Abstract
Background
Several genetic studies have evaluated the association between Toll-like receptor 4 (TLR4) A299G polymorphism and the risk of pneumonia. However, the results were not consistent. We thus did this meta-analysis.
Material/Methods
Relevant studies were systematically searched by using the NCBI, Medline, Web of Science, and Embase databases. Data were extracted independently by 2 investigators. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated.
Results
Eight case-control studies with 658 patients and 1862 controls were included in this meta-analysis. TLR4 A299G polymorphism was significantly associated with pneumonia risk (OR=1.74; 95% CI 1.19–2.53; P=0.004). The result was significant in adults. In addition, TLR4 A299G polymorphism was also associated with community-acquired pneumonia (CAP) risk. Results from cumulative meta-analysis and sensitivity analysis suggested that the results are reliable and robust.
Conclusions
The results of this meta-analysis suggest that susceptibility to pneumonia was associated with TLR4 A299G polymorphism.
MeSH Keywords: Meta-Analysis; Pneumonia; Polymorphism, Genetic; Toll-Like Receptor 4
Background
Pneumonia is an increasing problem among the elderly. In the U.S. the incidence rate of community-acquired pneumonia (CAP) is estimated to be 5.2 to 6.1 cases per 1000 adults, and the mortality rate may reach 2–3% [1]. Several independent risk factors for pneumonia have been identified, such as increasing age, comorbidities, swallowing dysfunction, and nutritional status. Host genetic susceptibility has also been reported to be an important risk factor for pneumonia [2,3].
Toll-like receptors (TLRs) are transmembrane proteins that recognize infection with various pathogens and damaged host cells, which lead to the subsequent inflammation responses [4]. TLR4 is a well-studied TLR. TLR4 can distinguish between lipopolysaccharide and Gram-negative bacteria, and can initiate intracellular signal cascades [5]. Standiford et al. showed that TLR4 played a protective role in lung epithelium during Gram-negative bacterial pneumonia [6]. Tang et al. found that decreased TLR4 expression occurred in deceased patients compared with survivors [7]. Furthermore, Tanaka et al. found that TLR4 agonistic monoclonal antibody UT12 could improve the prognosis of secondary pneumococcal pneumonia [8]. These results suggest that TLR4 might influence the development of pneumonia.
Previous studies have found 2 single-nucleotide polymorphisms (SNPs) of TLR4: TLR4 A299G and TLR4 T399I [9]. Figueroa et al. suggested that TLR4 A299G polymorphism impaired LPS-induced phosphorylation of p38 and TANK-binding kinase 1, activation of NF-κB and IFN regulatory factor 3, and induction of IL-8 and IFN-β mRNA [10]. In addition, Long et al. found that the TLR4 A299G variant, but not the T399I variant, was responsible for impaired responsiveness of TLR4 to LPS and corresponding activation of NF-κB [11]. Recently, studies assessed the association between TLR4 A299G polymorphism and the risk of pneumonia [12–19], but the results were inconclusive and mixed. We thus conducted this meta-analysis to investigate the association between TLR4 A299G polymorphism and the risk of pneumonia.
Material and Methods
Publication search
Relevant studies were systematically searched for by using the NCBI, Medline, Web of Science, and Embase databases (he last retrieval date was August 29, 2014, using the search terms: “pneumonia” and “‘Toll like receptor 4” or “TLR4”). All discovered studies were retrieved and only published studies with full-text articles were included. For publications with duplicates, only the newest study was used in this research.
Inclusion and exclusion criteria
The inclusion criteria was: (1) the diagnosis of pneumonia was confirmed by X-ray or CT; (2) the research was a case-control study or a cohort study; (3) the study focused on the association between the TLR4 A299G polymorphism and the risk of pneumonia; and (4) the TLR4 A299G genotype of individual groups were provided, The exclusion criteria were: (1) pneumonia was not confirmed by X-ray or CT; (2) animal studies; and (3) reviews or abstracts.
Data extraction
Based on the selection criteria, 2 reviewers (Xingjun Cai and Yihui Fu) extracted and sorted the data independently (including author, year, ethnicity, age, type, and sample size). Authors were contacted by email if further study details are needed.
Statistical analysis
Statistical analysis was conducted using Stata software 11.0 (StataCorp, College Station, Texas, USA). HWE test in the healthy control group was conducted using χ2 test. Odds ratio (OR) with a 95% confidence interval (CI) was presented for dichotomous data, and significant level was 0.05. Q statistic and I2 statistic were used to measure statistical heterogeneity and significant level was 0.10. Effect model selection was on the basis of heterogeneity test. A fixed-effects model was selected when no significant heterogeneity, otherwise we used a random-effects model. We performed a cumulative meta-analysis by sequential random-effects pooling, starting with the earliest studies. Each successive meta-analysis then summarized all the trials in the preceding years. Results were presented as a series of mini meta-analyses, which were ordered chronologically in a forest plot to show the consequence of adding studies on the effect size. To evaluate the effect of individual studies on overall risk of pneumonia, we conducted sensitivity analyses by excluding each study individually and recalculating the ORs and 95% CI. Funnel plots were performed to estimate potential publication bias, with an asymmetrical plot suggesting possible publication bias. The asymmetry was assessed using the Egger’s linear regression test and P<0.05 was considered to represent statistically significant publication bias.
Results
Study characteristics
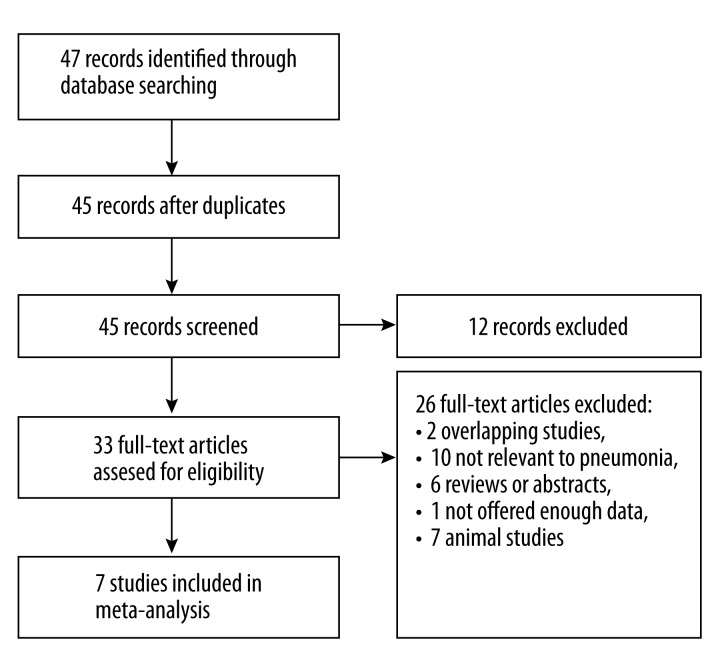
The study selection procedure is shown in Figure 1. All the studies were performed in Caucasian populations. Only 1 study used pediatric patients, while 7 studies used adult patients. Only 1 study assessed ventilator-associated pneumonia (VAP) patients, while other studies used CAP patients. A total of 658 patients and 1862 controls were included in our meta-analysis. The characteristics of the included studies are listed in Table 1.
Figure 1.
Flow of study identification, inclusion, and exclusion.
Table 1.
Characteristics of the studies included in meta-analysis.
| First author | Year | Ethnicity | Age group | Pneumonia type | Case number (n) | Control number (n) |
|---|---|---|---|---|---|---|
| Hawn | 2005 | Caucasian | Adult | CAP | 108 | 508 |
| Moens | 2007 | Caucasian | Adult | CAP | 72 | 178 |
| Everett | 2007 | Caucasian | Adult | CAP | 85 | 167 |
| Yuan | 2008 | Caucasian | Adult | CAP | 85 | 409 |
| Carvalho | 2009 | Caucasian | Adult | CAP | 87 | 134 |
| Kumpf | 2010 | Caucasian | Adult | VAP | 159 | 176 |
| Esposito | 2012 | Caucasian | Pediatric | CAP | 18 | 164 |
| Rodriguez-Osorio | 2013 | Caucasian | Adult | CAP | 44 | 126 |
CAP – community-acquired pneumonia; VAP – ventilator-associated pneumonia.
Quantitative data synthesis
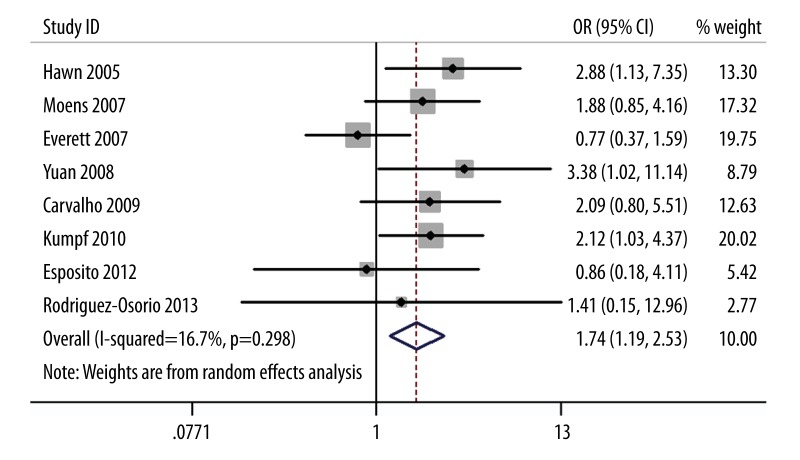
We investigated the association between TLR4 A299G polymorphism and pneumonia risk in the recessive models. Result of this meta-analysis showed that TLR4 A299G polymorphism was significantly associated with pneumonia risk (OR=1.74; 95% CI 1.19–2.53; P=0.004; Figure 2). When we deleted the pediatric study, the result was not changed (OR=1.81; 95% CI 1.22–2.69; P=0.003). When the VAP study was deleted, the result was also not changed (OR=1.67; 95% CI 1.06–2.62; P=0.03).
Figure 2.
Meta-analysis for the association between TLR4 A299G polymorphism and the risk of pneumonia.
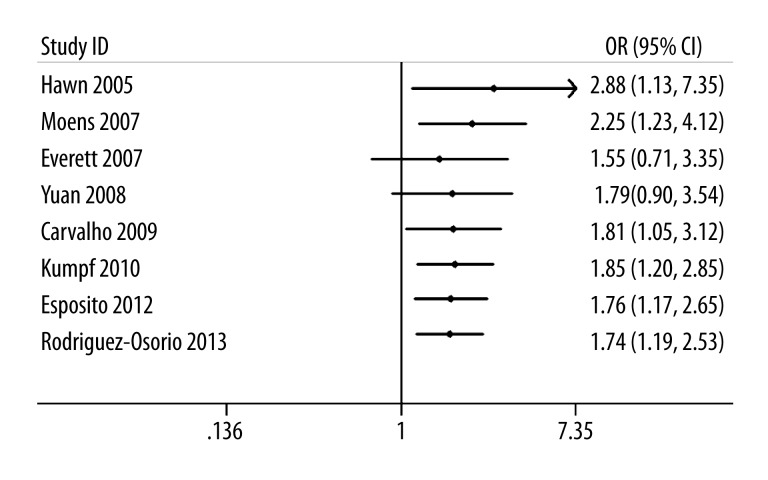
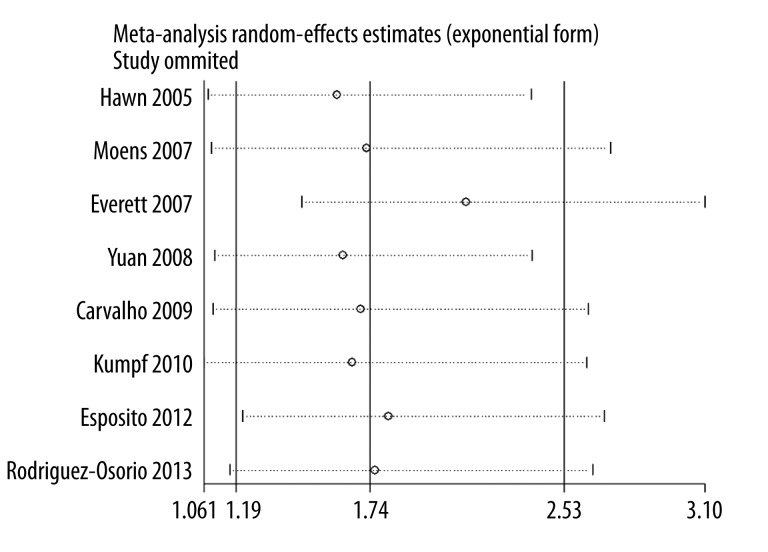
To determine the stability of the result, we performed sensitivity analysis by omitting 1 study at a time. We found that single study did not impact the pooled OR, indicating that the results of our research are statistically robust (Figure 3). Cumulative meta-analysis was also conducted by the assortment of studies by publication time (Figure 4).
Figure 3.
Cumulative meta-analysis for the association between TLR4 A299G polymorphism and the risk of pneumonia.
Figure 4.
Sensitivity analysis for the association between TLR4 A299G polymorphism and the risk of pneumonia.
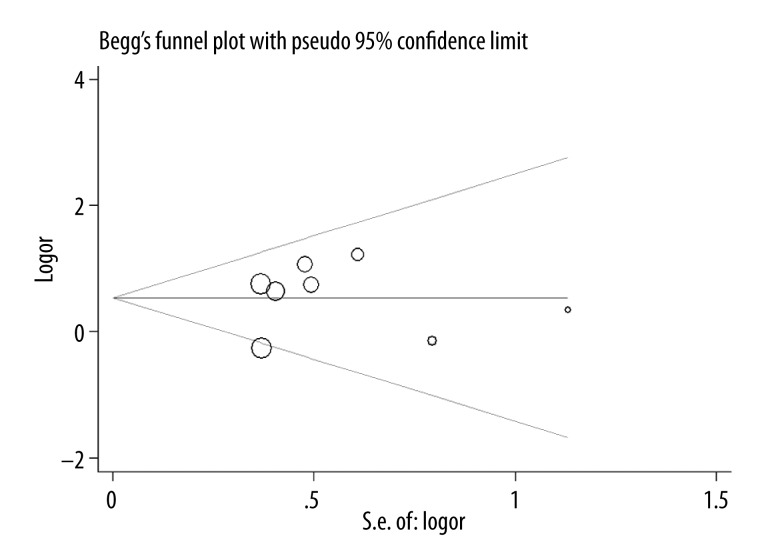
There was no publication bias in this meta-analysis, because no funnel plot asymmetry was detected (Figure 5). No significant publication bias was found with Begg’s test (P=0.912).
Figure 5.
Funnel plot for association between TLR4 A299G polymorphism and the risk of pneumonia.
Discussion
This meta-analysis of 8 case-control studies evaluated the association between TLR4 A299G polymorphism and the risk of pneumonia. We found that TLR4 A299G polymorphism might be a risk factor for developing pneumonia. This result suggests that TLR4 299AA genotype carriers might have increased pneumonia risk compared to the AG or GG carriers. The result remained significant in adults. In addition, we also found that TLR4 A299G polymorphism was associated CAP risk. Since there was only 1 study each with pediatric patients and VAP patients, more studies in these populations are needed to validate the result of this meta-analysis.
Previous studies have assessed the association between TLR4 A299G polymorphism and infectious diseases. For example, Panayiotis et al. did a meta-analysis and showed that TLR4 A299G polymorphism increased risk for all parasitic infections, malaria, brucellosis, cutaneous leishmaniasis, neurocysticercosis, Streptococcus pyogenes tonsillar disease, typhoid fever, and adult urinary tract infections [20]. However, no study evaluated the association between TLR4 A299G polymorphism and pneumonia. Thus, to the best of our knowledge, this is the first meta-analysis of the association between TLR4 A299G polymorphism and pneumonia risk.
A recent study suggested that deficient recruitment of signaling adapters MyD88 and TRIF to TLR4 was a mechanistic basis for A299G-mediated impairment of TLR4-elicited, LPS-induced activation of MyD88-dependent IL-8 and TNF-α genes, deficient TRIF-dependent phosphorylation of TBK1 and IRF3, transactivation of IRF3, and expression of IFN-β mRNA [10,21]. Thus, it is important to pay more attention to the subjects with TLR4 A299G polymorphism.
Conclusions
This meta-analysis had several limitations. Firstly, the sample size for this SNP was relatively small. Thus, the statistical power of genetic effects identified might be hampered. However, results from cumulative meta-analysis and sensitivity analysis suggest that our results are reliable and robust. Secondly, pneumonia is a complex process modulated by a series of genetic factors beyond TLR4. However, our analysis only tried to explore the effect of TLR4 A299G polymorphism on pneumonia, and did not link other gene variants that may be involved in pathophysiological pathways. Therefore, larger clinical studies are required to validate the hypothesis and findings obtained in this study. Once validated, they can be very helpful evidence in developing tailored therapeutics for individual patients.
The results of this meta-analysis suggest that susceptibility to pneumonia is associated with TLR4 A299G polymorphism.
Footnotes
Source of support: Self financing
References
- 1.Marrie TJ, Huang JQ. Epidemiology of community-acquired pneumonia in Edmonton, Alberta: an emergency department-based study. Can Respir J. 2005;12(3):139–42. doi: 10.1155/2005/672501. [DOI] [PubMed] [Google Scholar]
- 2.Waterer GW, Wunderink RG. Genetic susceptibility to pneumonia. Clin Chest Med. 2005;26(1):29–38. doi: 10.1016/j.ccm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Quasney MW, Waterer GW, Dahmer MK, et al. Association between surfactant protein B + 1580 polymorphism and the risk of respiratory failure in adults with community-acquired pneumonia. Crit Care Med. 2004;32(5):1115–19. doi: 10.1097/01.ccm.0000124872.55243.5a. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282(5396):2085–88. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Standiford LR, Standiford TJ, Newstead MJ, et al. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol. 2012;302(5):L447–54. doi: 10.1152/ajplung.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang L, Li Q, Bai J, et al. Severe pneumonia mortality in elderly patients is associated with downregulation of Toll-like receptors 2 and 4 on monocytes. Am J Med Sci. 2014;347(1):34–41. doi: 10.1097/MAJ.0b013e3182798583. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Nakamura S, Seki M, et al. Toll-like receptor 4 agonistic antibody promotes innate immunity against severe pneumonia induced by coinfection with influenza virus and Streptococcus pneumoniae. Clin Vaccine Immunol. 2013;20(7):977–85. doi: 10.1128/CVI.00010-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smirnova I, Mann N, Dols A, et al. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc Natl Acad Sci USA. 2003;100(10):6075–80. doi: 10.1073/pnas.1031605100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa L, Xiong Y, Song C, et al. The Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of MyD88 and TRIF. J Immunol. 2012;188(9):4506–15. doi: 10.4049/jimmunol.1200202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long H, O’Connor BP, Zemans RL, et al. The Toll-like receptor 4 polymorphism Asp299Gly but not Thr399Ile influences TLR4 signaling and function. PLoS One. 2014;9(4):e93550. doi: 10.1371/journal.pone.0093550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawn TR, Verbon A, Janer M, et al. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires’ disease. Proc Natl Acad Sci USA. 2005;102(7):2487–89. doi: 10.1073/pnas.0409831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett B, Cameron B, Li H, et al. Polymorphisms in Toll-like receptors-2 and -4 are not associated with disease manifestations in acute Q fever. Genes Immun. 2007;8(8):699–702. doi: 10.1038/sj.gene.6364428. [DOI] [PubMed] [Google Scholar]
- 14.Moens L, Verhaegen J, Pierik M, et al. Toll-like receptor 2 and Toll-like receptor 4 polymorphisms in invasive pneumococcal disease. Microbes Infect. 2007;9(1):15–20. doi: 10.1016/j.micinf.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Yuan FF, Marks K, Wong M, et al. Clinical relevance of TLR2, TLR4, CD14 and FcgammaRIIA gene polymorphisms in Streptococcus pneumoniae infection. Immunol Cell Biol. 2008;86(3):268–70. doi: 10.1038/sj.icb.7100155. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho A, Cunha C, Carotti A, et al. Polymorphisms in Toll-like receptor genes and susceptibility to infections in allogeneic stem cell transplantation. Exp Hematol. 2009;37(9):1022–29. doi: 10.1016/j.exphem.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Kumpf O, Giamarellos-Bourboulis EJ, Koch A, et al. Influence of genetic variations in TLR4 and TIRAP/Mal on the course of sepsis and pneumonia and cytokine release: an observational study in three cohorts. Crit Care. 2010;14(3):R103. doi: 10.1186/cc9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito S, Molteni CG, Giliani S, et al. Toll-like receptor 3 gene polymorphisms and severity of pandemic A/H1N1/2009 influenza in otherwise healthy children. Virol J. 2012;9:270. doi: 10.1186/1743-422X-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Osorio CA, Lima G, Herrera-Caceres JO, et al. Genetic variations in toll-like receptor 4 in Mexican-Mestizo patients with intra-abdominal infection and/or pneumonia. Immunol Lett. 2013;153(1–2):41–46. doi: 10.1016/j.imlet.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Ziakas PD, Prodromou ML, El Khoury J, et al. The role of TLR4 896 A>G and 1196 C>T in susceptibility to infections: a review and meta-analysis of genetic association studies. PLoS One. 2013;8(11):e81047. doi: 10.1371/journal.pone.0081047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puljiz I, Markotić A, Cvetko Krajinovic L, et al. Mycoplasma pneumoniae in adult community-acquired pneumonia increases matrix metalloproteinase-9 serum level and induces its gene expression in peripheral blood mononuclear cells. Med Sci Monit. 2012;18(8):CR500–5. doi: 10.12659/MSM.883270. [DOI] [PMC free article] [PubMed] [Google Scholar]