Abstract
Reinforcement signals in the striatum are known to be crucial for mediating the subjective rewarding effects of acute drug intake. It is proposed that these effects may be more involved in early phases of drug addiction, whereas negative reinforcement effects may occur more in later stages of the illness. This study used resting-state functional magnetic resonance imaging to explore whether acute heroin substitution also induced positive reinforcement effects in striatal brain regions of protracted heroin-maintained patients. Using independent component analysis and a dual regression approach, we compared resting-state functional connectivity (rsFC) strengths within the basal ganglia/limbic network across a group of heroin-dependent patients receiving both an acute infusion of heroin and placebo and 20 healthy subjects who received placebo only. Subsequent correlation analyses were performed to test whether the rsFC strength under heroin exposure correlated with the subjective rewarding effect and with plasma concentrations of heroin and its main metabolites morphine. Relative to the placebo treatment in patients, heroin significantly increased rsFC of the left putamen within the basal ganglia/limbic network, the extent of which correlated positively with patients' feelings of rush and with the plasma level of morphine. Furthermore, healthy controls revealed increased rsFC of the posterior cingulate cortex/precuneus in this network relative to the placebo treatment in patients. Our results indicate that acute heroin substitution induces a subjective rewarding effect via increased striatal connectivity in heroin-dependent patients, suggesting that positive reinforcement effects in the striatum still occur after protracted maintenance therapy.
Introduction
The initial development of drug addiction is mainly driven by pleasurable hedonic effects, which promote renewed drug intake through positive reinforcement mechanisms.1, 2, 3 Experimental evidence in animals revealed that the subjective rewarding effects of acute drug intake are crucially mediated by reinforcement signals in dopamine (DA)-innervated midbrain regions such as the striatum.4, 5 Imaging studies in humans have supported this hypothesis by showing that drug intake acutely increased the extracellular concentration of DA in the striatum and that these increases were associated with their rewarding effects.6 That is, the subjects who had the greatest striatal DA increases after acute drug administration were the ones who experienced the rewarding effects most intensely.7, 8
Behavioral,9 electrophysiological10 and neurochemical11 evidences from animal research have demonstrated that opiates also activate the DA system, which is essential for their acute rewarding effects.12 In more detail, heroin mainly exerts its effects through μ- and κ-opiate receptor agonism; the μ-opiate receptor subtype is critical for the rewarding effects of heroin and morphine, because blockade of μ-opiate receptors, but not of other receptors, attenuates opiate self-administration.13 The heroin-induced striatal DA release is mediated through binding the μ-opioid receptor of GABAergic cells, which is closely associated with subjective rewarding.14 Activation of μ-opioid receptors thus represents a key molecular trigger for the acute rewarding effects of opiates.15
Most recent imaging studies have been using resting-state functional magnetic resonance imaging (fMRI) to gain a mechanistic understanding of brain functions at the large-scale neural system level. Resting-state functional connectivity (rsFC) is based on the analysis of low-frequency fluctuations present in the blood-oxygenation-level-dependent signal.16 These low-frequency fluctuations have been shown to be temporally correlated within spatially distinct but functionally related resting-state networks,17 establishing an intrinsic functional architecture.18 Resting-state networks are associated with many known brain functions including sensory, cognitive or reward processes.18, 19 Thus, evaluating resting-state networks can provide important information regarding inherent brain function that may help identify networks key to addiction-related behaviors, which may be diagnostically or therapeutically useful. For instance, previous resting-state fMRI studies have detected a basal ganglia/limbic network during rest, which subsumes the striatum, the thalamus and the amygdala.20, 21 Many of these regions are strongly implicated in reward processes and DA function,22 and a recent imaging study revealed rsFC differences within this network between nicotine-dependent smokers and healthy controls (HCs).23 These evidences suggest that connectivity abnormalities within the basal ganglia/limbic network may contribute to aberrant rewarding effects in drug-dependent individuals.
This study used resting-state fMRI to explore how an acute heroin treatment modulated rsFC within the basal ganglia/limbic network and whether this change was related to the subjective rewarding effect induced by heroin. In particular, resting-state connectivity strengths in this network were compared between 20 heroin-dependent patients after receiving both an acute infusion of heroin and placebo and 20 healthy subjects who received placebo only. Although all patients are actively enrolled in a maintenance therapy, acute heroin substitution still induces positive emotions and a feeling of rush in these patients,24, 25 indicating that positive reinforcement effects continue during protracted maintenance treatments. Here, we examined whether these subjective rewarding effects after heroin substitution were mediated via connectivity changes in the basal ganglia/limbic network. Given that heroin26 and its psychoactive metabolite morphine augmented DA release in the striatum of rodents,11, 27, 28 we additionally performed correlation analysis between plasma levels of heroin and morphine and rsFC in the basal ganglia/limbic network after heroin administration. We hypothesized that the acute subjective heroin-induced reward effect (that is, feeling of ‘high') was associated with increased rsFC within the basal ganglia/limbic network compared with the placebo treatment in patients and HCs.
Materials and methods
Participants
The study was approved by the local ethics committee and registered with http://clinicaltrials.gov (ID NCT01174927). After receiving a written and oral description of the aim of this study, all participants gave written informed consent statements before inclusion.
Twenty outpatients with opioid dependence according to International Classification of Diseases (ICD)-10 criteria were recruited from the Centre of Substance Use Disorders of the University Hospital of Psychiatry in Basel. The included patients that were older than 18 years and had a past history of intravenous heroin consumption with a current heroin-assisted treatment for at least 6 months with an unchanged heroin dose during the previous 3 months. Patients were excluded from participation if they currently had additional physical diseases or a psychiatric disorder including other comorbid conditions such as substance dependencies. We did not exclude past history of psychiatric disorders or substance dependence. Clinically experienced psychiatrists (MW) conducted a Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Axis II Disorders Structured Clinical Interview for DSM Disorders (SCID-II) to assess the diagnosis of comorbid personality disorders.
Twenty HCs were recruited from the general population by advertisement in the same geographical area as patients. They were carefully screened using a semi-structured clinical interview to exclude psychiatric or physical illness or a family history of psychiatric illness. Analogous to the inclusion criteria for patients, controls were also excluded if they had a positive alcohol breathalyzer test, or any neurologic or severe medical illness history. Owing to ethical reasons, HC only received placebo but no heroin treatment.
Controls and patients were told to abstain from illicit drug consumption for the duration of the study, as well as to abstain from alcohol intake 72 h before scanning. Nevertheless, nine patients were tested positive for cocaine at one or both points of the measurement. We have incorporated this potential confounder into our analyses. Subjects' characteristics are summarized in Table 1.
Table 1. Characteristics of healthy controls and heroin-dependent patients.
| Healthy controls (n=20) | Heroin-dependent patients (n=20) | Between-group statistics | |
|---|---|---|---|
| Age (years±s.d.) | 40.24±10.91 | 41.45±6.70 | t(38)=0.423; P=0.675 |
| Gender (women/men) | 6/14 | 8/12 | χ2=0.440; P=0.507 |
| Education (years±s.d.) | 14.25±2.55 | 10.20±2.82 | t(38)=4.764; P=0.0001 |
| Employment (yes/no) | 20/0 | 10/10 | χ2=13.333; P=0.0003 |
| Smoker (yes/no) | 20/0 | 20/0 | NS |
| Cannabis consumption (yes/no) | 5/15 | 6/14 | χ2=0.125; P=0.723 |
| Cocaine consumption (yes/no) | 0/20 | 9/11 | χ2=11.613; P=0.007 |
| Age at first-time heroin use (years±s.d.) | — | 18.9±3.50 | — |
| Duration of dependence (years±s.d.) | — | 21.5±6.10 | — |
| Daily heroin dose (mg±s.d.) | — | 290±121.44 | — |
Abbreviation: NS, not significant.
Experimental design
Using a crossover, double-blind, vehicle-controlled design, placebo (saline) and heroin (diacetylmorphine) were administered through an indwelling intravenous catheter over a period of 30 s. Heroin hydrochloride was dissolved on site in 5 ml of sterile water and aspirated into a syringe as previously described.29 Each patient was scanned twice, with a short interval between scans (mean 9±3.8 days). Subjects (10) who received their individualized dose of heroin before the first scanning session received 5 ml of placebo before the second session, and vice versa. To preclude order effects, the application scenario was balanced so that 10 patients first received heroin and then placebo, whereas in the other 10 patients the sequence was reversed. During both sessions all heroin-dependent patients received both heroin and placebo. That is, the subjects who received heroin before scanning were administered vehicle after scanning (that is, 60 min after the first injection), whereas the subjects who received placebo before scanning were administered heroin after scanning. HC participated only in the placebo condition.
Subjective heroin effects and bioanalytical measurements
Patients reported their feelings of rush, sedation and intoxication on a visual analog scale (values ranging from 0 to 10) ~60 min after treatment. The plasma concentrations of heroin and morphine 3, 10 and 60 min after heroin administration have previously been reported.30 We briefly report here the measuring approach for the sake of completeness. The plasma concentrations of morphine were measured in venous ammonium-heparinized plasma and assessed using high-performance liquid chromatography on a 125 × 2-mm (inner diameter) Nucleosil 50 C-8 ec column with a particle size of 5 μm and a 8 × 3-mm (inner diameter) precolumn packed with Nucleosil 120 C-8 and a particle size of 3 μm followed by diode array detection. Sample preparation and instrumental conditions were as described previously in detail.31
Resting-state fMRI
For the resting-state scan (5 min), subjects were instructed to lie in dimmed light with their eyes open, to think of nothing in particular and not to fall asleep.
Image acquisition
Scanning was performed on a 3T scanner (Siemens Magnetom Verio, Siemens Healthcare, Erlangen, Germany) 20 min post treatment. Whole-brain functional imaging was performed using a gradient echo planar imaging sequence (repetition time (TR)=2000 ms, echo time (TE)=28 ms, flip angle=82°, field of view=228 × 228 mm2, 32 slices, slice thickness: 3.3 mm; voxel size=3.6 × 3.6 × 3.3 mm3). In total, 152 echo planar imaging volumes were acquired. In addition, a high-resolution T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) image was acquired (TR=2000 ms; TE=3.37 ms; flip angle=8° inversion time=1000 ms; 176 slices; slice thickness=1 mm; voxel size=1 × 1 × 1 mm3).
Functional connectivity analysis
In a first step, resting-state analysis was carried out using MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components),19 a part of FMRIB Software Library (www.fmrib.ox.ac.uk/fsl). MELODIC is a powerful data-driven model-free approach for finding independent patterns in multivariate data (that is, resting-state networks such as the basal ganglia/limbic network). Preprocessing consisted of motion correction using MCFLIRT (Motion Correction using FMRIB's Linear Image Registration Tool),32 removal of non-brain tissue with the Brain Extraction Tool,33 spatial smoothing using a 5-mm full-width-at-half-maximum Gaussian kernel and high-pass temporal filtering equivalent to 111.1 s. Registration of the functional echo planar imaging volumes to each individual subject's high-resolution MPRAGE image and registration of the MPRAGE data to the standard space template (Montreal Neurological Institute, MNI152 T1 1 mm3) were both done using FLIRT. Preprocessed functional data containing 152 time points for each subject were temporally concatenated across subjects to create a single four-dimensional data set. The data set was decomposed into independent components, with a free estimation for the number of components. The components of interest were selected by visual inspection based on previous literature19, 34 and the frequency spectra of the time courses of the components. We used an equal number of subjects from each group so as not to skew independent components in favor of a particular population/treatment. In this study, group-independent component analysis was used to identify the basal ganglia/limbic network. This network has been previously noted in other resting-state fMRI studies using independent component analysis20, 21 including studies in drug addiction.23 A dual regression approach35 with nonparametric permutation (5000) tests (randomize,36 FMRIB Software Library) were carried out to detect statistically significant differences among treatments (placebo vs heroin in patients) and groups (HC vs patients) within the boundaries of the spatial map (basal ganglia/limbic network) obtained with group-independent component analysis (Figure 1). Finally, a family-wise error correction for multiple comparisons was performed, implementing threshold-free cluster enhancement using a significance threshold of P<0.05.37
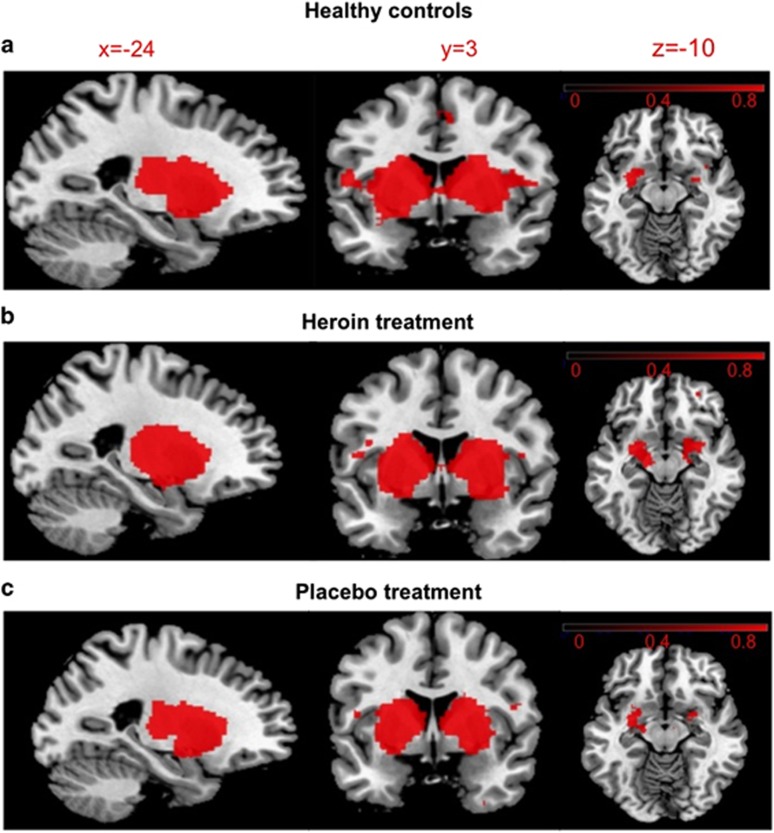
Figure 1.
Spatial maps representing the basal ganglia/limbic network during resting state detected by group-independent component analysis for (a) healthy controls, (b) heroin-dependent patients after heroin and (c) placebo administration. Maps were created using a one-sample t-test for each condition (family-wise error-corrected at P=0.001). Regions belonging to this network include the entire striatum, thalamus and amygdala. The right side of the brain is displayed on the right side of the figure. Color bars represent signal intensity (one-P-value) (obtained from the one-sample t-test).
Results
Subjective heroin effects and plasma levels
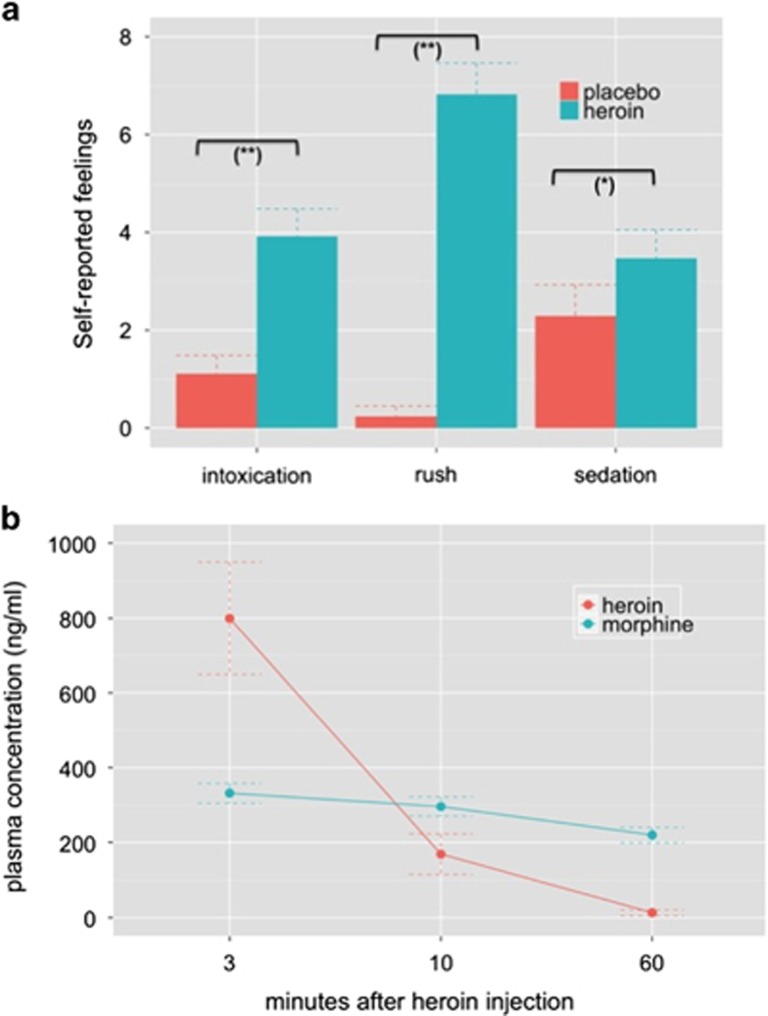
These data have been previously published.24, 30, 38 We adapted them to the subjects who completed resting-state fMRI scanning. Subjective feelings of rush (t(19)=9.570, P=0.0001), sedation (t(19)=2.111, P=0.049) and intoxication (t(19)=4.040, P=0.0008) were significantly higher after heroin than placebo administration (Figure 2a). Measurements of heroin's and morphine's plasma concentrations indicated the much longer half-life period of morphine compared with acetylated compounds.39 Although heroin's plasma concentration rapidly decreased from 800 ng ml−1 (s.d.: 655) to 170 ng ml−1 (s.d.: 236) and 13 ng ml−1 (s.d.: 31) at 3, 10 and 60 min after heroin injection, the mean concentrations of morphine decreased from 332 ng ml−1 (s.d.: 117) to 296 ng ml−1 (s.d.: 114) and 220 ng ml−1 (s.d.: 89). Plasma concentrations of heroin and morphine are depicted in Figure 2b.
Figure 2.
(a) Self-reported psychological heroin effects. Heroin significantly increased feelings of intoxication, rush and sedation relative to the placebo treatment. Note: significant differences between treatment conditions at *P<0.05 and at **P<0.001. (b) Plasma concentrations of heroin (diacetylmorphine) and its main psychoactive metabolite morphine 3, 10 and 60 min after heroin injection. Means and s.e. are displayed.
Functional resting-state connectivity results
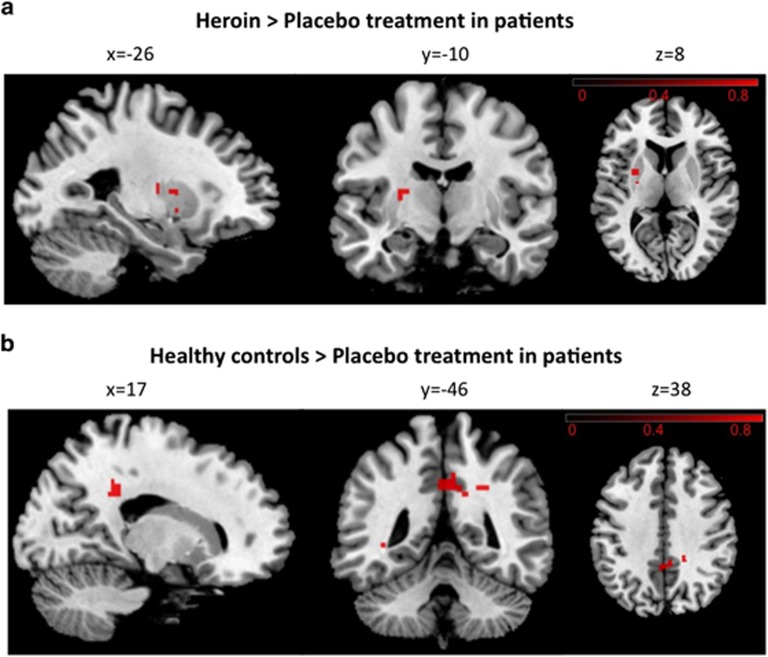
Dual regression of the basal ganglia/limbic network identified with group-independent component analysis (Figure 1) revealed increased rsFC of the left putamen within this network in patients after heroin administration compared with the placebo treatment (family-wise error-corrected) (Figure 3a). Importantly, this heroin-induced increase in rsFC of the left putamen relative to placebo did not depend on whether the patients were positive tested for cocaine or not (t(18)=1.075, P=0.297). There were no regions where the placebo treatment showed increased rsFC to the network compared with the heroin treatment. No difference between HC and the heroin treatment in patients was found, but HC revealed increased rsFC of the posterior cingulate cortex (PCC)/precuneus in the basal ganglia/limbic network relative to the placebo treatment in patients (family-wise error-corrected) (Figure 3b).
Figure 3.
Dual regression results. (a) Results of the comparison between the placebo and heroin treatment in heroin-dependent patients. The left putamen demonstrates greater functional connectivity (FC) within the basal ganglia/limbic network after the heroin compared with the placebo treatment (family-wise error (FWE) corrected at P<0.05) (peak maxima of cluster 1: x=−26, y=–10 and z=8; cluster 2: x=−26, y=2 and z=8 and cluster 3: x=−26, y=2 and z=−4). (b) Results between healthy controls and patients after placebo administration. The posterior cingulate cortex/precuneus (peak maxima: x=17, y=−46 and z=38) demonstrates greater resting-state functional connectivity within the basal ganglia/limbic network in healthy controls compared with the placebo treatment in patients (FWE-corrected at P<0.05). The right side of the brain is displayed on the right side of the figure. Color bars represent signal intensity (one–P-value) (obtained from the paired t-test (a) and the two-sample t-test (b), respectively).
Relation of functional connectivity, subjective heroin effect and plasma levels
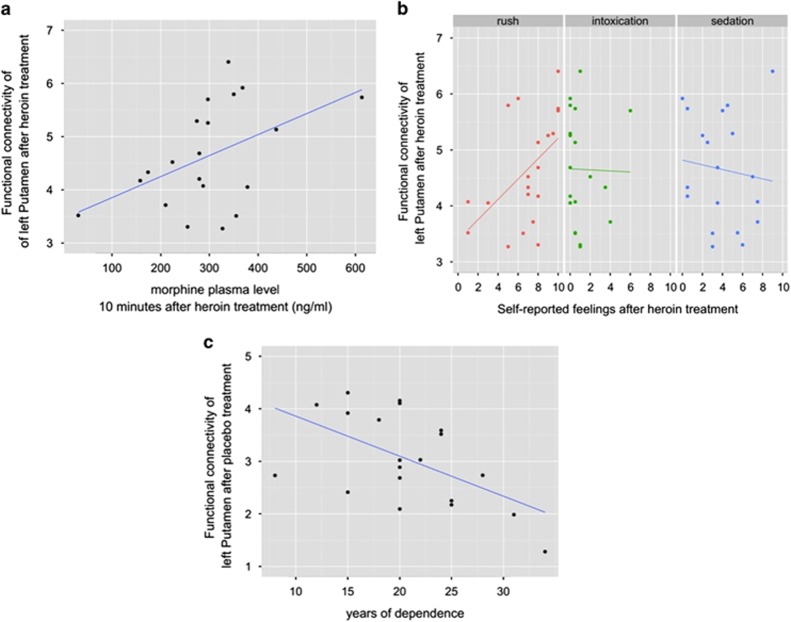
We further tested by using Pearson correlation whether the degree of rsFC strength of the left putamen under heroin exposure correlated with subjective heroin effects (intoxication, rush and sedation) and with plasma levels of heroin and morphine 10 min after heroin administration (nearest time point to resting-state recording). Bonferroni correction was used to adjust for multiple testing. On the basis of previous resting-state fMRI evidence showing a relation between the left putamen and the duration of heroin use in abstinent heroin-dependent subjects,40 we also tested whether rsFC strengths of the left putamen after placebo exposure was related to the duration of heroin dependence. The FC strength of the left putamen within the basal ganglia/limbic network was indexed by parameter estimates (z-scaled) only of the significant voxels obtained from the heroin vs placebo comparison in patients (cf. Figure 3a).
The rsFC strength after heroin injection positively correlated with the plasma level of morphine (r=0.480, P=0.032, uncorrected for multiple comparisons) but not of heroin (r=−0.267, P=0.255) (Figure 4a). Furthermore, we found a significant positive correlation between the FC strength of the left putamen within the basal ganglia/limbic network under heroin exposure and the subjectively perceived feeling of ‘rush' (r=0.507, P=0.023, uncorrected for multiple testing), but not with feelings of sedation (r=−0.113, P=0.635) or intoxication (r=−0.016, P=0.947) (Figure 4b). Finally, there was a significant negative correlation between the rsFC strength after placebo treatment and years of dependence (r=-0.552, P=0.012) (Figure 4c).
Figure 4.
(a) Significant positive correlation between resting-state functional connectivity (rsFC) strength of the left putamen and morphine's plasma level 10 min after heroin injection (r=0.480, P=0.032). (b) Positive correlation between rsFC strength under heroin exposure and subjective feelings of ‘rush' (r=0.507, P=0.023). Importantly, no correlation was found with heroin-induced feelings of sedation (r=−0.113, P=0.635) or intoxication (r=−0.016, P=0.947). (c) Significant negative correlation between the rsFC strength following placebo treatment and years of heroin dependence (r=−0.552, P=0.012).
Discussion
This study explored the neural mechanism underlying heroin's acute subjective rewarding effect in protracted heroin-dependent patients. In particular, we investigated how acute heroin substitution modulated rsFC within the basal ganglia/limbic network and whether this change was related to the subjective rewarding effect induced by heroin. We found that heroin acutely increased rsFC of the left putamen within the basal ganglia/limbic network compared with the placebo treatment in heroin-dependent patients. Notably, the extent of rsFC strength of the left putamen after heroin administration correlated positively with the subjective experience of rush and with the plasma levels of heroin's main psychoactive metabolite morphine. Furthermore, we found that HCs had increased rsFC of the PCC/precuneus in this network relative to the placebo treatment in patients. We discuss these findings in the following within a positive reinforcement framework of reward processing in drug addiction1, 41 and infer on the basis of this perspective a possible neuropharmacological mechanism for the subjective rewarding effect of heroin.
Reinforcement signals in the striatum are known to be crucial for the rewarding effect of heroin and thus have a key role in the development of opioid dependence.5 In accordance with this, our results show that acute heroin administration increased rsFC of the left putamen in the basal ganglia/limbic network compared with the placebo treatment, whereas the extent of left putamen connectivity under heroin exposure correlated with patients' feelings of rush. Remarkably, this relation was specific for the euphoric effect of the drug and not for its sedative and intoxicant effect, albeit this correlation did not survive after correcting for multiple testing. Nevertheless, this finding is in line with a previous fMRI study, which revealed an association between putamen activity and the pleasurable relaxation and enjoyment of cigarette smoking.42 It is interesting to note that acute heroin administration selectively exerted its effect on the left, but not right putamen connectivity. This hemispheric asymmetry is in line with previous evidence showing that intravenous injection of μ-opioid agonist induces left-lateralized brain activation, which correlated with the euphoric effect of the drug.43 However, more evidence is needed to confirm this laterality effect of acute heroin substitution.
It is further emphasized that drugs of abuse increase striatal DA concentrations and that these increases were associated with their reinforcing effects.6 For instance, amphetamine-induced DA release in the human ventral striatum correlates with euphoria,7 whereas subjects having the greatest DA increases after methylphenidate intake were those that perceived the most intense ‘high'.8 Increased striatal DA levels have also been implicated in the rewarding and locomotor-stimulatory properties of morphine in animals,41, 44 likely mediated via major dopaminergic projections from the substantia nigra and the ventral tegmental area.5 In more detail, the morphine-evoked DA release is considered to be due to inhibition of the inhibitory GABAergic interneurons in the ventral tegmental area through activation of μ-opioid receptors to activate mesocorticolimbic dopaminergic neurons.4, 45 According to this evidence, it is conceivable that our finding of increased putamen connectivity after heroin administration was mediated at least in part through heroin-induced DA release into the striatum. This interpretation is supported by the correlation between morphine's plasma level and left putamen connectivity under heroin exposure, having in mind that morphine evokes DA in the striatum.11, 46 That is, patients revealing the greatest morphine plasma concentrations 10 min after heroin injection showed the strongest putamen connectivity to the basal ganglia/limbic network, which subsumes the entire striatum, thalamus and amygdala. The putative striatal DA release after acute heroin injection resonances with evidence showing that detoxified heroin users have lower DA transporter availability that in turn leads to increased DA levels.47
Baseline differences in striatal DA levels might also explain why heroin only increased rsFC of the left putamen compared with the placebo treatment in patients, but not compared with HCs. In particular, chronic heroin-dependent subjects have lower striatal D2 receptor availability and low presynaptic DA compared with HCs48, 49 (see Hou et al.14 for a comprehensive review on the DA system in heroin addiction). Supportive, acute states of withdrawal, as in this study reflected by the placebo condition,50 have been associated with decreased striatal DA level.51 Therefore, we might speculate that the placebo treatment in our study (experimentally induced state of acute withdrawal50) was accompanied by lower levels of striatal DA compared with HCs, resulting in a more pronounced difference of striatal DA level compared with the DA level induced by acute heroin treatment.
Furthermore, we found that the left putamen connectivity within the basal ganglia/limbic network after placebo treatment correlated negatively with the duration of heroin use, perhaps reflecting disease-related long-term effects on reward-related pathways. In line with this results, previous resting-state fMRI studies have already highlighted the importance of the putamen for the pathophysiology of heroin addiction by showing that decreased FC of the putamen,52 as well as abnormal topological properties of the left putamen as indicated by graph metrics (that is, the shortest absolute path length) were negatively related to the duration of heroin dependence.40 Furthermore, it has also been shown that D2 receptor availability in the putamen correlated negatively with years of opiate use in recently abstinent opiate-dependent subjects.48 These changes in the neural reward system may be part of functional alterations, besides those on the brain stress system, postulated to underlie the chronic relapsing nature of prescription opioid addiction.53, 54
However, although acute heroin injection induced DA release in rats,26 several imaging studies in humans failed to detected striatal DA release induced by acute heroin administration55, 56, 57 or by the expectation of heroin reward.58 This can either be explained by the small patient samples used in these studies, the limited sensitivity of the applied technologies or because activation of the dopaminergic system is not necessarily critical for the rewarding effects of heroin.4, 59, 60 The hypothesis that the positive reinforcement effect of acute heroin injection is not directly mediated by DA is supported by studies showing that neurochemically specific lesions of DA in the nucleus accumbens with 6-hydroxydopamine failed to block heroin self-administration.61 It has also been shown that DA is not required for morphine-induced reward as measured by conditioned place preference.62
Finally, we also found that HCs had increased rsFC of the PCC/precuneus in the basal ganglia/limbic network relative to the placebo treatment in patients. Decreased FC of the PCC and thalamus to different brain regions has previously been found in heroin abusers.63 A recent study suggests that high impulsive smokers have greater difficulty in controlling their cravings, and that this weakness may be mediated by lower PCC activity.64 It has also been shown that positive emotionality correlated positively with activity in the precuneus and PCC65 and that they activate with just the presence of an immediate reward,66 a mechanism that is likely related to positive emotional feelings. Given that the placebo treatment in this study represents an acute state of withdrawal characterized by high levels of craving, anxiety and stress hormone releases,50 the reduced PCC/precuneus connectivity might have partly contributed to this negative emotional state. In accordance with this view, metabolic activity of the ventral striatum tended to be negatively correlated with withdrawal symptoms, including craving, negative effect and somatic anxiety symptoms in nicotine-dependent subjects.42
This study has limitations that may influence the interpretation of our findings. We acknowledge the lack of a direct measure of DA concentration induced by acute heroin administration, which aggravates inferences about the underlying neuropharmacological mechanism induced by μ-opioid receptor agonism. Thus, further research is needed to understand the subjective rewarding effects of acute heroin administration by unifying different levels starting from μ-opioid receptor binding to effects of this binding on neurotransmitter pathways and further to the neural system level. Furthermore, we cannot exclude effects on vasoactivity induced by heroin,67 which might have confounded our results. We have also not considered physiological noise that may increase the risk for false positives in pharmacological resting-state fMRI analysis.68 Finally, although differences in formal education may have confounded the comparisons between patients and HCs, this could not account for the differences between the heroin and placebo treatment among patients.
In summary, our findings indicate that increased rsFC of the putamen may contribute to the subjective rewarding effect of heroin and thus provide evidence at the large-scale neural system level of how continued heroin consumption is motivated through positive reinforcement effects. Notably, this positive reinforcement effect was found in heroin-maintained patients. Although it has been proposed that the initial phase of drug addiction is dominated by positive reinforcement mechanisms and that antireward processes (that is, negative reinforcement) increase over the duration of the dependence,69, 70 our data suggest that acute heroin substitution in long-lasting patients still induces a positive reinforcement effect in the striatum. We have recently shown that acute heroin substitution in these patients also led to a negative reinforcement effect by reducing state-anxiety and stress hormone responses, an effect that was mediated through reduced amygdala activity and connectivity.38, 71 These studies together indicate that both positive and negative reinforcement mechanisms still occur after protracted heroin maintenance therapy. The prescription of pharmaceutical heroin has repeatedly been found to be an effective treatment for severe heroin addiction72, 73, 74 and for heroin addicts who have failed to benefit from methadone maintenance treatment.75 However, although heroin substitution strategies can successfully reduce harm and criminality, as well as the additional intake of heroin besides the daily substitution doses,73, 74 this study also shows that heroin-assisted treatment further drives the addiction circle by inducing a positive reinforcement effect. In general, neuroimaging and pychopharmacological imaging needs to translate results in the clinical field, targeting clinical outcomes including response to preventive interventions.76 This additionally impedes withdrawal and abstinence efforts from opioid consumption and should be considered in substitution therapies. However, the relative importance of positive and negative reinforcement mechanisms for the transition from early to late phases of drug addiction is currently being discussed controversially.2 Future research is thus needed to gain a better understanding of the reinforcement mechanisms underlying the development and maintenance of heroin addiction. This study demonstrates that the assessment of resting-state fMRI is a promising technique for this purpose.
Acknowledgments
We would like to acknowledge the infrastructural support of the Medical Image Analysis Center, University Hospital Basel. This study was supported by the Swiss National Science Foundation (32003B-127544) (MW and SB) and FAG Basel (AS).
The authors declare no conflict of interest.
References
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- Holmes LJ, Bozarth MA, Wise RA. Circling from intracranial morphine applied to the ventral tegmental area in rats. Brain Res Bull. 1983;11:295–298. doi: 10.1016/0361-9230(83)90163-6. [DOI] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Heroin reward is dependent on a dopaminergic substrate. Life Sci. 1981;29:1881–1886. doi: 10.1016/0024-3205(81)90519-1. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci. 2002;22:3321–3325. doi: 10.1523/JNEUROSCI.22-09-03321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Tian M, Zhang H. Positron emission tomography molecular imaging of dopaminergic system in drug addiction. Anat Rec (Hoboken) 2012;295:722–733. doi: 10.1002/ar.22457. [DOI] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, et al. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 2012;22:2784–2793. doi: 10.1093/cercor/bhr354. [DOI] [PubMed] [Google Scholar]
- Moussa MN, Steen MR, Laurienti PJ, Hayasaka S. Consistency of network modules in resting-state FMRI connectome data. PLoS One. 2012;7:e44428. doi: 10.1371/journal.pone.0044428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick BEB, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier N, Gerber H, Vogel M, Klarhöfer M, Riecher-Rossler A, Wiesbeck GA, et al. Reduction in cerebral perfusion after heroin administration: a resting state arterial spin labeling study. PLoS One. 2013;8:e71461. doi: 10.1371/journal.pone.0071461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J, Gerber H, Gerhard U, Schmid O, Petitjean S, Riecher-Rössler A, et al. Acute effects of heroin on emotions in heroin-dependent patients. Am J Addict. 2013;22:598–604. doi: 10.1111/j.1521-0391.2013.12025.x. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki Y, Nagayasu K, Kitaichi M, Shirakawa H, Kaneko S. Repeated exposure to methamphetamine, cocaine or morphine induces augmentation of dopamine release in rat mesocorticolimbic slice co-cultures. PLoS One. 2011;6:e24865. doi: 10.1371/journal.pone.0024865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S, et al. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Stohler R, Dürsteler KM, Störmer R, Seifritz E, Hug I, Sattler-Mayr J, et al. Rapid cortical hemoglobin deoxygenation after heroin and methadone injection in humans: a preliminary report. Drug Alcohol Depend. 1999;57:23–28. doi: 10.1016/s0376-8716(99)00036-8. [DOI] [PubMed] [Google Scholar]
- Walter M, Denier N, Gerber H, Schmid O, Lanz C, Brenneisen R, et al. Orbitofrontal response to drug-related stimuli after heroin administration Addict Biol 2014. doi: 10.1111/adb.12145 [DOI] [PubMed]
- Bourquin D, Bundeli P, Lehmann T, Brenneisen R. Diacetylmorphine and its metabolites in plasma by HPLC with diode-array and atmospheric pressure ionization mass spectrometric detection. J Liq Chrom Rel Technol. 1999;22:2663–2674. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Walter M, Gerber H, Seifritz E, Brenneisen R, Wiesbeck GA, et al. Normalizing effect of heroin maintenance treatment on stress-induced brain connectivity. Brain. 2014;138:217–228. doi: 10.1093/brain/awu326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopp G, Ganssmann B, Cone EJ, Aderjan R. Plasma concentrations of heroin and morphine-related metabolites after intranasal and intramuscular administration. J Anal Toxicol. 1997;21:105–111. doi: 10.1093/jat/21.2.105. [DOI] [PubMed] [Google Scholar]
- Yuan K, Qin W, Liu J, Guo Q, Dong M, Sun J, et al. Altered small-world brain functional networks and duration of heroin use in male abstinent heroin-dependent individuals. Neurosci Lett. 2010;477:37–42. doi: 10.1016/j.neulet.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, et al. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Strain EC, Greenberg BD, Preston KL, Lancaster E, Bigelow GE, et al. Site of opioid action in the human brain: mu and kappa agonists' subjective and cerebral blood flow effects. Am J Psychiatry. 1998;155:470–473. doi: 10.1176/ajp.155.4.470. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Co C, Freeman ME, Sands MP, Lane JD. Neurotransmitter turnover in rat striatum is correlated with morphine self-administration. Nature. 1980;287:152–154. doi: 10.1038/287152a0. [DOI] [PubMed] [Google Scholar]
- Jia SW, Wang W, Liu Y, Wu ZM. Neuroimaging studies of brain corpus striatum changes among heroin-dependent patients treated with herbal medicine, U'finer capsule. Addict Biol. 2005;10:293–297. doi: 10.1080/13556210500222456. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18:262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, et al. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71:192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, et al. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2013;76:289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ. Opiates, opioids and addiction. Mol Psychiatry. 1996;1:232–254. [PubMed] [Google Scholar]
- Daglish MR, Williams TM, Wilson SJ, Taylor LG, Eap CB, Augsburger M, et al. Brain dopamine response in human opioid addiction. Br J Psychiatry. 2008;193:65–72. doi: 10.1192/bjp.bp.107.041228. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Tellez-Jacques K, Pittman B, Petrakis I, Baldwin RM, Tamagnan G, et al. Dopamine and serotonin transporter availability in chronic heroin users: a [¹23I]β-CIT SPECT imaging study. Psychiatry Res. 2010;184:192–195. doi: 10.1016/j.pscychresns.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosel M, Noss RS, Hämmig R, Wielepp P, Bundeli P, Heidbreder R, et al. Cerebral blood flow effects of acute intravenous heroin administration. Eur Neuropsychopharmacol. 2008;18:278–285. doi: 10.1016/j.euroneuro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Watson BJ, Taylor LG, Reid AG, Wilson SJ, Stokes PR, Brooks DJ, et al. Investigating expectation and reward in human opioid addiction with [(11) C]raclopride PET. Addict Biol. 2013;19:1032–1040. doi: 10.1111/adb.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction. Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang YR, Qin W, Yuan K, Tian J, Li Q, et al. Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chin Med J (Engl) 2010;123:1582–1588. [PubMed] [Google Scholar]
- Bourque J, Mendrek A, Dinh-Williams L, Potvin S. Neural circuitry of impulsivity in a cigarette craving paradigm. Front Psychiatry. 2013;4:67. doi: 10.3389/fpsyt.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, et al. Positive emotionality is associated with baseline metabolism in orbitofrontal cortex and in regions of the default network. Mol Psychiatry. 2011;16:818–825. doi: 10.1038/mp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht K, Volz KG, Sutter M, von Cramon DY. What do I want and when do I want it: brain correlates of decisions made for self and other. PLoS One. 2013;8:e73531. doi: 10.1371/journal.pone.0073531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey G, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25:366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Chang C, van Osch MJ, Veer IM, van Buchem MA, Dahan A, et al. The impact of ‘physiological correction' on functional connectivity analysis of pharmacological resting state fMRI. Neuroimage. 2013;65:499–510. doi: 10.1016/j.neuroimage.2012.09.044. [DOI] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rössler A, et al. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014;76:289–296. doi: 10.1016/j.biopsych.2013.10.019. [DOI] [PubMed] [Google Scholar]
- van den Brink W, Hendriks VM, Blanken P, Koeter MW, van Zwieten BJ, van Ree JM. Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ. 2003;327:310. doi: 10.1136/bmj.327.7410.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry. 2007;191:55–62. doi: 10.1192/bjp.bp.106.026112. [DOI] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Brissette S, Marsh DC, Lauzon P, Guh D, Anis A, et al. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med. 2009;361:777–786. doi: 10.1056/NEJMoa0810635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken P, Hendriks VM, van Ree JM, van den Brink W. Outcome of long-term heroin-assisted treatment offered to chronic, treatment-resistant heroin addicts in the Netherlands. Addiction. 2010;105:300–308. doi: 10.1111/j.1360-0443.2009.02754.x. [DOI] [PubMed] [Google Scholar]
- Borgwardt S, Fusar-Poli P. Third-generation neuroimaging in early schizophrenia: translating research evidence into clinical utility. Br J Psychiatry. 2012;200:270–272. doi: 10.1192/bjp.bp.111.103234. [DOI] [PubMed] [Google Scholar]