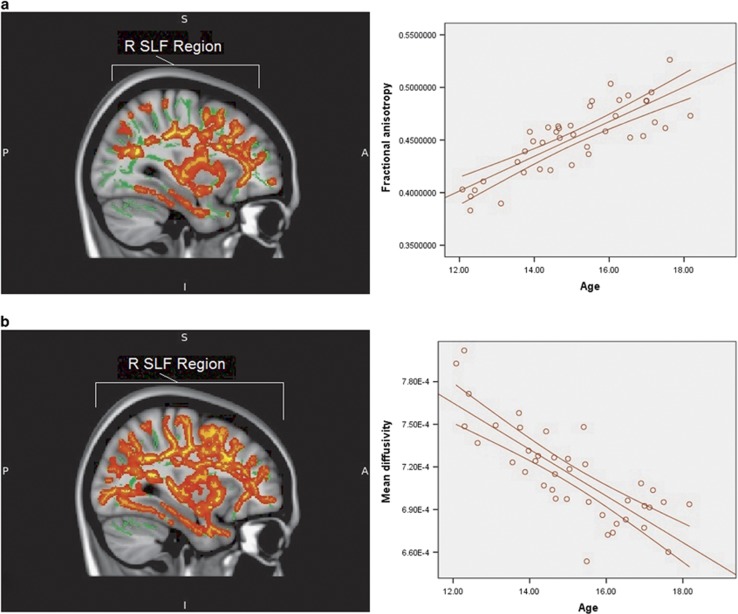
Typically developing individuals displayed a significant positive correlation between age and FA as well as negative correlation between age and MD (Figure 1). Structures displaying correlation between FA and age involved left and right superior and inferior longitudinal fasciculus, right inferior fronto-occipital, corticospinal tract, thalamic radiation and corpus callosum (Table 2). The correlation between MD and age was observed in typically developing individuals throughout all WM and included both long- and short-range connections in both hemispheres (Table 2).
Figure 1.
(a) Areas displaying positive correlation between age and fractional anisotropy values in typically developing adolescents (red area). The graph to the right presents correlation between age and fractional anisotropy values in those areas (red line). (b) Areas displaying negative correlation between age and mean diffusivity values in typically developing adolescents (red area). The graph to the right presents correlation between age and mean diffusivity values in those areas (red line). The results for both a and b are TFCE whole-brain corrected with a threshold at P<0.05. The position of the largest region is indicated. R SLF represents the right-hemispheric region centred on superior longitudinal fasciculus. Background represents the mean tract skeleton (green area) and T1-weighted MNI152 1 mm standard FSL brain (grey area). FSL, FMRIB Software Library; TFCE, threshold-free cluster enhancement.
Table 2. Areas displaying significant correlations between white matter microstructure measurements and age.
| Tracts | Cluster size (N voxels) | T value | P-value (TFCE corrected) |
Coordinates of the peak in aligned anatomical space |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Positive correlation between fractional anisotropy and age in typically developing individuals | ||||||
| Superior longitudinal fasciculus R External capsule R Corpus callosum (forceps minor, body, splenium, tapetum, forceps major) Inferior fronto-occipital fasciculus R Inferior longitudinal fasciculus R Corona radiata (anterior, superior and posterior part) R Internal capsule (anterior and posterior limb, retrolenticular part) R Thalamic radiation (anterior and posterior part) R Fornix R | 15 032 | 2.66 | 0.021 | 42 | −13 | −14 |
| Inferior longitudinal fasciculus L Corticospinal tract L Thalamic radiation (anterior and posterior part) L Medial lemniscus L Inferior fronto-occipital fasciculus L Superior longitudinal fasciculus L Cingulum (cingulate gyrus and hippocampus) L Posterior corona radiata L | 2033 | 3.63 | 0.026 | −24 | −71 | 26 |
| Corticospinal tract R Anterior thalamic radiation R | 1628 | 3.22 | 0.039 | 9 | −29 | −19 |
| Cingulum in hippocampus R Inferior longitudinal fasciculus R | 1102 | 3.84 | 0.034 | 30 | −20 | −28 |
| Uncinate fasciculus R Inferior fronto-occipital fasciculus R | 241 | 3.69 | 0.042 | 28 | 14 | −9 |
| Cerebellum (peduncles and pons) | 98 | 3.94 | 0.046 | 11 | −75 | −33 |
| Anterior thalamic radiation R Medial lemniscus R | 80 | 3.46 | 0.048 | 8 | −20 | −8 |
| Inferior longitudinal fasciculus L | 72 | 3.76 | 0.046 | −34 | −57 | −12 |
| Forceps major Inferior fronto-occipital fasciculus R | 66 | 2.89 | 0.047 | 24 | −87 | 23 |
| Inferior fronto-occipital fasciculus R | 21 | 2.79 | 0.049 | 28 | 48 | −6 |
| Middle cerebellar peduncle | 12 | 3.37 | 0.049 | 14 | −29 | −37 |
| Inferior longitudinal fasciculus L | 4 | 3.53 | 0.049 | −34 | −48 | −14 |
| Negative correlations between mean diffusivity and age in typically developing individuals | ||||||
| Corpus callosum (forceps minor, genu, body, splenium, tapetum, forceps major) Superior longitudinal fasciculus R+L Cerebellum (peduncles and pons) Corona radiata (anterior, superior and posterior part) R+L Inferior fronto-occipital fasciculus R+L Internal capsule (anterior and posterior limb, retrolenticular part) L+R Inferior longitudinal fasciculus R+L Corticospinal tract R+L Thalamic radiation (anterior and posterior part) R+L External capsule R+L Cingulum (cingulate gyrus and hippocampus) R+L Uncinate fasciculus L+R Medial lemniscus R+L Fornix L+R | 79 764 | 2.12 | <0.001 | 16 | −79 | 22 |
| Negative correlation between mean diffusivity and age: ASC < typically developing individuals | ||||||
| Superior longitudinal fasciculus R Corona radiata (anterior, superior and posterior part) R Corpus callosum (forceps minor, genu, body, splenium, tapetum, forceps major) Inferior fronto-occipital fasciculus R External capsule R Inferior longitudinal fasciculus R Thalamic radiation (anterior and posterior part) R Internal capsule (anterior and posterior limb, retrolenticular part) R Corticospinal tract R Cingulum (cingulate gyrus and hippocampus) R Uncinate fasciculus R Fornix R | 20 963 | 3.38 | 0.011 | 20 | −74 | 37 |
| Negative correlation between second diffusivity direction and age: ASC < typically developing individuals | ||||||
| Superior longitudinal fasciculus R Corona radiata (anterior, superior and posterior part) R Corpus callosum (forceps minor, genu, body, splenium, tapetum, forceps major) Corticospinal tract R Thalamic radiation (anterior and posterior part) R Inferior fronto-occipital fasciculus R Internal capsule (anterior and posterior limb, retrolenticular part) R External capsule R Inferior longitudinal fasciculus R Cingulum (cingulate gyrus and hippocampus) R Uncinate fasciculus R Fornix R | 14 391 | 2.82 | 0.017 | 50 | 0 | 18 |
| Corpus callosum (splenium and forceps major) | 4924 | 3.68 | 0.031 | −20 | −49 | 22 |
| Anterior thalamic radiation L | 4489 | 3.24 | 0.037 | −6 | −4 | 8 |
| Forceps minor Anterior thalamic radiation R | 3835 | 3.98 | 0.029 | 20 | 37 | 19 |
| Uncinate fasciculus L Inferior fronto-occipital fasciculus L Anterior thalamic radiation L | 1622 | 3.24 | 0.038 | −39 | 38 | 6 |
| Inferior longitudinal fasciculus L Uncinate fasciculus L Superior longitudinal fasciculus L | 807 | 3.63 | 0.04 | −44 | 3 | −21 |
| Superior longitudinal fasciculus L | 770 | 3.87 | 0.047 | −39 | 6 | 42 |
| Inferior longitudinal fasciculus L Inferior fronto-occipital fasciculus L Superior longitudinal fasciculus L | 406 | 2.45 | 0.047 | −42 | −17 | −17 |
| Forceps minor Cingulate gyrus Anterior corona radiata L | 275 | 2.57 | 0.047 | −17 | 33 | 14 |
| Corticospinal tract L | 249 | 3.98 | 0.043 | −19 | −31 | 48 |
| Inferior longitudinal fasciculus L | 232 | 3.03 | 0.048 | −34 | −54 | −13 |
| Middle cerebellar peduncle | 128 | 3.18 | 0.048 | 24 | −65 | −37 |
| Inferior fronto-occipital fasciculus L Inferior longitudinal fasciculus L Forceps major | 94 | 2.3 | 0.048 | −26 | −72 | 0 |
| Inferior longitudinal fasciculus L Uncinate fasciculus L | 91 | 2.68 | 0.049 | −40 | −3 | −24 |
| Corticospinal tract L | 81 | 2.88 | 0.048 | −9 | −29 | 60 |
| Uncinate fasciculus L | 61 | 3.08 | 0.049 | −26 | 22 | −17 |
| Negative correlation between second diffusivity direction and age: ASC < siblings | ||||||
| Superior longitudinal fasciculus R | 77 | 2.68 | 0.041 | 47 | −3 | 24 |
| Superior longitudinal fasciculus R | 47 | 2.84 | 0.047 | 34 | −2 | 29 |
| Negative correlation between third diffusivity direction and age: ASC < typically developing individuals | ||||||
| Superior longitudinal fasciculus R | 68 | 4 | 0.046 | 53 | −33 | 36 |
| Superior longitudinal fasciculus R | 67 | 3.85 | 0.047 | 42 | −28 | 37 |
Abbreviations: ASC, autism spectrum condition; L, left; R, right; TFCE, threshold-free cluster enhancement.
Group differences in correlation between WM microstructure measures and age
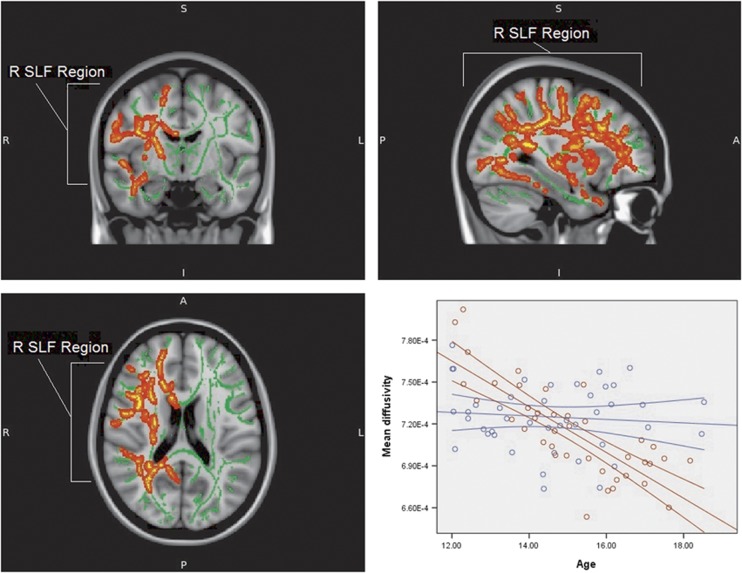
There was no observed interaction between age and group for age-related changes in FA. However, there was a highly significant difference between typically developing adolescents and individuals with ASC in terms of the correlation between age and MD (Figure 2). Typically developing individuals showed a far stronger negative correlation between MD and age than adolescents with ASC. The difference was observed in both short- and long-range fibres, predominantly in the right hemisphere (Table 2). After excluding the six participants with the lowest IQ scores from the ASC group, this result remained (Supplementary Material 1). The difference in age-MD correlation between the typically developing adolescents and siblings of individuals with ASC trended towards significance with the P-value of the centre of gravity of the most significant cluster at 0.059 (Supplementary Figure 2). After adding sex to the analysis as a confounding variable, the changes in the observed results were not large enough to change our conclusions (Supplementary Figure 3).
Figure 2.
Areas displaying the difference between typically developing adolescents and individuals with ASC in correlation between age and mean diffusivity values (red area). The graph presents correlation between age and mean diffusivity values for both typically developing adolescents (red line) and individuals with ASC (blue line) in the area of significant interaction between age and diagnosis. The results are TFCE whole-brain corrected with a threshold at P<0.05. The position of the region is indicated. R SLF represents the right-hemispheric region centred on superior longitudinal fasciculus. Background represents the mean tract skeleton (green area) and T1-weighted MNI152 1 mm standard FSL brain (grey area). ASC, autism spectrum condition; FSL, FMRIB Software Library; TFCE, threshold-free cluster enhancement.
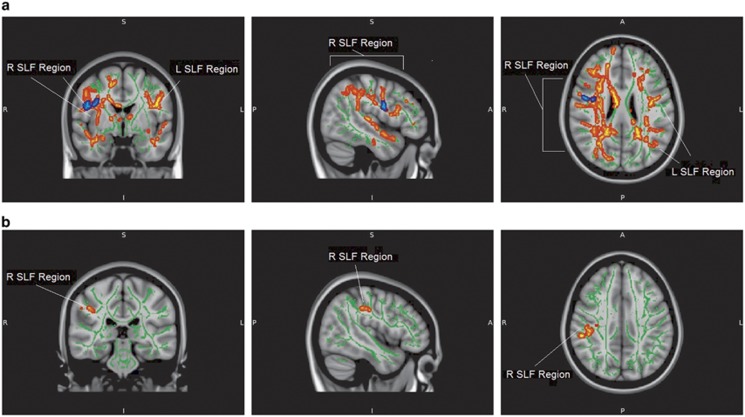
With individual tensor directions, we did not observe any difference in correlation of age and L1 between the compared groups. On the other hand, typically developing individuals showed stronger negative correlations between age and L2, as well as age and L3, in comparison with individuals with ASC with difference in L2 being observed in a broader area of the brain than the difference in L3 (Figure 3). Typically developing adolescents displayed a stronger negative correlation between L2 and age also in comparison with siblings of individuals with ASC in the right superior longitudinal fasciculus (Figure 3). Even though all the comparisons were performed for the entire brain skeleton, 98.4% of the area observed in the difference between siblings and controls spatially overlapped with the area of the difference between individuals with ASC and controls in the corresponding WM microstructure measurement (Figure 3). After adding sex to the analysis as a confounding variable, the changes in the observed results were not large enough to change our conclusions (Supplementary Figure 3).
Figure 3.
(a) Areas displaying the difference in correlation between age and second diffusivity direction between typically developing adolescents and individuals with ASC (red area) and between typically developing adolescents and siblings of individuals with ASC (blue area); 98.4% of the blue area is within the boundaries of the red area. (b) Areas displaying the difference in correlation between age and the third diffusivity direction between typically developing adolescents and individuals with ASC (red area). The results for both a and b are TFCE whole-brain corrected with a threshold at P<0.05. The position of the regions is indicated. R SLF represents the right-hemispheric region centred on superior longitudinal fasciculus. Background represents mean tract skeleton (green area) and T1-weighted MNI152 1 mm standard FSL brain (grey area). ASC, autism spectrum condition; FSL, FMRIB Software Library; TFCE, threshold-free cluster enhancement.
Discussion
For typically developing adolescents, our study found significant correlations of WM microstructure with age, corresponding to the developmental changes previously observed in longitudinal studies.11, 12 This suggests that these correlations are indicative of the previously observed changes in FA and in MD reported in adolescence.3, 4, 5, 6, 7, 8, 10, 11, 12, 74, 75 Thus, age-related development in adolescence is associated with increasing fibre coherence and directionality,3, 13 as well as with diminishing axon calibre, growing axon density and developing myelination,3, 13, 14, 15 leading to increasing consolidation and maturation of WM fibres.4 The change may result in a more coordinated information flow in the central nervous system14 and consequently in a more controlled and appropriate response to the surrounding physical and social environment of an individual.
The difference between healthy participants and individuals with ASC occurred in the correlation between MD and age and encompassed a broad area of the brain. Although at the beginning of puberty, individuals with ASC show lower MD and therefore more maturity in WM microstructure than typically developing teenagers,29, 34 by the end of adolescence, the trend is reversed and typically developing individuals display more mature WM than their counterparts with ASC.27, 28, 30 These results can be explained in two ways. First, in spite of shared core symptoms,76 the autistic population is heterogeneous.77, 78 Thus, the maturation of each individual with ASC may be idiosyncratic and possibly shifted in time for some individuals but not for the others. Therefore, when aggregated, individuals do not form a uniform line of maturation and thus large-scale longitudinal studies may be required to observe the diverse individual WM developmental trajectories. This would suggest that when genetic influences are considered, WM maturation in the ASC group could be highly impacted by loci such as 3q27 and 15q25,48 which contribute to individual differences in WM development and are simultaneously linked to increased susceptibility to ASC.49 Second, there is a possibility that WM development in the entire ASC group is shifted in time, and during adolescence it has already reached its plateau. Evidence for early brain overgrowth observed in autism79, 80, 81, 82, 83, 84, 85 supports this notion, suggesting that genes such as NLGN3, NLGN4, NRXN1, CNTNAP2 and SHANK3—potential genetic candidates for ASC susceptibility which simultaneously affect WM development49, 86, 87—may contribute to the observed differences. Their selected variants predispose individuals to developing autism, potentially through shifting their WM maturation in time and thus changing the flexibility of the entire nervous system.
The differences between individuals with ASC and typically developing controls were associated with MD. This suggests that WM alterations in the phenotype of autism are related to axon calibre, density or myelination. The difference is driven by diffusivity directions perpendicular to the axon axis (L2 and L3). These measures of fibre diameter provide information about the axon membrane and its surrounding myelin sheath.14 The biological role of the myelin sheath is to accelerate signal transmission and to enhance fibre regeneration.88 During adolescence, myelination of the frontal and parietal lobes is an ongoing process.2 Since altered correlation between age and myelination is shown in ASC, one may speculate that signal transmission and fibre regeneration are also affected. Greater myelination observed in children with ASC may intensify information flow in some of their neural networks, reinforcing particular behaviours while limiting their variety. This may explain restricted, intense yet more advanced interests displayed by some children with autism.89, 90 The trend, however, reverses during adolescence and typically developing individuals are the ones with neural structures allowing for increasingly coordinated flow of information.
In our study, we observed that the largest area displaying the differences between individuals with ASC and typically developing adolescents in age-MD correlation was the right superior longitudinal fasciculus. The right superior longitudinal fasciculus is a long-range inter-lobe fibre, which underpins attentional processes in an individual.91, 92 According to longitudinal studies it undergoes substantial growth during adolescence.11, 12 Motor behaviour, visual-spatial coherence and language production, domains affected in ASC,40 all depend on smooth information flow in the superior longitudinal fasciculus93 and in each of these domains, attention has a crucial role. Impaired disengagement of attention,94 reduced capacity for joint attention95, 96 and lessened attention to social details97 are features central to autism. Inflexibility of attention, both situational and developmental, may prove to be a crucial feature of the mechanism leading to ASC associated with WM changes.98 Interestingly, withdrawing participants with the lowest IQ scores from the ASC group did not change this result, at least in our sample, providing a hint that this feature may be independent of intelligence measured with Wechsler Abbreviated Scale of Intelligence.
We also observed a difference in age-L2 correlation between typically developing adolescents and siblings of individuals with ASC. This difference was not as extensive as the one observed in phenotype and was limited to one of the perpendicular diffusivity directions which, however, carries information about predominance of the main diffusivity direction in a voxel and about the possibility of crossing fibres.17 The occurrence of the endophenotypic difference in L2 suggested that individuals with ASC and their siblings may display less crossing fibres in the right superior longitudinal fasciculus than typically developing teenagers at the beginning of adolescence but this trend reversed at the end of this period. What is important the endophenotypic difference followed the same pattern, with typically developing individuals displaying stronger negative correlation between L2 and age than siblings of individuals with ASC in the right superior longitudinal fasciculus, at mostly the same region as that found in the ASC-control comparison. A similar relation between individuals with ASC and their siblings, where the latter displayed a milder form of the changes characteristic for the former, was observed in a younger cohort by Barnea-Goraly et al.28 As the differences noted in first-degree relatives are a more subtle variant of those observed in the autism phenotype, it is possible that alterations in WM maturation aggregate with increasing presence of genetic mutations associated with ASC. However, autistic symptoms appear when the level of WM alterations surpasses a certain threshold.46 Therefore, although these features form a continuum, the diagnosis can be disjunctive. The detected difference is especially striking as siblings of individuals with ASC did not differ in autism symptoms from typically developing adolescents. It suggests that these alterations can be more fundamental than observed symptoms.
Limitations
In our study, we observed some differences between the diagnostic groups in the distribution of sex and IQ. This poses a question as to whether such features of the samples might be responsible for the differences noted in WM. Differences of distribution in IQ have been observed in previous studies of ASC, and are difficult to control through statistical modelling.61, 62 This is not to say that the ASC group displays lower general intelligence than the typically developing population, but rather that individuals of this group may have specific difficulties related to Wechsler Abbreviated Scale of measured intelligence.99 However, after excluding the participants with lowest IQ scores from the ASC group, and thus diminishing the difference in IQ between the two groups to a level of non-significance, we still observed the difference between the groups in WM. This suggests that the observed differences in MD go beyond the differences in IQ. Dissimilarity of sex ratio is of lesser importance for our conclusions as we did not investigate the difference between individuals with ASC and their siblings directly, which were the only two groups to differ significantly in gender distribution. Furthermore, after adding sex to the analysis as a confounding variable, the changes in the observed results were not large enough to change our conclusions.
Our study is additionally limited by the fact that it uses a cross-sectional design to observe changes associated with age. However, our observations in typically developing individuals concord with the results of previous longitudinal studies in this age group.11, 12 Furthermore, entry of our participants to the examined groups were statistically independent in age, thus preventing any bias linked with non-random entry of the participants into the sample.100 We cannot entirely guarantee that age-trajectories of all individuals in the ASC group or in the siblings group were parallel, and therefore we cannot ascertain whether weaker correlations between diffusivity measures and age in these groups indicate changes in the development trajectory of the entire group or increased developmental variability within each group. For this reason we offer two possible explanations of the observed differences in the Discussion section above.100
Conclusion
We conclude that the correlation between age and WM microstructure measurements differs between individuals with and without ASC. The difference in its milder form also appears in siblings of individuals with ASC, compared with the typically developing population, suggesting that WM development during adolescence may be a potential endophenotype of autism and may be associated with altered number of crossing fibres. The difference is observed in areas that have been associated with attention and cognitive flexibility in the typically developing population. This suggests that attention flexibility and its association with WM development may be an interesting topic for future studies of the ASC endophenotype. Our findings emphasize the importance of WM development in pathophysiology of ASC and in integrating its genes-to-symptoms mechanisms. They also signal that a potential treatment for ASC may be extended to the period of adolescence.
Acknowledgments
We are grateful to all the participants and their families and to the autism support organizations that helped with recruitment. We are grateful for the technical assistance of Dr Cinly Ooi, and the help of Dr Erik O'Hanlon, Dr Kirstie Whitaker and Dr Marta Correia. This research was funded by an MRC Clinician Scientist Fellowship to MDS from the UK Medical Research Council (G0701919). LRC was supported by the Gates Cambridge Scholarship Trust. SB-C was supported by the Wellcome Trust, the MRC and the Autism Research Trust, during the period of this work.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan L-H, Zhou K, Khong P-L. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41:223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson L-A, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Regional brain axial and radial diffusivity changes during development. J Neurosci Res. 2012;90:346–355. doi: 10.1002/jnr.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins K, Douaud G, James A, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins K, Chadwick M, James S, Winmill L, Douaud G, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon. Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Sinha S, Sinha U, Edgerton VR. In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging. 2006;24:182–190. doi: 10.1002/jmri.20593. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association. DSM-5 Task Force Diagnostic and Statistical Manual of Mental Disorders: DSM-55th edn, American Psychiatric Association: Washington, DC, USA; 2013. p 947. [Google Scholar]
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. J Child Psychol Psychiatry. 2005;46:401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Mengotti P, D'Agostini S, Terlevic R, De Colle C, Biasizzo E, Londero D, et al. Altered white matter integrity and development in children with autism: a combined voxel-based morphometry and diffusion imaging study. Brain Res Bull. 2011;84:189–195. doi: 10.1016/j.brainresbull.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou K-H, Chen I-Y, Fan Y-T, Decety J, Lin C-P. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Cook E, Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Jou R, Mateljevic N, Kaiser M, Sugrue D, Volkmar F, Pelphrey K. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. Am J Neuroradiol. 2011;32:1607–1613. doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry. 2010;67:1052. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 2012;12:148. doi: 10.1186/1471-2377-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Buitelaar JK, van der Gaag RJ, Zwiers MP. Pervasive microstructural abnormalities in autism: a DTI study. J Psychiatry Neurosci. 2011;36:32. doi: 10.1503/jpn.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Walker L, Gozzi M, Lenroot R, Thurm A, Behseta B, Swedo S, et al. Diffusion tensor imaging in young children with autism: biological effects and potential confounds. Biol Psychiatry. 2012;72:1043–1051. doi: 10.1016/j.biopsych.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou E, Taylor MJ. Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Mol Autism. 2011;2:4. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol. 2008;67:829. doi: 10.1097/NEN.0b013e318184482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156:255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucksmith E, Roth I, Hoekstra R. Autistic traits below the clinical threshold: re-examining the broader autism phenotype in the 21st century. Neuropsychol Rev. 2011;21:360–389. doi: 10.1007/s11065-011-9183-9. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167:1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry. 2005;46:963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- Grønborg TK, Schendel DE, Parner ET. Recurrence of autism spectrum disorders in full-and half-siblings and trends over time: a population-based cohort study. JAMA Pediatr. 2013;167:947–953. doi: 10.1001/jamapediatrics.2013.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelwright S, Auyeung B, Allison C, Baron-Cohen S. Research defining the broader, medium and narrow autism phenotype among parents using the Autism Spectrum Quotient (AQ) Mol Autism. 2010;1:10. doi: 10.1186/2040-2392-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. J Autism Dev Disord. 2007;37:1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Chiang M-C, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, et al. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage. 2011;54:2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster JL, Winkler AM, Smith S, Thompson PM, et al. Genetics of microstructure of cerebral white matter using diffusion tensor imaging. Neuroimage. 2010;53:1109–1116. doi: 10.1016/j.neuroimage.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, Christian SL. Genetics of autism spectrum disorders. Curr Neurol Neurosci Rep. 2009;9:188–197. doi: 10.1007/s11910-009-0029-2. [DOI] [PubMed] [Google Scholar]
- Spencer M, Holt R, Chura L, Suckling J, Calder A, Bullmore E, et al. A novel functional brain imaging endophenotype of autism: the neural response to facial expression of emotion. Transl Psychiatry. 2011;1:e19. doi: 10.1038/tp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised. Western Psychological Services: Los Angeles, CA, USA; 2003. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Spencer MD, Holt RJ, Chura LR, Calder AJ, Suckling J, Bullmore ET, et al. Atypical activation during the Embedded Figures Task as a functional magnetic resonance imaging endophenotype of autism. Brain. 2012;135:3469–3480. doi: 10.1093/brain/aws229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire: Manual. Western Psychological Services: Torrance, CA, USA; 2003. [Google Scholar]
- Spencer MD, Chura LR, Holt RJ, Suckling J, Calder AJ, Bullmore ET, et al. Failure to deactivate the default mode network indicates a possible endophenotype of autism. Mol Autism. 2012;3:1–9. doi: 10.1186/2040-2392-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males. PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DS. Wechsler Abbreviated Scale of Intelligence (WASI) Psychological Corporation: London, UK; 1999. [Google Scholar]
- Chiang H-M, Tsai LY, Cheung YK, Brown A, Li H. A meta-analysis of differences in IQ profiles between individuals with Asperger's disorder and high-functioning autism. J Autism Dev Disord. 2014;44:1577–1596. doi: 10.1007/s10803-013-2025-2. [DOI] [PubMed] [Google Scholar]
- Gizzonio V, Avanzini P, Fabbri-Destro M, Campi C, Rizzolatti G. Cognitive abilities in siblings of children with autism spectrum disorders. Exp Brain Res. 2014;232:2381–2390. doi: 10.1007/s00221-014-3935-8. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Pauley G, Richards T, Neuhaus E, Martin N, Corrigan NM, et al. Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Res. 2012;1479:1–16. doi: 10.1016/j.brainres.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Chung S, Berman J, Hess C, Henry R. Diffusion tensor MR imaging and fiber tractography: technical considerations. Am J Neuroradiol. 2008;29:843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens T, Robson M, Drobnjak I, Rushworth M, Brady J, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Denton ER, Sonoda LI, Rueckert D, Rankin SC, Hayes C, Leach MO, et al. Comparison and evaluation of rigid and nonrigid registration of breast MR images. Medical Imaging'99. International Society for Optics and Photonics. J Comput Assist Tomogr. 1999;23:800–805. doi: 10.1097/00004728-199909000-00031. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, Smith S. Non-Linear Optimisation FMRIB Technical Report TR07JA1. University of Oxford FMRIB Centre: Oxford, UK; 2007. [Google Scholar]
- Andersson JL, Jenkinson M, Smith S.Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2 FMRIB Analysis Group of the University of Oxford 2007 ; http://www.fmrib.ox.ac.uk/analysis/techrep/ .
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher L. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Simms ML, Kemper TL, Timbie CM, Bauman ML, Blatt GJ. The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathol. 2009;118:673–684. doi: 10.1007/s00401-009-0568-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns C, Davis H, Ziccardi R, Carper R, Tigue Z, et al. Unusual brain growth patterns in early life in patients with autistic disorder an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck SM. Genetics of autism spectrum disorder. Eur J Hum Genet. 2006;14:714–720. doi: 10.1038/sj.ejhg.5201610. [DOI] [PubMed] [Google Scholar]
- Hartline DK. What is myelin. Neuron Glia Biol. 2008;4:153–163. doi: 10.1017/S1740925X09990263. [DOI] [PubMed] [Google Scholar]
- Walsh P, Elsabbagh M, Bolton P, Singh I. In search of biomarkers for autism: scientific, social and ethical challenges. Nat Rev Neurosci. 2011;12:603–612. doi: 10.1038/nrn3113. [DOI] [PubMed] [Google Scholar]
- Klin A, Danovitch JH, Merz AB, Volkmar FR. Circumscribed interests in higher functioning individuals with autism spectrum disorders: an exploratory study. Res Pract Persons Severe Disabil. 2007;32:89–100. [Google Scholar]
- De Schotten MT, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, et al. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K. Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia. 2009;47:2600–2603. doi: 10.1016/j.neuropsychologia.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Merchant RE. Encyclopedia of Clinical Neuropsychology. New York: Springer; 2011. Superior longitudinal fasciculus; pp. 2435–2436. [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. J Child Psychol Psychiatry. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol. 2004;40:271. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc B Biol Sci. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170:899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Ability profiles in children with autism influence of age and IQ. Autism. 2003;7:65–80. doi: 10.1177/1362361303007001006. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we. Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.