Abstract
Serum cholesterol, both total and lipoprotein fractions, has been associated with mid- and late-life depression. Using longitudinal data on a large and ethnically diverse sample of urban adults, the associations of serum lipid profile measured by high or low total cholesterol (TC; >200 mg dl−1; <160 mg dl−1) and by atherogenic indices, namely high total cholesterol and low-density lipoprotein cholesterol relative to high-density lipoprotein cholesterol, with change in total and domain-specific depressive symptoms over time were examined. Findings were compared by sex. (Hypothesis 1) In addition, baseline depressive symptoms as predictors for longitudinal change in lipid profile trajectory were tested. (Hypothesis 2) Mixed-effects regression analyses stratified by sex was used. Sample sizes of participants (n) and repeated observations (n') were: Hypothesis 1 (Men: n=826 ; n'=1319; Women: n=1099 ; n'=1817); Hypothesis 2 (Men: n=738; n'=1230; Women: n=964; n'=1678). As hypothesized, a higher level of atherogenic indices was linked to faster increase in depressive symptom scores, particularly depressed affect and interpersonal problems, though this relationship was found only among women. Among men a U-shaped relationship between baseline TC and longitudinal increase in somatic complaints and a direct link between low TC and longitudinal putative improvement in positive affect was found. On excluding statin users among women, low TC was associated with slower increase in depressed affect over time, whereas high TC was associated with faster increase in interpersonal problems. In summary, atherogenic indices were directly linked to faster increase in depressive symptoms among women only. More studies are needed to explain these sex-specific associations.
Introduction
Unipolar depression, a potentially life-long illness,1, 2 is currently among the most prevalent diseases in the health care spectrum.3 In the US, its lifetime prevalence among men and women is 12 and 21%, respectively.3 Serum cholesterol (total and lipoprotein fractions) has been associated with mid- and late-life depression. However, research findings from observational and intervention studies have been inconsistent ranging from direct to inverse and often null associations between total cholesterol (TC) and depressive symptoms or depression.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 These inconsistencies may reflect the complexity of such an association, although variations in methodology (for example, study design, measures used to assess depressive symptoms and types of covariates assessed as confounders) along with sample selection characteristics (for example, age, ethnic background and dietary patterns) may explain some discrepancies. Moreover, other serum lipid components, namely triglycerides, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) may determine the development of depression.4, 5, 6, 8, 9, 10, 11, 12, 13, 14, 15, 18, 19, 20, 22, 23, 24, 25, 27, 30, 32, 33, 34, 35, 36, 37, 39, 40, 42, 45, 46, 48, 49 However, few population-based studies have examined associations between lipid markers and depressive symptoms among middle-aged or older adults, using a prospective cohort design40, 46, 47, 48 and none so far have tested reverse causality within the same cohort study. In a recent study using Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) longitudinal data, we found a putative protective effect of a higher n-3:n-6 fatty acid ratio on the change in Center for Epidemiologic Studies-Depression (CES-D) scores over time among women only. Moreover, in this study, the association between this ratio and depressive symptoms was specific to some subscales of CES-D but not others52 Given the known association between fatty acid consumption patterns and serum lipids, we speculated that the longitudinal relationship between serum lipids and depressive symptoms might also be sex-specific as well as domain-specific.
Using longitudinal data on a large and ethnically diverse sample of urban adults, we tested whether the serum lipid profile (that is, high or low TC (>200 mg dl−1; <160 mg dl−1) and high TC or LDL-C relative to HDL-C) are associated with change in depressive symptoms over time;2 whether these associations were specific to certain domains of depressive symptomatology;3 and whether those associations differed between men and women. (Hypothesis 1) Finally, we also tested the reverse causality of the association: whether baseline level of depressive symptoms had an effect on lipid profile trajectory and whether that effect was sex-specific. (Hypothesis 2)
Materials and methods
Database and study sample
The HANDLS study is an ongoing prospective cohort study. Initiated in 2004, HANDLS recruited a representative sample of African Americans and whites (30–64 years old) living in Baltimore, MD, USA.53 Using an area probability sampling design of 13 neighborhoods (groups of contiguous census tracts), phase 1 consisted of screening, recruitment and household interview, whereas phase 2 consisted of examinations in mobile Medical Research Vehicles. Written informed consent was obtained from all the participants following their access to a protocol booklet in layman's terms and a video describing all procedures and future re-contacts. Materials' approval was obtained from the MedStar Institutional Review Board. We use longitudinal data from baseline and the first follow-up examination (visit 2 ended in 2013). Time between visits 1 and 2 ranged from <1 year to ~8 years, with a mean±SD of 4.65±0.93 years.
Initially, 3720 participants were recruited (Sample 1), of whom 2177 (58.5%) had complete baseline phase 2 examination and non-missing dietary data (Sample 2, 2 days of dietary recall). Of those, all participants had non-missing data on depressive symptoms (Sample 3, n=2177). This study restricted data to participants with 2 days of dietary recall and CES-D data at visits 1 or 2 or both (n=2053; Sample 4). Out of Sample 4, we restricted our sample further to those with complete data on baseline serum cholesterol, HDL-C and LDL-C (Sample 5A, n=1925; 826 men and 1099 women). Repeated observations for Hypothesis 1 were n'=3136 (1319 in men and 1817 in women). Participants selected into Sample 5A, compared with the remaining HANDLS participants in Sample 1, had a lower percentage with Poverty Index Ratio (PIR) >125% (57 vs 61%, P=0.011), and a higher percentage of women (57 vs 52%, P=0.003) though no significant differences were found in age and race distributions.
In addition, for Hypothesis 2, the final sample size consisted of participants with complete lipid profile variables at visits 1, 2 or both and had complete data on CES-D total score at visit 1 (Sample 5B, n=1702; 738 men and 964 women), with similar selectivity patterns as for Sample 5A. Repeated observations for Hypothesis 2 were n'=2908 (1230 in men and 1678 in women).
Depressive symptoms
Depressive symptoms were measured using the CES-D scale, at both baseline and follow-up HANDLS visits. The CES-D is a 20-item self-report symptom rating scale that assesses affective and depressed mood.54 A score ⩾16 on the CES-D is a commonly used indicator for elevated depressive symptoms (EDSs),55 associated with clinical depression based on the diagnostic and statistical manual, fourth edition criteria.56 Four CES-D subscales were derived and shown to have an invariant factor structure between The National Health and Nutrition Examination Survey I and pilot HANDLS data, an indication of reliability across samples.57 We examined both total and domain-specific CES-D scores: (1) somatic complaints; (2) depressive affect; (3) positive affect and (4) interpersonal problems.57
Serum cholesterol and atherogenic indices
TC, HDL-C and TA (triacylglycerols) were assessed using a spectrophotometer (Olympus 5400, Olympus, Melville, NY, USA). LDL-C was calculated as TC−(HDL-C+TA/5) and directly measured in a subsample (N=236) also using a spectrophotometer (Olympus 5400). The correlation between those with baseline calculated LDL-C and those with measured LDL-C was r~0.95. From these measures, two relative measures were obtained, namely TC:HDL-C and LDL-C:HDL-C ratios. Those two relative measures, also termed ‘atherogenic indices' were previously studied in relation to various cardiovascular outcomes and were found to be positively associated with measures of atherosclerosis and coronary heart disease.58, 59, 60 Moreover, TC, categorized as low (<160 mg dl−1), medium (160–200 mg dl−1) and high (>200 mg dl−1) was considered as a third main exposure in the analysis predicting depressive symptoms from serum lipid levels.16, 17, 21, 31, 43
Covariates
Socio-demographic, lifestyle and health-related potential confounders
Some covariates were considered potential confounders: age, sex, race (white vs African American), marital status (married vs unmarried), completed years of education (0⩽ High School; 1=High School and 2⩾ High School), poverty income ratio (0=PIR <125% 1=PIR ⩾125%), measured body mass index (kg/m2), current use of drugs (‘opiates, marijuana or cocaine'=1 vs not=0), and current smoking status (0: ‘never or former smoker' and 1 ‘current smoker'). Finally, ‘statin use' vs not at baseline was included as an indicator variable to conduct a sensitivity analysis among non-users as well as to control for statin use in the models. This was done given the known relationship between statin use and serum lipid change over time as well as statin use and baseline elevated serum TC.
Dietary potential confounders
Potentially confounding nutrients (per 1000 kcal) formerly linked to depression were: vitamins B-6, folate and B-12, total carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, lycopene), vitamin C and α-tocopherol,61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 and the ratio of n-3 PUFA:n-6 PUFA.75 To emulate a multivariate nutrient density model,76 total energy intake was included as a covariate. Moreover, overall dietary quality, measured by the Healthy Eating Index (HEI-2010) was also included. The National Cancer Institute's Applied Research website provided steps for calculating HEI-2010 component and total scores (http://appliedresearch.cancer.gov/tools/hei/tools.html). Detailed description of the procedure used is available on the HANDLS website (http://handls.nih.gov/06Coll-dataDoc.htm). Total and component HEI-2010 scores were calculated for each recall day (day 1 and day 2) and then averaged to obtain the mean HEI-2010 total and component scores for both days combined. In the present study, only total HEI-2010 score was considered.
Statistical analysis
Analyses were conducted using Stata release 13.0 (StataCorp, College Station, TX, USA).77 First, socio-demographic characteristics were assessed by sex and EDS status (CES-D score ⩾16 vs <16, based on mean score across visits). We tested mean differences between groups using t-tests and analysis of variance; relationships among categorical variables were evaluated with χ2 tests. Second, mixed-effects regression on continuous CES-D total or on domain-specific score(s) was used to test the association between the three serum lipid exposure variables and depressive symptoms, controlling for potential confounders. Moderating effect of sex was tested by adding interaction terms to the multivariable mixed-effects regressions and stratifying by sex. The mixed-effects regression analyses are discussed in detail in Appendix I and in the following papers.78, 79 A similar approach was used to test Hypothesis 2 whereby baseline CES-D total score was the predictor for each of the three serum lipid variables (TC, TC:HDL-C and LDL-C:HDL-C) modeled over-time in a linear mixed-effects regression model. A sensitivity analysis was conducted to examine if the key findings were robust to the exclusion of statin users at baseline.
To account for potential selection bias in mixed-effects regression models (due to the non-random selection of participants with complete data from the target study population), a two-stage Heckman selection model was constructed,80 using a probit model to obtain an inverse mills ratio at the first stage (derived from the predicted probability of being selected, conditional on the covariates in the probit model, mainly baseline age, sex, race, poverty status and education), as was done in an earlier study.81 A type I error of 0.05 was used for all analyses, with P-values between 0.05 and 0.10 considered as borderline significant for main effects, and P-value <0.10 considered significant for interaction terms,82 before multiple testing correction. The latter was done using a family-wise Bonferroni procedure, with family defined by CES-D total or subscale scores, assuming content-wise independence. As defined in a methodological report, a ‘family' of inferences in the context of confirmatory analysis is ‘any collection of inferences for which it is meaningful to take into account some combined measure of error'. 83 Thus, accounting for three main exposures assuming that a family is each of the subscales and the total score type I error was reduced to 0.05/3=0.017 for main effects and 0.10/3=0.033 for interaction terms (that is, two-way and three-way interactions). A similar approach was followed in a recent study.52
Results
EDS across visits had an estimated unweighted prevalence of 29.2% in men and 37.9% in women (P<0.001, χ2 test; Table 1). Participants classified as EDS+ differed significantly from their EDS− counterparts. Most notably, they had lower income based on PIR <125%, they were more likely unemployed, and had lower educational attainment. Among women, EDS+ (vs EDS−) was associated with a lower likelihood of marriage (22.6 vs 32.1%, P=0.002), and a higher proportion of current smokers (50.5 vs 34.7%, P<0.001) or users of any type of illicit drugs (41.0 vs 30.4%, P=0.001). Moreover, mean body mass index was higher among women vs men (31.3 vs 28.0), but was not associated with EDS status.
Table 1. Characteristics of HANDLS study participants by sex and Center for Epidemiologic Studies Depression Scale (CES-D) score (mean across visits)a.
| % |
Men |
Women |
Pb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| <16 (n=587) | ⩾16 (n=240) | All men (n=827) | <16 (n=675) | ⩾16 (n=424) | All women (n=1099) | Men vs women | Low vs high CES-D score among men | Low vs high CES-D score among women | |
| Depressive symptoms | |||||||||
| CES-D, mean±s.e.m. | 8.3±0.2 | 23.0±0.4 | 12.5±0.3 | 8.00±0.17 | 24.1±0.34 | 14.2±0.3 | <0.001 | <0.001 | <0.001 |
| TC, mean±s.e.m. | 182.9±1.9 | 186.0±3.0 | 183.8±1.6 | 190.1±1.5 | 189.6±2.0 | 189.9±1.2 | 0.002 | 0.39 | 0.84 |
| <160 mg dl−1, % | 22.8 | 19.6 | 21.9 | 15.1 | 16.5 | 15.7 | <0.001 | 0.55 | 0.65 |
| >200 mg dl−1, % | 31.2 | 33.8 | 31.9 | 37.3 | 38.7 | 37.9 | |||
| TC:HDL-C, mean±s.e.m. | 4.08±0.06 | 4.17±0.12 | 4.11±0.06 | 3.60±0.04 | 3.76±0.06 | 3.66±0.04 | <0.001 | 0.44 | 0.045 |
| LDL-C:HDL-C, mean±s.e.m. | 2.45±0.05 | 2.44±0.09 | 2.45±0.04 | 2.13±0.04 | 2.24±0.05 | 2.17±0.03 | <0.001 | 0.91 | 0.07 |
| Socio-demographic and lifestyle factors | |||||||||
| Age (years), mean ±s.e.m. | 48.6±0.4 | 48.3±0.6 | 48.5±0.3 | 48.6±0.4 | 48.0±0.4 | 48.4±0.3 | 0.73 | 0.68 | 0.25 |
| African-American, % | 58.4 | 60.8 | 59.1 | 57.3 | 55.0 | 56.4 | 0.23 | 0.52 | 0.44 |
| Marital status, % | |||||||||
| Currently married | 36.3 | 28.8 | 34.1 | 32.1 | 22.6 | 28.5 | 0.017 | 0.05 | 0.002 |
| Missing | 4.1 | 6.7 | 4.8 | 4.4 | 3.8 | 4.2 | |||
| Education, % | 0.26 | <0.001 | <0.001 | ||||||
| <HS | 6.6 | 11.3 | 8.0 | 5.0 | 7.6 | 6.0 | |||
| HS | 55.0 | 67.1 | 58.5 | 53.6 | 66.0 | 58.4 | |||
| >HS | 38.3 | 21.7 | 33.5 | 41.3 | 26.2 | 35.5 | |||
| Missing | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | |||
| PIR ⩾125%, % | 63.5 | 52.5 | 60.3 | 60.1 | 44.3 | 54.1 | 0.006 | 0.003 | <0.001 |
| Employed, % | <0.001 | <0.001 | <0.001 | ||||||
| Yes | 59.1 | 37.5 | 52.8 | 51.7 | 32.3 | 44.2 | |||
| Missing | 13.3 | 18.3 | 14.8 | 14.5 | 15.6 | 14.9 | |||
| Current smoking status, % | |||||||||
| Currently smoking | 48.2 | 54.2 | 49.9 | 34.7 | 50.5 | 40.8 | <0.001 | 0.28 | <0.001 |
| Missing | 3.2 | 3.3 | 3.3 | 5.6 | 6.4 | 5.9 | |||
| Current use of illicit drugs, % | |||||||||
| Used any type | 57.4 | 57.1 | 57.3 | 30.4 | 41.0 | 34.5 | <0.001 | 0.80 | 0.001 |
| Missing | 6.6 | 7.9 | 7.0 | 9.0 | 6.8 | 8.2 | |||
| Body mass index, kg/m2; mean±s.e.m. | 28.0±0.2 | 27.9±0.4 | 28.0±0.2 | 31.4±0.3 | 31.1±0.4 | 31.3±0.26 | <0.001 | 0.76 | 0.56 |
| Dietary factors, daily intakes | |||||||||
| Energy, kcal | 2290±44 | 2302±75 | 2364±38 | 1740±29 | 1734±36 | 1738±23 | <0.001 | 0.30 | 0.89 |
| Total carotenoids, mg/1000 kcal | 3847±192 | 3164±236 | 3649±153 | 4371±187 | 3817±226 | 4158±145 | 0.017 | 0.042 | 0.06 |
| Vitamin A, RE per 1000 kcal | 302±19 | 311±36 | 304±17 | 347±17 | 343±36 | 345±17 | 0.10 | 0.82 | 0.90 |
| Vitamin C, mg per 1000 kcal | 38.0±1.6 | 33.9±2.8 | 36.8±1.4 | 42.2±1.5 | 36.5±2.0 | 40.0±1.2 | 0.08 | 0.18 | 0.021 |
| Vitamin E, mg per 1000 kcal | 3.16±0.08 | 2.86±0.10 | 3.1±0.1 | 3.6±0.1 | 3.3±0.1 | 3.5±0.1 | <0.001 | 0.030 | 0.013 |
| Vitamin B-6, mg per 1000 kcal | 0.95±0.02 | 0.88±0.03 | 0.93±0.02 | 0.95±0.02 | 0.86±0.02 | 0.91±0.01 | 0.45 | 0.06 | 0.002 |
| Vitamin B-12, μg per 1000 kcal | 3.08±0.21 | 3.16±0.34 | 3.10±0.18 | 2.97±0.17 | 3.15±0.36 | 3.04±0.17 | 0.79 | 0.84 | 0.62 |
| Folate, μg per 1000 kcal | 181.7±4.2 | 173.4±5.9 | 179.3±3.5 | 193.7±3.9 | 178.3±4.5 | 187.8±3.0 | 0.06 | 0.28 | 0.012 |
| n-3 PUFA:n-6 PUFA ratioc | 0.114±0.004 | 0.111±0.003 | 0.113±0.002 | 0.115±0.002 | 0.113±0.002 | 0.114±0.002 | 0.75 | 0.69 | 0.55 |
| Healthy Eating Index-2010 | 42.6±0.4 | 39.7±0.6 | 41.8±0.4 | 45.0±0.5 | 40.9±0.5 | 43.4±0.4 | 0.002 | <0.001 | <0.001 |
Abbreviations: AA, arachidonic acid; ALA, α-linolenic acid; CES-D, Center for Epidemiologic Studies-Depression Scale; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Life Span; HDL-C, high-density lipoprotein-cholesterol; HS, high school; LA, linoleic acid; LDL-C, low-density lipoprotein-cholesterol; n-3, omega-3; n-6, omega-6; PIR, poverty income ratio; PUFAs, polyunsaturated fatty acids; TC, total cholesterol.
Values are percent or mean±s.e.m.
P-value was based on independent samples t-test when row variable is continuous and χ2-test when row variable is categorical.
n-3 PUFAs included DHA+EPA+n-3 DPA+ALA. n-6 PUFA included AA+LA.
Sex differences were also detected for marital status (28.5% in women, 34.1% in men, P=0.017), PIR ⩾125% (60.3% in men, 54.1% in women, P<0.001), employed (52.8% in men, 44.2% in women, P<0.001), current smoking status (49.9% in men vs 40.8% in women) and current use of any illicit drug (57.3% in men vs 34.5% in women).
Of the three serum lipid exposures of interest, women had a higher mean TC (189.9 vs 183.8, P=0.002) and higher proportion >200 mg dl−1 (37.9 vs 31.9, P<0.001) compared with men. However, both atherogenic indices were significantly higher among men (TC:HDL-C → 4.11 vs 3.66, P<0.001; LDL-C:HDL-C → 2.45 vs 2.17, P<0.001; Table 1). Moreover, ~14.7% of the selected sample were statin users. Statin use did not differ by sex or EDS status. Importantly, statin use was not associated with baseline CES-D score or its rate of change over time. This also pertained to the subscales of CES-D. Thus, statin use was not an important confounder in the relationship between serum lipids in longitudinal change in depressive symptoms. Its inclusion in the models did not alter the key findings. (data not shown)
Overall dietary quality measured by the HEI-2010 was significantly lower among men compared with women and significantly lower among both men and women with EDS+ status compared with EDS− (Table 1). EDS+ status was also associated with lower intakes of energy-adjusted total carotenoids (men), vitamin C (women), vitamin E (both), vitamin B-6 and folate (women). Women had higher energy-adjusted intakes of total carotenoids and vitamin E compared with men, though the reverse pattern was true for total energy intake.
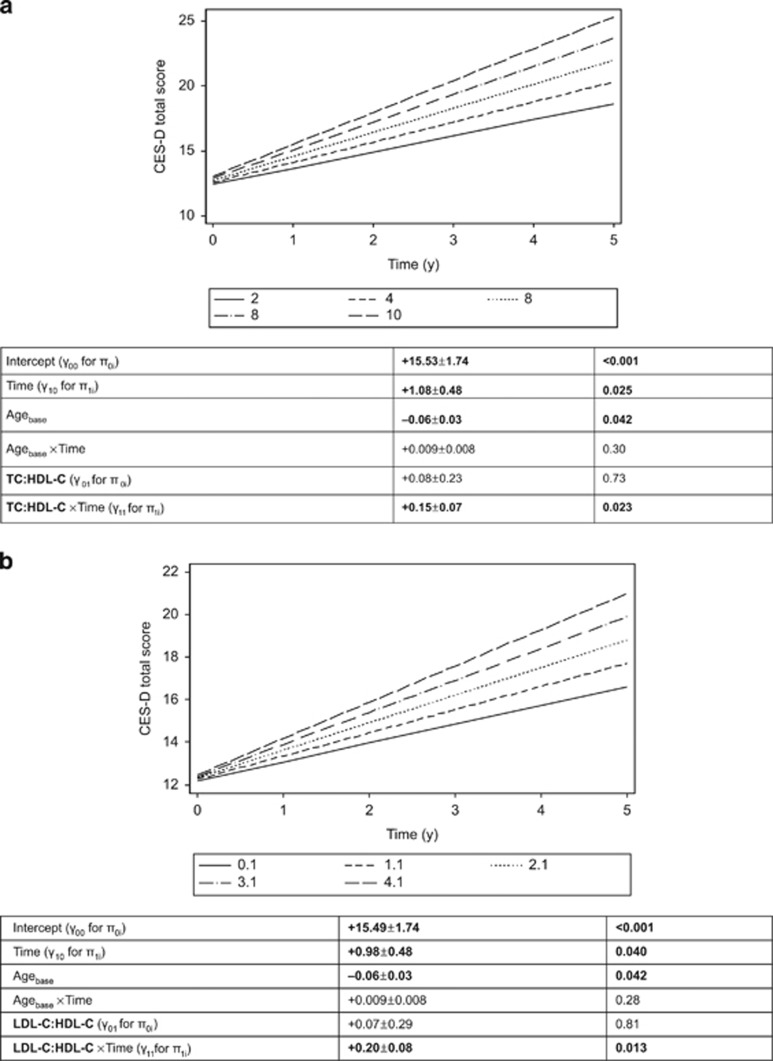
Using a mixed-effects regression model without adjustment for covariates or inclusion of exposure variables, the annual rate of change over time in the CES-D score was estimated at 0.88 with 95% CI (0.78; 0.99), P<0.001. Table 2 displays key findings from mixed-effects regression models with CES-D score as the main outcome and the three lipid exposures, stratified by sex and adjusting for multiple covariates. The fixed effects of baseline age indicate that younger participants at baseline had a higher CES-D score than older participants (γage>0, P<0.05). Among men, the CES-D score increased over time (γ10>0, P<0.050). Importantly, among women, higher baseline atherogenic indices (both TC:HDL-C and LDL-C:HDL-C) were associated with a faster rate of increase in CES-D scores over time, after correction for multiple testing (γ11>0, P<0.030). The total CES-D scores' trajectories over time by level of atherogenic indices among women are illustrated in Figures 1a and b. In contrast, the total CES-D score's trajectory over time was not significantly altered by baseline TC level. These findings remained unaltered when excluding statin users, though slightly attenuated in the case of LDL-C:HDL-C (P<0.05, data not shown).
Table 2. Analysis of baseline TC (low vs medium; high vs medium) and atherogenic indices (TC:HDL-C; LDL-C:HDL-C) exposures and longitudinal change in CES-D score (sex-stratified), mixed-effects linear regression analysis, HANDLS study, 2004–2013.
|
Men: Model 1a |
Women: Model 2a |
|||
|---|---|---|---|---|
| γ±s.e.e. (N=826) | P-value (N′=1319) | γ±s.e.e. (N=1099) | P-value (N′=1817) | |
| Total cholesterol (1=TClow:<160 mg dl−1; 2=TChigh:>200 mg dl−1 vs 0=TCmedium: 160–200 mg dl−1) | ||||
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +15.92±1.78 | <0.001 | +15.85±1.76 | <0.001 |
| Time (γ10 for π1i) | +1.18±0.50 | 0.018 | +0.83±0.49 | 0.09 |
| Agebase | −0.07±0.03 | 0.019 | −0.06±0.03 | 0.06 |
| Agebase × time | +0.011±0.009 | 0.20 | +0.006±0.009 | 0.50 |
| TClow (γ011 for π0i) | −0.87±0.67 | 0.19 | −0.38±0.76 | 0.62 |
| TClow × time (γ111 for π1i) | −0.81±0.58 | 0.17 | −0.25±0.22 | 0.25 |
| TChigh (γ012 for π0i) | −0.03±0.19 | 0.86 | −0.61±0.59 | 0.30 |
| TChigh × time (γ112 for π1i) | +0.14±0.16 | 0.40 | +0.23±0.17 | 0.17 |
| Random effects | ||||
| Level 1 residuals (Rij) | 2.52±0.49 | <0.001 | 4.69±0.32 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 6.46±0.24 | <0.004 | 6.77±0.25 | <0.001 |
| Linear slope (ξ1i) | 1.46±0.09 | <0.001 | 1.50±0.09 | <0.001 |
| LDL-C:HDL-C, continuous | ||||
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +15.46±1.78 | <0.001 | +15.49±1.74 | <0.001 |
| Time (γ10 for π1i) | +1.17±0.50 | 0.018 | +0.98±0.48 | 0.040 |
| Agebase | −0.07±0.03 | 0.014 | −0.06±0.03 | 0.042 |
| Agebase × time | +0.012±0.009 | 0.17 | +0.009±0.008 | 0.28 |
| LDL-C:HDL-C (γ01 for π0i) | −0.29±0.22 | 0.18 | +0.07±0.29 | 0.81 |
| LDL-C:HDL-C × time (γ11 for π1i) | −0.02±0.07 | 0.73 | +0.20±0.08 | 0.013 |
| TC:HDL-C, continuous | ||||
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +15.48±1.79 | <0.001 | +15.53±1.74 | <0.001 |
| Time (γ10 for π1i) | +1.21±0.50 | 0.016 | +1.08±0.48 | 0.025 |
| Agebase | −0.07±0.03 | 0.015 | −0.06±0.03 | 0.042 |
| Agebase × time | +0.012±0.009 | 0.17 | +0.009±0.008 | 0.30 |
| TC:HDL-C (γ01 for π0i) | −0.10±0.17 | 0.57 | +0.08±0.23 | 0.73 |
| TC:HDL-C × time (γ11 for π1i) | +0.01±0.05 | 0.82 | +0.15±0.07 | 0.023 |
Abbreviations: CES-D, Center for Epidemiologic Studies-Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Lifespan; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; n-3, omega-3; n-6, omega-6; PUFAs, polyunsaturated fatty acids; TC, total cholesterol.
Models were further adjusted for other covariates (main effects and interaction with time). See Materials and Methods section for more details on covariate coding and model specifications. Time at baseline visit was set to zero. Baseline age was centered at 50 years, total energy intake at 2000 kcal per day, total carotenoid intake at 3 mg per 1000 kcal per d, vitamin C intake at 30 mg per 1000 kcal per day, vitamin A intake at 300 RE per 1000 kcal per day, vitamin E at 3 mg per 1000 kcal per day, vitamin B-6 at 0.8 mg per 1000 kcal per day, vitamin B-12 at 3 μg per 1000 kcal per day, folate at 170 μg per 1000 kcal per day, n-3 PUFA:n-6 PUFA at 0.11. Healthy Eating Index-2010 was centered at 42.
N=number of participants in the analysis; N'=total number of visits included in the analysis. Findings that were significant at a type I error of 0.05 are in bold. γ±s.e.e. refer to the estimated regression coefficients from the mixed-effects regression models with their associated standard error. Random effects are presented only for TC models, but were comparable in the remaining models.
Figure 1.
(a) Predictive margins of CES-D total score, mixed-effects regression model with TC:HDL-C atherogenic index, controlling for selected covariates, among women. (b) Predictive margins of CES-D total score, mixed-effects regression model with LDL-C:HDL-C atherogenic index, controlling for selected covariates, among women. CES-D, Center for Epidemiologic Studies-Depression; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
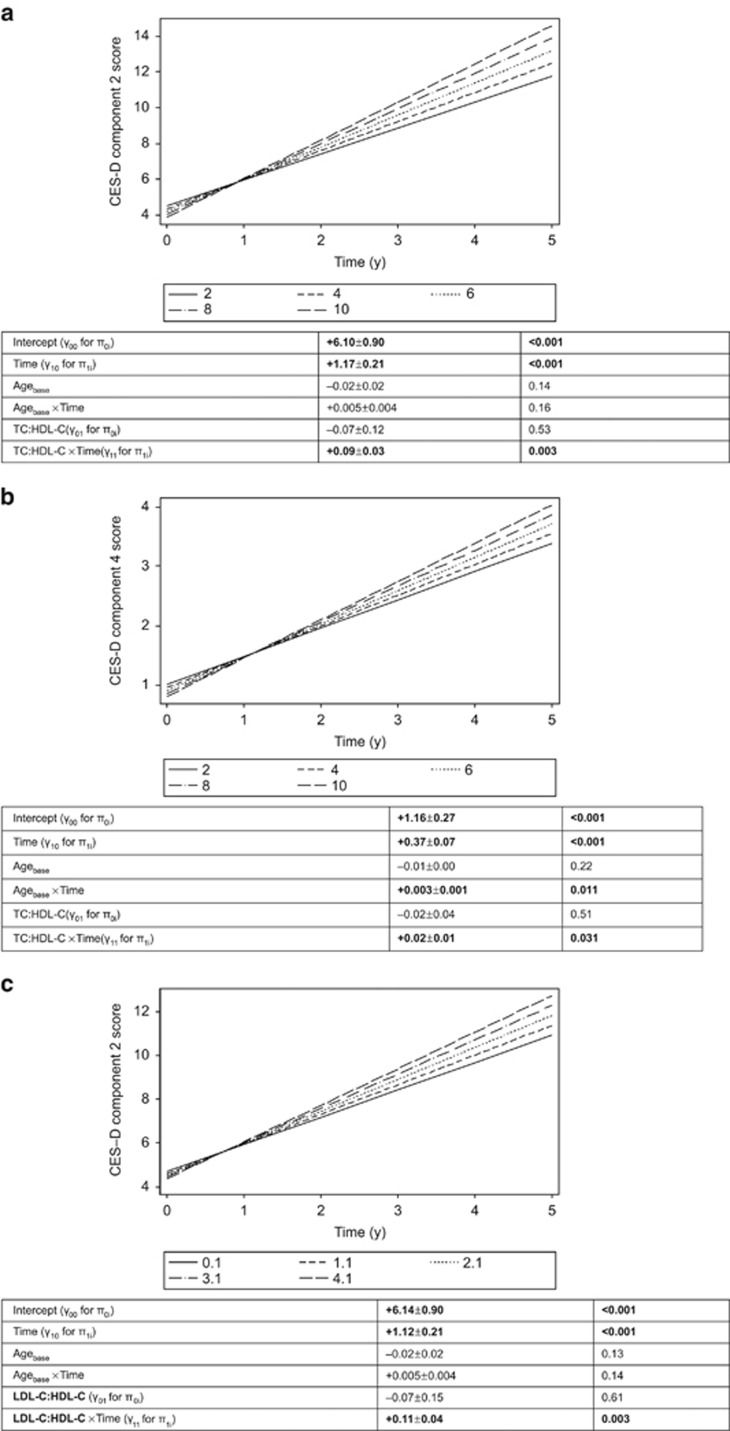
Findings from domain-specific analyses among women (Table 3) indicated that atherogenic indices were associated with a faster rate of increase in depressed affect (component 2: both indices) and interpersonal problems (component 4: TC:HDL-C; P<0.033), findings illustrated in Figures 2a–c. No significant associations between baseline atherogenic indices and CES-D components were found among men (Table 4). Sex differences were significant for both atherogenic indices for depressed affect using a three-way interaction to test sex differences in the trajectories of this domain over time by levels of exposure (P=0.023 for TC:HDL-C exposure and P=0.028 for LDL-C:HDL-C exposure). Moreover, after excluding statin users among women, TClow was linked to slower increase in depressed affect over time (γ11<0; P<0.033), whereas TChigh was associated with faster increase in interpersonal problems (γ11>0; P<0.033).
Table 3. Analysis of baseline atherogenic indices and longitudinal change in CES-D component scores among women, mixed-effects linear regression analysis, HANDLS study, 2004–2013a.
|
X=LDL-C:HDL-C |
X=TC:HDL-C |
|||
|---|---|---|---|---|
| γ±s.e.e. | P-value | γ±s.e.e. | P-value | |
| Y=CES-D component 1: somatic complaints | N=1099 | N′=1816 | N=1099 | N′'=1816 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +8.81±0.91 | <0.001 | +8.83±0.91 | <0.001 |
| Time (γ10 for π1i) | +1.35±0.22 | <0.001 | +1.37±0.22 | <0.001 |
| Agebase | −0.02±0.02 | 0.31 | −0.02±0.02 | 0.31 |
| Agebase × time | +0.004±0.004 | 0.21 | +0.004±0.004 | 0.21 |
| Atherogenic index (γ01 for π0i) | +0.03±0.15 | 0.83 | +0.02±0.12 | 0.86 |
| Atherogenic index (γ11 for π1i) | +0.04±0.04 | 0.23 | +0.03±0.03 | 0.30 |
| Random effects | ||||
| Level 1 residuals (Rij) | 3.25±0.09 | <0.001 | 3.25±0.09 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 2.88±0.13 | <0.001 | 2.88±0.13 | <0.001 |
| Linear slope (ξ1i) | 0.00±0.00 | <0.05 | 0.00±0.00 | <0.001 |
| Y=CES-D component 2: depressed affect | N=1098 | N'′=1815 | N=1099 | N′′=1815 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +6.14±0.90 | <0.001 | +6.10±0.90 | <0.001 |
| Time (γ10 for π1i) | +1.12±0.21 | <0.001 | +1.17±0.21 | <0.001 |
| Agebase | −0.02±0.02 | 0.13 | −0.02±0.02 | 0.14 |
| Agebase × time | +0.005±0.004 | 0.14 | +0.005±0.004 | 0.16 |
| Atherogenic index (γ01 for π0i) | −0.07±0.15 | 0.61 | −0.07±0.12 | 0.53 |
| Atherogenic index (γ11 for π1i) | +0.11±0.04 | 0.003b | +0.09±0.03 | 0.003b |
| Random effects | ||||
| Level 1 residuals (Rij) | 2.97±0.12 | <0.001 | 2.95±0.12 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 3.11±0.12 | <0.001 | 3.11±0.12 | <0.001 |
| Linear slope (ξ1i) | 0.25±0.09 | <0.001 | 0.25±0.09 | <0.001 |
| Y=CES-D component 3: positive affect | N=1099 | N′=1816 | N=1099 | N′=1816 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +8.52±0.55 | <0.001 | +8.48±0.55 | <0.001 |
| Time (γ10 for π1i) | +0.71±0.13 | <0.001 | +0.69±0.13 | <0.001 |
| Agebase | +0.03±0.01 | 0.009 | +0.03±0.01 | 0.008 |
| Agebase × time | −0.000±0.002 | 0.89 | −0.000±0.002 | 0.90 |
| Atherogenic index (γ01 for π0i) | −0.06±0.09 | 0.53 | +0.13±0.38 | 0.74 |
| Atherogenic index × time (γ11 for π1i) | −0.04±0.02 | 0.09 | −0.07±0.07 | 0.32 |
| Random effects | ||||
| Level 1 residuals (Rij) | 1.94±0.05 | <0.001 | 1.94±0.05 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 1.76±0.08 | <0.001 | 1.76±0.08 | <0.001 |
| Linear slope (ξ1i) | 0.00±0.00 | <0.05 | 0.00±0.00 | <0.05 |
| Y=CES-D component 4: interpersonal problems | N=1099 | N′=1817 | N=1099 | N′=1817 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +1.17±0.27 | <0.001 | +1.16±0.27 | <0.001 |
| Time (γ10 for π1i) | +0.36±0.07 | <0.001 | +0.37±0.07 | <0.001 |
| Agebase | −0.01±0.00 | 0.21 | −0.01±0.00 | 0.22 |
| Agebase × time | +0.003±0.001 | 0.010 | +0.003±0.001 | 0.011 |
| Atherogenic index (γ01 for π0i) | −0.02±0.05 | 0.75 | −0.02±0.04 | 0.51 |
| Atherogenic index × time (γ11 for π1i) | +0.02±0.01 | 0.045 | +0.02±0.01 | 0.031 |
| Random effects | ||||
| Level 1 residuals (Rij) | 0.97±0.04 | <0.001 | 0.97±0.04 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 0.87±0.04 | <0.001 | 0.87±0.04 | <0.001 |
| Linear slope (ξ1i) | 0.12±0.02 | <0.001 | 0.12±0.01 | <0.001 |
Abbreviations: CES-D, Center for Epidemiologic Studies-Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Lifespan; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; n-3, omega-3; n-6, omega-6; PUFAs, polyunsaturated fatty acids; TC, total cholesterol.
Models were further adjusted for other covariates (main effects and interaction with time). aSee Materials and Methods section for more details on covariate coding and model specifications. Time at baseline visit was set to zero. Baseline age was centered at 50 years, total energy intake at 2000 kcal per day, total carotenoid intake at 3 mg per 1000 kcal per day, vitamin C intake at 30 mg per 1000 kcal per day, vitamin A intake at 300 RE per 1000 kcal per day, vitamin E at 3 mg per 1000 kcal per day, vitamin B-6 at 0.8 mg per 1000 kcal per day, vitamin B-12 at 3 μg per 1000 kcal per day, folate at 170 μg per 1000 kcal per day, n-3 PUFA:n-6 PUFA at 0.11. Healthy Eating Index-2010 was centered at 42. N=number of participants in the analysis; N'=total number of visits included in the analysis. Findings that were significant at a type I error of 0.05 are in bold. γ±s.e.e. refer to the estimated regression coefficients from the mixed-effects regression models with their associated standard error. bIn a separate model with interaction of lipid exposures by TIME by sex, including all other terms in the current model, P<0.10 for null hypothesis that this interaction term is=0.
Figure 2.
(a) Predictive margins of CES-D component 2 score (depressed affect), mixed-effects regression model with TC:HDL-C atherogenic index, controlling for selected covariates, among women. (b) Predictive margins of CES-D component 4 (interpersonal problems), mixed-effects regression model with TC:HDL-C atherogenic index, controlling for selected covariates, among women. (c) Predictive margins of CES-D component 2 score (depressed affect), mixed-effects regression model with LDL-C:HDL-C atherogenic index, controlling for selected covariates, among women. CES-D, Center for Epidemiologic Studies-Depression; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
Table 4. Analysis of baseline atherogenic indices and longitudinal change in CES-D component scores among men, mixed-effects linear regression analysis, HANDLS study, 2004–2013.
|
X=LDL-C:HDL-C |
X=TC:HDL-C |
|||
|---|---|---|---|---|
| γ±s.e.e. | P-value | γ±s.e.e. | P-value | |
| Y=CES-D component 1: somatic complaints | N=826 | N′=1319 | N=826 | N′=1319 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +8.20±1.00 | <0.001 | +8.20±1.00 | <0.001 |
| Time (γ10 for π1i) | +1.40±0.23 | <0.001 | +1.41±0.24 | <0.001 |
| Agebase | −0.03±0.02 | 0.037 | −0.03±0.02 | 0.040 |
| Agebase × time | +0.004±0.004 | 0.34 | +0.004±0.004 | 0.34 |
| Atherogenic index (γ01 for π0i) | −0.19±0.12 | 0.11 | −0.07±0.10 | 0.47 |
| Atherogenic index (γ11 for π1i) | −0.03±0.03 | 0.36 | −0.01±0.02 | 0.71 |
| Random effects | ||||
| Level 1 residuals (Rij) | 2.57±0.14 | <0.001 | 2.57±0.14 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 2.84±0.13 | <0.001 | 2.85±0.13 | <0.001 |
| Linear slope (ξ1i) | 0.22±0.10 | <0.05 | 0.23±0.10 | <0.001 |
| Y=CES-D component 2: depressed affect | N=824 | N′=1317 | N=824 | N′=1317 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | 6.98±0.99 | <0.001 | +7.00±0.99 | <0.001 |
| Time (γ10 for π1i) | 0.93±0.23 | <0.001 | +0.92±0.24 | <0.001 |
| Agebase | −0.02±0.02 | 0.35 | −0.02±0.02 | 0.37 |
| Agebase × time | +0.003±0.004 | 0.48 | +0.003±0.004 | 0.48 |
| Atherogenic index (γ01 for π0i) | −0.21±0.12 | 0.09 | −0.07±0.10 | 0.44 |
| Atherogenic index (γ11 for π1i) | −0.01±0.03 | 0.67 | −0.01±0.02 | 0.63 |
| Random effects | ||||
| Level 1 residuals (Rij) | 2.56±0.13 | <0.001 | 2.56±0.13 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 2.83±0.13 | <0.001 | 2.84±0.13 | <0.001 |
| Linear slope (ξ1i) | 0.23±0.10 | <0.001 | 0.23±0.10 | <0.001 |
| Y=CES-D component 3: positive affect | N=825 | N′=1317 | N=825 | N′=1317 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +8.24±0.61 | <0.001 | +8.26±0.61 | <0.001 |
| Time (γ10 for π1i) | +0.58±0.16 | <0.001 | +0.57±0.16 | <0.001 |
| Agebase | +0.02±0.01 | 0.07 | +0.02±0.01 | 0.07 |
| Agebase × time | −0.004±0.003 | 0.09 | −0.005±0.003 | 0.09 |
| Atherogenic index (γ01 for π0i) | +0.05±0.07 | 0.53 | +0.03±0.06 | 0.55 |
| Atherogenic index × time (γ11 for π1i) | −0.02±0.02 | 0.26 | −0.02±0.02 | 0.17 |
| Random effects | ||||
| Level 1 residuals (Rij) | 1.64±0.08 | <0.001 | 1.64±0.09 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 1.66±0.08 | <0.001 | 1.66±0.08 | <0.001 |
| Linear slope (ξ1i) | 0.20±0.05 | <0.001 | 0.20±0.05 | <0.001 |
| Y=CES-D component 4: interpersonal problems | N=825 | N′=1319 | N=825 | N=1319 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +1.89±0.04 | <0.001 | +1.87±0.35 | <0.001 |
| Time (γ10 for π1i) | +0.39±0.09 | <0.001 | +0.39±0.09 | <0.001 |
| Agebase | −0.01±0.01 | 0.037 | −0.01±0.01 | 0.038 |
| Agebase × time | +0.002±0.002 | 0.17 | +0.002±0.002 | 0.17 |
| Atherogenic index (γ01 for π0i) | −0.06±0.04 | 0.14 | −0.04±0.03 | 0.27 |
| Atherogenic index × time (γ11 for π1i) | +0.01±0.01 | 0.58 | +0.00±0.01 | 0.70 |
| Random effects | ||||
| Level 1 residuals (Rij) | 1.05±0.05 | <0.001 | 1.06±0.05 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 0.84±0.05 | <0.001 | 0.84±0.05 | <0.001 |
| Linear slope (ξ1i) | 0.06±0.05 | <0.001 | 0.06±0.05 | <0.05 |
Abbreviations: CES-D, Center for Epidemiologic Studies-Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Lifespan; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; n-3, omega-3; n-6, omega-6; PUFAs, polyunsaturated fatty acids; TC, total cholesterol.
Models were further adjusted for other covariates (main effects and interaction with time). See Materials and Methods section for more details on covariate coding and model specifications. Time at baseline visit was set to zero. Baseline age was centered at 50 years, total energy intake at 2000 kcal per day, total carotenoid intake at 3 mg per 1000 kcal per day, vitamin C intake at 30 mg per 1000 kcal per day, vitamin A intake at 300 RE per 1000 kcal per day, vitamin E at 3 mg per 1000 kcal per day, vitamin B-6 at 0.8 mg per 1000 kcal per day, vitamin B-12 at 3 μg per 1000 kcal per day, folate at 170 μg per 1000 kcal per day, n-3 PUFA:n-6 PUFA at 0.11. Healthy Eating Index-2010 was centered at 42. N=number of participants in the analysis; N'=total number of visits included in the analysis. Findings that were significant at a type I error of 0.05 are in bold. γ±s.e.e. refer to the estimated regression coefficients from the mixed-effects regression models with their associated standard error.
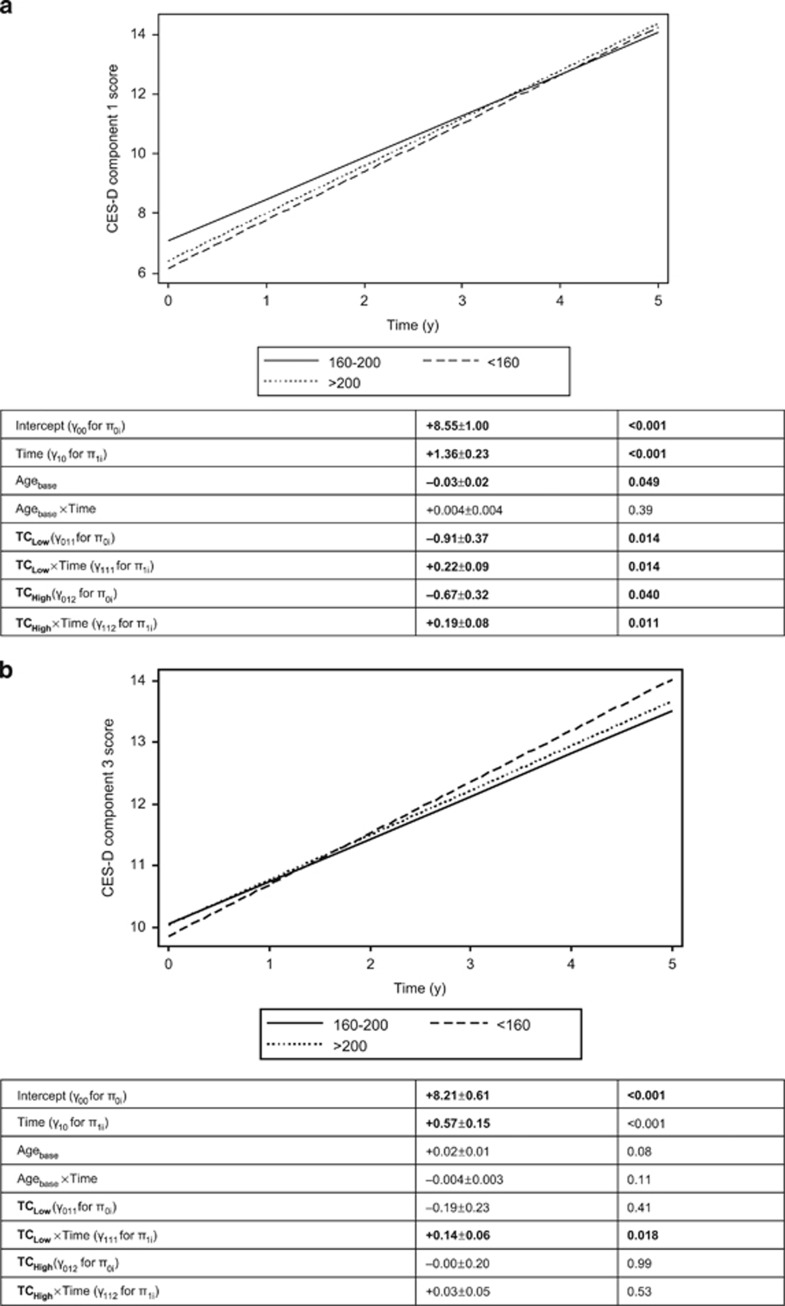
Among men, a U-shaped pattern was found between TC and somatic complaints whereby TClow and TChigh (vs TCmedium) were both associated with a faster rate of increase over time even though TClow was linked to significantly lower score compared with TCmedium (Figure 3a; γ011>0 (P<0.033), γ111>0 (P<0.05) and γ112>0, P<0.033). After excluding statin users, the results remained similar. In contrast, and unlike its potential adverse effect on somatic complaints, TClow at baseline was associated with a higher rate of increase over time on positive affect (component 3), indicating a better outcome when TC level is <160 mg dl−1 when compared with TCmedium (160–200 mg dl−1; Figure 3b).
Figure 3.
(a) Predictive margins of CES-D domain 1 score (somatic complaints), mixed-effects regression model with TC, categorical, controlling for selected covariates, among men. (b) Predictive margins of CES-D domain 3 score (positive affect), mixed-effects regression model with TC, categorical, controlling for selected covariates, among men. CES-D, Center for Epidemiologic Studies-Depression; TC, total cholesterol.
None of the longitudinal associations were statistically significant when baseline CES-D total score was modeled as a predictor of change in lipid profile over time (Table 5). This finding indicates that the direction of the relationship in this sample is serum lipids → depressive symptoms rather than depressive symptoms → serum lipids.
Table 5. Analysis of baseline CES-D score (X) and longitudinal change in total cholesterol (TC) and atherogenic indices (Y): sex-stratified mixed-effects linear regression analysis, HANDLS study, 2004–2013.
|
Men: Model 1a |
Women: Model 2a |
|||
|---|---|---|---|---|
| γ±s.e.e. | P-value | γ±s.e.e. | P-value | |
| Y=TC | N=738 | N′=1230 | N=964 | N′=1678 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +177.5±12.0 | <0.001 | +209.1±8.5 | <0.001 |
| Time (γ10 for π1i) | +3.67±2.73 | 0.18 | −1.53±1.82 | 0.40 |
| Agebase | +0.11±0.20 | 0.58 | +0.75±0.14 | <0.001 |
| Agebase × time | −0.01±0.05 | 0.88 | −0.014±0.031 | 0.65 |
| CES-D (γ01 for π0i) | −0.17±0.24 | 0.47 | −0.04±0.16 | 0.78 |
| CES-D × time (γ11 for π1i) | +0.05±0.06 | 0.35 | +0.02±0.03 | 0.49 |
| Random effects | ||||
| Level 1 residuals (Rij) | 29.24±1.55 | <0.001 | 25.66±0.68 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 33.1±1.5 | <0.001 | 29.00±1.04 | <0.001 |
| Linear slope (ξ1i) | 1.61±1.53 | <0.001 | 0.00±0.00 | <0.001 |
| Y=LDL-C:HDL-C ratio | N=738 | N′=1230 | N=964 | N′=1673 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +1.49±0.30 | <0.001 | +2.01±0.19 | <0.001 |
| Time (γ10 for π1i) | +0.07±0.06 | 0.22 | −0.08±0.04 | 0.035 |
| Agebase | −0.01±0.00 | 0.25 | +0.00±0.00 | 0.29 |
| Agebase × time | −0.001±0.001 | 0.49 | −0.000±0.001 | 0.88 |
| CES-D (γ01 for π0i) | −0.01±0.01 | 0.17 | +0.00±0.00 | 0.31 |
| CES-D × time (γ11 for π1i) | +0.01±0.00 | 0.48 | +0.00±0.00 | 0.48 |
| Random effects | ||||
| Level 1 residuals (Rij) | 0.59±0.02 | <0.001 | 0.52±0.01 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 0.93±0.03 | <0.001 | 0.71±0.02 | <0.001 |
| Linear slope (ξ1i) | 0.00±0.00 | <0.001 | 0.00±0.00 | <0.001 |
| Y=TC:HDL-C ratio | N=738 | N′=1230 | N=964 | N′=1678 |
| Fixed effect | ||||
| Intercept (γ00 for π0i) | +2.48±0.38 | <0.001 | +3.36±0.25 | <0.001 |
| Time (γ10 for π1i) | +0.13±0.08 | 0.09 | −0.13±0.05 | 0.007 |
| Agebase | −0.01±0.01 | 0.26 | +0.01±0.00 | 0.23 |
| Agebase × Time | −0.000±0.001 | 0.84 | −0.001±0.001 | 0.55 |
| CES-D (γ01 for π0i) | −0.00±0.01 | 0.58 | +0.01±0.00 | 0.25 |
| CES-D × time (γ11 for π1i) | +0.00±0.00 | 0.41 | +0.00±0.00 | 0.38 |
| Random effects | ||||
| Level 1 residuals (Rij) | 0.82±0.03 | <0.001 | 0.69±0.02 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | 1.14±0.04 | <0.001 | 0.90±0.03 | <0.001 |
| Linear slope (ξ1i) | 0.00±0.00 | <0.001 | 0.00±0.02 | <0.001 |
Abbreviations: CES-D, Center for Epidemiologic Studies-Depression Scale; HANDLS, Healthy Aging in Neighborhoods of Diversity Across the Lifespan; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; n-3, omega-3; n-6, omega-6; PUFAs, polyunsaturated fatty acids; TC, total cholesterol.
Models were further adjusted for other covariates (main effects and interaction with time). See Materials and Methods section for more details on covariate coding and model specifications. Time at baseline visit was set to zero. Baseline age was centered at 50 years, total energy intake at 2000 kcal per day, total carotenoid intake at 3 mg per 1000 kcal per day, vitamin C intake at 30 mg per 1000 kcal per day, vitamin A intake at 300 RE per 1000 kcal per day, vitamin E at 3 mg per 1000 kcal per day, vitamin B-6 at 0.8 mg per 1000 kcal per day, vitamin B-12 at 3 μg per 1000 kcal per day, folate at 170 μg per 1000 kcal per day, n-3 PUFA:n-6 PUFA at 0.11. Healthy Eating Index-2010 was centered at 42.
N=number of participants in the analysis; N'=total number of visits included in the analysis. Findings that were significant at a type I error of 0.05 are in bold. γ±s.e.e. refer to the estimated regression coefficients from the mixed-effects regression models with their associated standard error.
Discussion
The present study found that among adult women residing in Baltimore, MD, USA, higher levels of atherogenic indices were linked to faster increases in depressive symptom scores, particularly depressed affect and interpersonal problems. Three of 11 case–control studies4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 corroborated our findings of a positive relationship between atherogenic indices and depression.9, 11, 12 Unlike the current study, many case–control studies tested only TC as the exposure4, 7 and commonly found an inverse relationship between TC and mood disorders, particularly major depressive disorder.4, 6 Furthermore, most did not utilize atherogenic indices but examined the metabolic syndrome and components14 or lipoprotein fractions as they relate to mood disorders.5, 6, 13, 15, 24 With only few exceptions,13, 14 most case–control studies had limited sample size (<200).
The results from cross-sectional studies that focused on the relationship between serum lipid profiles and depression are highly mixed. Some results suggested an atherogenic profile characterized by low HDL-C and/or higher LDL-C to be related to higher depressive symptoms.20, 22, 25, 33, 35, 36 Others found that depression was related to both low LDL-C and/or HDL-C,18, 19, 32 and a third group found no association between depression and TC, HDL-C or LDL-C.23, 27, 28, 30
Among men, there was a U-shaped relationship between baseline TC and longitudinal increase in somatic complaints and a direct link between low TC and longitudinal putative improvement in positive affect compared with medium TC. A recent study using national data compared lipid profiles of severely depressed with mild or moderately depressed participants. Among men, there was also a U-shaped relationship between LDL-C and the odds of severe depression remained significant after adjustment for potential confounders.34
Baseline depressive symptoms were not longitudinally related to changes in TC or atherogenic indices in our study. These associations were independent of basic socio-demographic variables, as well as selected lifestyle and behavioral factors.
Among 12 prospective cohort studies37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 that examined similar research questions, findings were mixed. In fact, in one of the largest cohort studies to date (N=29 133),38 lower baseline TC was associated with low mood and increased risk of hospitalization for depression and death from suicide after 5–8 years of follow-up. This finding was corroborated by at least three other studies. In the first study conducted among 504 Japanese older adults, higher baseline TC was linked to slower increase in the Geriatric Depression Scale score over 4 years, among men only.39 In a study conducted among 421 older Finnish adult men (70–89 years), TC was inversely related to risk of depression measured by the Zung Self-Rating Depression scale (score >48).40 A TC decline over 5 years was linked to incident depression on the basis of the third study also conducted among Finnish men (70–89 years).44 No other cohort studies examined atherogenic indices in relation to mood changes or incident depression. Some studies with TC as an exposure did not find associations with depression,37, 41 whereas one found that higher TC was linked to greater risk of suicide attempts among depressed in-patients.43 However, several population-based cohort studies examined lipoprotein fractions in relation to well-being and psychosocial outcomes. Some confirmed the vascular depression hypothesis whereby lower HDL-C was associated with worse outcome,42, 45, 46 whereas others yielded null or mixed findings.37, 39 At least two studies examined the relationship between lipid profiles and depression in the opposite temporal direction. The first study conducted among 1186 male workers (19–68 years) who were followed for 5 years showed that higher baseline depressive symptoms, particularly psychomotor agitation, were associated with increased risk of incident metabolic syndrome and hypertriglyceridemia.47 The second study conducted in the Netherlands among 2126 adults followed for 2 years found that higher baseline depressive and anxiety symptoms predicted a decrease in HDL-C and a concomitant increase in waist circumference, thus predisposing to cardiovascular disease. In contrast, reduction in depressive symptoms did not correlate with a lipid profile improvement.48
A number of intervention studies examined the effects of cholesterol-lowering diets and medications, mainly statins, on affective disorders. In an earlier population-based intervention on ~300 middle-aged adults, depression and aggressive hostility were reduced significantly by consuming cholesterol-lowering diets, compared with controls.50 A meta-analysis of seven randomized controlled trials of statin use that included 2105 adults showed that statin use was linked to improvement in mood scores, thus refuting the evidence of negative effects of statins on psychological well-being.51 When examining the association in the reverse direction, an open-label prospective trial of 65 depressed in-patients was conducted whereby remission vs non-remission over 4–5 years was the primary exposure and lipid profile was the outcome. One key finding was that remission from depression was associated with a reduction in LDL-C/HDL-C ratio and thus a change in lipid profile towards a lesser atherogenic composition.49 Moreover, in our study, some findings emerged after exclusion of statin users among women, while others were attenuated (for example, LDL-C:HDL-C vs CES-D total score trajectory among women).
Despite the inclusion of both men and women in most reviewed studies, only some conducted sex-stratified analyses.4, 19, 22, 24, 33, 34, 39, 46 Notably, in one large cross-sectional study, a higher depression prevalence was observed among women with HDL-C ⩽35 mg dl−1.22 In contrast, another study found that LDL-C was inversely related to depressive symptoms among women but not men, after controlling for adiposity and other cardiovascular and socio-demographic factors.33 In men only, a cohort study found that a higher TC (but not HDL-C) was associated with slower increase in depressive symptoms among Japanese older adults.39 In another large cohort study that stratified analyses by sex, and similar to our key finding, low HDL-C was associated to higher EDS prevalence (based on CES-D score ⩾16) among women only. Both incident and prevalent EDS were linked to lower baseline LDL-C among men, particularly those who were at increased risk of depression by carrying the s/l or s/s genotype of the 5-serotonin transporter gene.46
The CNS, accounting only for 2% of body mass, contains 25% of body cholesterol. Cholesterol, being an integral part of cell membranes and a major myelin component, has a vital role in the development, function and stability of synapses. Low serum cholesterol may directly influence brain lipids and cell membrane fluidity, reducing serotonergic neurotransmission leading to mood and behavioral disorders. Cholesterol level may also be a ‘bystander' for omega-3 fatty acid levels rather than underlying suicide or self-harm. Sudden fall in cholesterol after delivery currently serves as a ‘natural model' for the cholesterol–postpartum mood relationship. Adverse psychological changes associated with cholesterol-lowering dietary and drug treatments might be due to effects on intakes and tissue concentrations of omega-3 fatty acids, and not due to a reduction of cholesterol levels. Docosahexaenoic acid, an omega-3 fatty acid obtained from fish consumption can affect synaptic function and cognitive abilities by providing plasma membrane fluidity at synaptic regions.84 Nevertheless, our study controlled for n-3 PUFA exposure, relative to n-6 PUFA without altering the key findings.
Our study has several notable strengths. First, the prospective cohort study design allows one to ascertain temporality of associations between serum lipids and depression. In fact, our analyses found significant associations in one temporal direction (that is, lipids→depression) but not the other (depression→lipids). Second, the sample size was large enough to allow for sex stratification without major loss in statistical power. Third, mixed-effects regression models were used adjusting for a large number of potentially confounding covariates. Despite those strengths, our study is also limited by potential selection bias due to non-participation or missing data on key covariates compared with the initially sampled group of individuals. However, the use of two-stage Heckman selection models minimized this bias. Furthermore, even though the key exposures were directly measured, the outcome and many of the covariates were self-reported, with potential for measurement error leading to residual confounding.
In summary, atherogenic indices were linked to a faster rate of increase in depressive symptoms among women only. Future intervention studies should examine changes in atherogenic indices with lipid-lowering treatment and how such changes may affect mood, particularly among women. Future longitudinal studies with at least three waves of data should also address the potential mediating effects of cardiovascular disease and type 2 diabetes in the associations between serum lipids and depressive symptoms. Finally, more studies are needed to uncover gene–atherogenic index interactions and their effect on depressive symptoms and mood disorders.
Acknowledgments
This work was fully supported by the Intramural Reasearch Program of the National Institutes of Health, National Insitute on Aging, NIA/NIH/IRP.
Appendix I
The mixed-effects regression models can be summarized as follows:
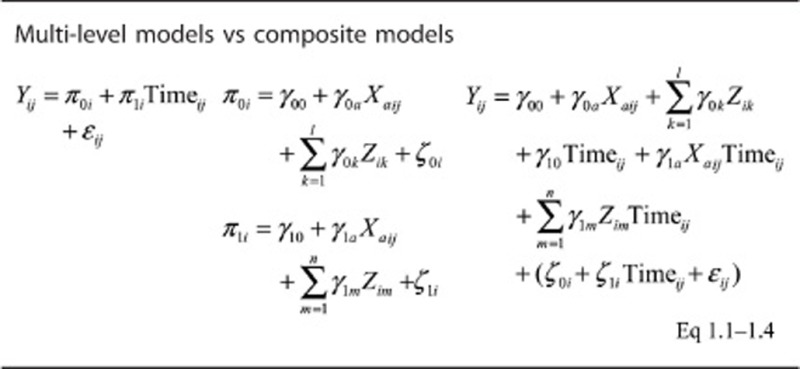
Where Yij is the outcome (CES-D total or domain-specific score) for each individual ‘i' and visit ‘j' π0i is the level-1 intercept for individual i; π1i is the level-1 slope for individual i; γ00 is the level-2 intercept of the random intercept π0i; γ10 is the level-2 intercept of the slope π1i; Zik is a vector of fixed covariates for each individual i that are used to predict level-1 intercepts and slopes and included baseline age (Agebase) among other covariates. Xija, represents the main predictor variables (1 absolute and 2 relative serum lipid exposures); ζ0i and ζ1i are level-2 disturbances; ɛij is the within-person level-1 disturbance. Of primary interest are the main effects of each exposure Xa (γ0a) and their interaction with time (γ1a), as described in a previous methodolgical paper.78
The authors declare no conflict of interest.
References
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, et al. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Olusi SO, Fido AA. Serum lipid concentrations in patients with major depressive disorder. Biol Psychiatry. 1996;40:1128–1131. doi: 10.1016/S0006-3223(95)00599-4. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Christophe A, Vandoolaeghe E, Van Gastel A, Neels H, et al. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta Psychiatr Scand. 1997;95:212–221. doi: 10.1111/j.1600-0447.1997.tb09622.x. [DOI] [PubMed] [Google Scholar]
- Rabe-Jablonska J, Poprawska I. Levels of serum total cholesterol and LDL-cholesterol in patients with major depression in acute period and remission. Med Sci Monit. 2000;6:539–547. [PubMed] [Google Scholar]
- Huang TL. Serum cholesterol levels in mood disorders associated with physical violence or suicide attempts in Taiwanese. Chang Gung Med J. 2001;24:563–568. [PubMed] [Google Scholar]
- Huang TL, Wu SC, Chiang YS, Chen JF. Correlation between serum lipid, lipoprotein concentrations and anxious state, depressive state or major depressive disorder. Psychiatry Res. 2003;118:147–153. doi: 10.1016/s0165-1781(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Huang TL. Serum lipid profiles in major depression with clinical subtypes, suicide attempts and episodes. J Affect Disord. 2005;86:75–79. doi: 10.1016/j.jad.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Mitsonis C, Liappas I, et al. Characterization of the lipid profile in dementia and depression in the elderly. J Geriatr Psychiatry Neurol. 2007;20:138–144. doi: 10.1177/0891988707301867. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Hintikka J, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, et al. Low HDL cholesterol associates with major depression in a sample with a 7-year history of depressive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1557–1561. doi: 10.1016/j.pnpbp.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Sagud M, Mihaljevic-Peles A, Pivac N, Jakovljevic M, Muck-Seler D. Lipid levels in female patients with affective disorders. Psychiatry Res. 2009;168:218–221. doi: 10.1016/j.psychres.2008.06.048. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AKB, Giltay EJ, van Veen T, van Pelt J, Zitman FG, Penninx BWJH. Associations between serum lipids and major depressive disorder: results from the Netherlands Study of Depression and Anxiety (NESDA) J Clin Psychiatry. 2010;71:729–736. doi: 10.4088/JCP.08m04865blu. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AKB, Giltay EJ, van Veen T, Zitman FG, Penninx BWJH. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand. 2010;122:30–39. doi: 10.1111/j.1600-0447.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Williams C, Diesch J, Steptoe A, Leeson P, Cowen PJ. Cardiovascular and metabolic risk profile in young people at familial risk of depression. Br J Psychiatry. 2013;203:18–23. doi: 10.1192/bjp.bp.113.126987. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Palinkas LA, Barrett-Connor EL, Wingard DL. Plasma cholesterol and depressive symptoms in older men. Lancet. 1993;341:75–79. doi: 10.1016/0140-6736(93)92556-9. [DOI] [PubMed] [Google Scholar]
- Brown SL, Salive ME, Harris TB, Simonsick EM, Guralnik JM, Kohout FJ. Low cholesterol concentrations and severe depressive symptoms in elderly people. BMJ. 1994;308:1328–1332. doi: 10.1136/bmj.308.6940.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck CJ, Tieger M, Kunkel R, Hamer T, Tracy T, Speirs J. Hypocholesterolemia and affective disorders. Am J Med Sci. 1994;308:218–225. doi: 10.1097/00000441-199430840-00002. [DOI] [PubMed] [Google Scholar]
- Lindberg G, Larsson G, Setterlind S, Rastam L. Serum lipids and mood in working men and women in Sweden. J Epidemiol Commun Health. 1994;48:360–363. doi: 10.1136/jech.48.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsten M, Wamala SP, Vingerhoets A, Orth-Gomer K. Depressive symptoms, social support, and lipid profile in healthy middle-aged women. Psychosom Med. 1997;59:521–528. doi: 10.1097/00006842-199709000-00009. [DOI] [PubMed] [Google Scholar]
- Steegmans PH, Hoes AW, Bak AA, van der Does E, Grobbee DE. Higher prevalence of depressive symptoms in middle-aged men with low serum cholesterol levels. Psychosom Med. 2000;62:205–211. doi: 10.1097/00006842-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lu FH, Wu JS, Chang CJ. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2001;102:153–162. doi: 10.1016/s0165-1781(01)00231-1. [DOI] [PubMed] [Google Scholar]
- Ergün UGO, Uguz S, Bozdemir N, Güzel R, Burgut R, Saatçi E, et al. The relationship between cholesterol levels and depression in the elderly. Int J Geriatr Psychiatry. 2004;19:291–296. doi: 10.1002/gps.1078. [DOI] [PubMed] [Google Scholar]
- Huang TL, Chen JF. Lipid and lipoprotein levels in depressive disorders with melancholic feature or atypical feature and dysthymia. Psychiatry Clin Neurosci. 2004;58:295–299. doi: 10.1111/j.1440-1819.2004.01235.x. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Shin IS, Yoon JS. Vascular disease/risk and late-life depression in a Korean community population. Br J Psychiatry. 2004;185:102–107. doi: 10.1192/bjp.185.2.102. [DOI] [PubMed] [Google Scholar]
- Nakao M, Yano E. Relationship between major depression and high serum cholesterol in Japanese men. Tohoku J Exp Med. 2004;204:273–287. doi: 10.1620/tjem.204.273. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Flicker L, Norman P, Hankey GJ, Vasikaran S, van Bockxmeer FM, et al. Association of cardiovascular risk factors and disease with depression in later life. Am J Geriatr Psychiatry. 2007;15:506–513. doi: 10.1097/01.JGP.0000246869.49892.77. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, McConnell S, Williams LJ, Kotowicz MA, Nicholson GC, et al. Self-reported depression and cardiovascular risk factors in a community sample of women. Psychosomatics. 2007;48:54–59. doi: 10.1176/appi.psy.48.1.54. [DOI] [PubMed] [Google Scholar]
- Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29:1110–1117. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- Ji-Rong Y, Bi-Rong D, Chang-Quan H, Yan-Ling Z. Depression and serum lipids and lipoprotein in Chinese nonagenarians and centenarians. J Am Geriatr Soc. 2009;57:732–733. doi: 10.1111/j.1532-5415.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- Tyrovolas S, Lionis C, Zeimbekis A, Bountziouka V, Micheli M, Katsarou A, et al. Increased body mass and depressive symptomatology are associated with hypercholesterolemia, among elderly individuals; results from the MEDIS study. Lipids Health Dis. 2009;8:10. doi: 10.1186/1476-511X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SM, Ruusunen A, Niskanen L, Tolmunen T, Voutilainen S, Viinamaki H, et al. Elevated depressive symptoms and compositional changes in LDL particles in middle-aged men. Eur J Epidemiol. 2010;25:403–409. doi: 10.1007/s10654-010-9457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MC, Katzel LI, Waldstein SR. Sex-specific associations of depressive symptoms and cardiovascular risk factors in older adults. Aging Ment Health. 2010;14:405–410. doi: 10.1080/13607860903586185. [DOI] [PubMed] [Google Scholar]
- Tedders SH, Fokong KD, McKenzie LE, Wesley C, Yu L, Zhang J. Low cholesterol is associated with depression among US household population. J Affect Disord. 2011;135:115–121. doi: 10.1016/j.jad.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Liang Y, Yan Z, Cai C, Jiang H, Song A, Qiu C. Association between lipid profile and depressive symptoms among chinese older people: mediation by cardiovascular diseases. Int J Behav Med. 2014;21:590–596. doi: 10.1007/s12529-013-9358-2. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AKB, Vreeburg SA, Giltay EJ, Licht CMM, Vogelzangs N, van Veen T, et al. The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology. 2013;38:209–218. doi: 10.1016/j.psyneuen.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Markovitz JH, Smith D, Raczynski JM, Oberman A, Williams OD, Knox S, et al. Lack of relations of hostility, negative affect, and high-risk behavior with low plasma lipid levels in the Coronary Artery Risk Development in Young Adults Study. Arch Int Med. 1997;157:1953–1959. [PubMed] [Google Scholar]
- Partonen T, Haukka J, Virtamo J, Taylor PR, Lonnqvist J. Association of low serum total cholesterol with major depression and suicide. Br J Psychiatry. 1999;175:259–262. doi: 10.1192/bjp.175.3.259. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kumagai S, Watanabe S, Suzuki T. Relationship of serum cholesterols and vitamin E to depressive status in the elderly. J Epidemiol. 1999;9:261–267. doi: 10.2188/jea.9.261. [DOI] [PubMed] [Google Scholar]
- Aijanseppa S, Kivinen P, Helkala EL, Kivela SL, Tuomilehto J, Nissinen A. Serum cholesterol and depressive symptoms in elderly Finnish men. Int J Geriatr Psychiatry. 2002;17:629–634. doi: 10.1002/gps.666. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Burchett BB, Fillenbaum GG. APOE epsilon4 and low cholesterol as risks for depression in a biracial elderly community sample. Am J Geriatr Psychiatry. 2002;10:515–520. [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Vascular risk factors and incident late-life depression in a Korean population. Br J Psychiatry. 2006;189:26–30. doi: 10.1192/bjp.bp.105.015032. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152:11–20. doi: 10.1016/j.psychres.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots S, Tijhuis M, Giampaoli S, Kromhout D, Nissinen A. Lifestyle- and diet-related factors in late-life depression—a 5-year follow-up of elderly European men: the FINE study. Int J Geriatr Psychiatry. 2008;23:478–484. doi: 10.1002/gps.1919. [DOI] [PubMed] [Google Scholar]
- Mast BT, Miles T, Penninx BW, Yaffe K, Rosano C, Satterfield S, et al. Vascular disease and future risk of depressive symptomatology in older adults: findings from the Health, Aging, and Body Composition study. Biol Psychiatry. 2008;64:320–326. doi: 10.1016/j.biopsych.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Ancelin ML, Carriere I, Boulenger JP, Malafosse A, Stewart R, Cristol JP, et al. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study) Biol Psychiatry. 2010;68:125–132. doi: 10.1016/j.biopsych.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kamezaki F, Sonoda S, Nakata S, Okazaki M, Tamura M, Abe H, et al. Elevated depressive symptoms are associated with hypertriglyceridemia in Japanese male workers. Intern Med. 2011;50:2485–2490. doi: 10.2169/internalmedicine.50.5667. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosom Med. 2013;75:83–89. doi: 10.1097/PSY.0b013e318274d30f. [DOI] [PubMed] [Google Scholar]
- Hummel J, Westphal S, Weber-Hamann B, Gilles M, Lederbogen F, Angermeier T, et al. Serum lipoproteins improve after successful pharmacologic antidepressant treatment: a randomized open-label prospective trial. J Clin Psychiatry. 2011;72:885–891. doi: 10.4088/JCP.09m05853blu. [DOI] [PubMed] [Google Scholar]
- Weidner G, Connor SL, Hollis JF, Connor WE. Improvements in hostility and depression in relation to dietary change and cholesterol lowering. The Family Heart Study. Ann Intern Med. 1992;117:820–823. doi: 10.7326/0003-4819-117-10-820. [DOI] [PubMed] [Google Scholar]
- O'Neil A, Sanna L, Redlich C, Sanderson K, Jacka F, Williams LJ, et al. The impact of statins on psychological wellbeing: a systematic review and meta-analysis. BMC Med. 2012;10:154. doi: 10.1186/1741-7015-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Rostant O, Evans MK, Zonderman AB.Associations between n-3 to n-6 dietary fatty acid ratio and longitudinal change in depressive symptoms among US women Am J EpidemiolIn press. [DOI] [PMC free article] [PubMed]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr. 2004;80:1024–1028. doi: 10.1093/ajcn/80.4.1024. [DOI] [PubMed] [Google Scholar]
- Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB. Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res. 2004;126:177–187. doi: 10.1016/j.psychres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, Fujiyoshi A, Miura K, Ohkubo T, Kadota A, Kadowaki S, et al. Lipoprotein particle profiles compared with standard lipids in association with coronary artery calcification in the general Japanese population. Atherosclerosis. 2014;236:237–243. doi: 10.1016/j.atherosclerosis.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Manickam P, Rathod A, Panaich S, Hari P, Veeranna V, Badheka A, et al. Comparative prognostic utility of conventional and novel lipid parameters for cardiovascular disease risk prediction: do novel lipid parameters offer an advantage. J Clin Lipidol. 2011;5:82–90. doi: 10.1016/j.jacl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Nair D, Carrigan TP, Curtin RJ, Popovic ZB, Kuzmiak S, Schoenhagen P, et al. Association of total cholesterol/ high-density lipoprotein cholesterol ratio with proximal coronary atherosclerosis detected by multislice computed tomography. Prev Cardiol. 2009;12:19–26. doi: 10.1111/j.1751-7141.2008.00011.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Morris DW, Trivedi MH, Rush AJ. Folate and unipolar depression. J Altern Complement Med. 2008;14:277–285. doi: 10.1089/acm.2007.0663. [DOI] [PubMed] [Google Scholar]
- Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12:631–638. doi: 10.1176/appi.ajgp.12.6.631. [DOI] [PubMed] [Google Scholar]
- D'Anci KE, Rosenberg IH. Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7:659–664. doi: 10.1097/00075197-200411000-00011. [DOI] [PubMed] [Google Scholar]
- Tolmunen T, Hintikka J, Ruusunen A, Voutilainen S, Tanskanen A, Valkonen VP, et al. Dietary folate and the risk of depression in Finnish middle-aged men. A prospective follow-up study. Psychother Psychosom. 2004;73:334–339. doi: 10.1159/000080385. [DOI] [PubMed] [Google Scholar]
- Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;54:382–390. doi: 10.1111/j.1753-4887.1996.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Kivela SL, Pahkala K, Eronen A. Depression in the aged: relation to folate and vitamins C and B12. Biol Psychiatry. 1989;26:210–213. doi: 10.1016/0006-3223(89)90027-9. [DOI] [PubMed] [Google Scholar]
- Levitt AJ, Joffe RT. Folate, B12, and life course of depressive illness. Biol Psychiatry. 1989;25:867–872. doi: 10.1016/0006-3223(89)90266-7. [DOI] [PubMed] [Google Scholar]
- Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. 2009;63:9–17. doi: 10.18926/AMO/31854. [DOI] [PubMed] [Google Scholar]
- Owen AJ, Batterham MJ, Probst YC, Grenyer BF, Tapsell LC. Low plasma vitamin E levels in major depression: diet or disease. Eur J Clin Nutr. 2005;59:304–306. doi: 10.1038/sj.ejcn.1602072. [DOI] [PubMed] [Google Scholar]
- Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58:241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y, et al. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–147. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62:939–945. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Martin A, Andres-Lacueva C, Di Iorio A, Lamponi M, Mecocci P, et al. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26:987–994. doi: 10.1016/j.neurobiolaging.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Hibbeln JR, Evans MK, Zonderman AB. Omega-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J Nutr. 2013;143:1743–1752. doi: 10.3945/jn.113.179119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willet WC.Nutritional Epidemiology2nd edn, Oxford University Press: New York, NY, USA; 1998 [Google Scholar]
- STATA . Statistics/Data Analysis: Release 13.0. Stata Corporation: College Station, TX, USA; 2013. [Google Scholar]
- Blackwell E, de Leon CF, Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med. 2006;68:870–878. doi: 10.1097/01.psy.0000239144.91689.ca. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Gamaldo AA, Beydoun HA, Tanaka T, Tucker KL, Talegawkar SA, et al. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. J Nutr. 2014;144:890–901. doi: 10.3945/jn.113.189027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline Neurobiol Aging 201233720–731.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin S.Statistical Analysis of Epidemiologic Data3rd edn, Oxford University Press: New York, NY; 2004 [Google Scholar]
- Hochberg Y, Tamhane AC. Multiple Comparison Procedures. Wiley: New York, NY, USA; 1987. [Google Scholar]
- Troisi A. Cholesterol in coronary heart disease and psychiatric disorders: same or opposite effects on morbidity risk. Neurosci Biobehav Rev. 2009;33:125–132. doi: 10.1016/j.neubiorev.2008.09.003. [DOI] [PubMed] [Google Scholar]