Abstract
Background
The expression of Src and phospho-Src (p-Src) is closely related to tumor invasion and metastasis. The aim of the present study was to investigate the expression of these molecules in osteosarcoma and their relationship with each other, to provide a theoretical basis to understand the prognosis of osteosarcoma.
Material/Methods
We selected surgically resected osteosarcoma specimens from 116 patients of Zhongnan Hospital of Wuhan University and Hubei Cancer Hospital, Hubei, China, between January 2000 and January 2010 with detailed follow-up data. Twenty osteochondroma specimens from the corresponding period were used as controls. Expression of Src and p-Src was detected in osteosarcoma and osteochondroma by immunohistochemistry. We analyzed the relationship of the 2 proteins and osteosarcoma patient prognosis.
Results
The expression of Src and p-Src in osteosarcoma was significantly higher than the expression level in osteochondroma (P<0.05). The expression levels of the 2 proteins, clinical stage, and tumor metastasis were significantly associated with survival time (P<0.05), but there was no correlation between age or sex and survival time. The expression of Src and p-Src in osteosarcoma was positively correlated.
Conclusions
Src and p-Src can be used as an auxiliary indicator to determine a malignant phenotype of bone tumors, and the combined detection of Src and p-Src may indicate the prognosis of osteosarcoma.
MeSH Keywords: Genes, src; Osteosarcoma; Prognosis
Background
Osteosarcoma is a malignant tumor that originates from mesenchymal cells and is characterized by fusiform stromal cells producing osteoid. It is the most common malignant tumor of the skeletal system [1–3], and mainly occurs in young people aged 15 to 19 years. It usually appears in the metaphysis and has a rich blood supply. The hematogenous metastasis rate is high, with about 85% of metastasis seeding the lung [4]. Due to its rapid progression and poor prognosis, osteosarcoma is a major death-causing disease in adolescence. Sixty to seventy percent of osteosarcoma patients achieve 5-year disease-free survival due to the current treatments, including surgery, chemotherapy, and radiation therapy [5–8]. However, 20–25% of patients have lung metastases when diagnosed [9], and there are still further patients that develop lung metastasis during treatment. The patients with lung metastases have a worse prognosis and the long-term survival rate is only 10–30% [8]. Moreover, patient prognoses remain poor even after receiving systemic treatment. Therefore, it is crucial to choose suitable survival prognostic indicators to estimate the prognosis of patients with osteosarcoma. Recently, some molecules, including Src and p-Src, have been correlated with the genesis and development of osteosarcoma and may be potential prognostic markers.
Src family kinases include Src and another 11 members, and they are jointly involved in intracellular signal transduction processes [10–13]. Src can be activated by multiple signaling pathways to become phospho-Src (p-Src), and p-Src can activate specific signaling pathways by phosphorylating the target proteins [12,14]. Thus, Src and the intracellular signaling pathways constitute an amplification cascade of a network regulation system, and Src is the central hub of a variety of signaling pathways. Recent studies have found that Src family kinases mediate a variety of malignancies [15–19], including osteosarcoma cell signal transduction, and play an important role in promoting the genesis and development of osteosarcoma [20–22].
This study detected the expression of Src and p-Src in osteosarcoma and osteochondroma by immunohistochemistry and explored the proteins’ relationship with the clinicopathological features of osteosarcoma patients as well as their correlation in osteosarcoma to evaluate whether Src and p-Src are risk factors for osteosarcoma, and to provide important information for clinical treatment and for a prognosis estimate of osteosarcoma.
Material and Methods
Patients and samples
The local research ethics committee (Zhongnan Hospital of Wuhan University) approved this study, taking out exemption for informed consent procedure. All patients or their carer providers gave informed consent for participation in this study. We selected surgically resected osteosarcoma specimens from 116 patients of Zhongnan Hospital of Wuhan University and the Hubei Cancer Hospital, Hubei, China, between January 2000 and January 2010 with detailed follow-up data (except for the patients that received preoperative radiotherapy and chemotherapy). Twenty osteochondroma specimens from the corresponding period were used as controls. All of the samples were evaluated for diagnosis by 3 experienced pathologists. Of the 116 osteosarcoma patients, 73 cases had metastases and 43 cases did not; 71 were male and 45 were female; patients were aged from 8 to 60 years with an average age of 19.1 years. Lesions were located as follows: 43 femoral, 34 tibial, 21 humerus, 4 upper jaw, 7 ilium, 5 fibula, and 2 withers. According to the Ennecking method, there were 5 cases of stage IB, 17 cases of IIA, 64 cases of IIB, and 30 cases of IIIA. Of the 20 osteochondroma patients, 12 were male and 8 were female, aged from 21 to 78 years with an average age of 60.4 years.
Immunohistochemistry
The streptavidin-peroxidase immunohistochemistry method was used to inspect Src and p-Src expression in specimens. The paraffin sections were routinely dewaxed 3 times with dimethyl benzene, and hydrated with an ethanol gradient. Subsequently, sections were washed with distilled water and phosphate buffered saline (PBS). Dewaxed paraffin sections were treated with 3% hydrogen peroxide for 20 min to block endogenous peroxidases. Then the sections were blocked with 2% goat serum in PBS for 1 h at room temperature and incubated with rat antibody against human Src and p-Src (Cell Signaling Technologies, Danvers, USA). Afterwards, the sections were washed with PBS and subjected to immunohistochemical staining using a PV6000 kit (Bioss Inc., Beijing, China). Then, the sections were washed with PBS and developed with 3,3′-diaminobenzidine (DAB) for 5 min and counterstained with hematoxylin. Finally, the sections were assessed under a light microscope by 2 independent investigators.
Evaluation of immunohistochemistry
We used a semi-quantitative method to calculate an immunohistochemical score (IHS). Five fields were randomly selected under a light microscope (×200) to count the positive cells in each slice and to calculate the percentage of positive cells to total cells. We divided the cell staining density into 4 grades: 1=≤25% positive cells; 2=26–50% positive cells; 3=51–75% positive cells; 4=≥76% positive cells. The cell staining intensities were divided into 4 grades: 0=uncolored; 1=light brown; 2=brown; 3=dark brown. The final IHS was obtained by multiplying the score of density and intensity. The IHS of each specimen was categorized into 4 groups: 0–2 −; 3–5 +; 6–8 ++, 9–12 +++. Scores of 0–5 were designated as low expression, while scores of 6–12 were designated as high expression. The immunohistochemistry results were tested by 3 of the authors in all specimens using a blinded method.
Statistical analysis
SPSS version 18.0 software (SPSS Inc., Chicago, USA) was used to perform statistical analysis. The semi-quantitative data and sample rate difference were estimated by the chi-square test. Survival rates were assessed by Kaplan-Meier statistics and survival curves were compared by the log-rank test. Multivariate analysis was estimated by the Cox regression method. The correlation between Src and p-Src was assessed by Spearman’s correlation analysis. P<0.05 was considered statistically significant for all tests.
Results
Expression of Src and p-Src and their correlation with clinicopathological features of patients with osteosarcoma
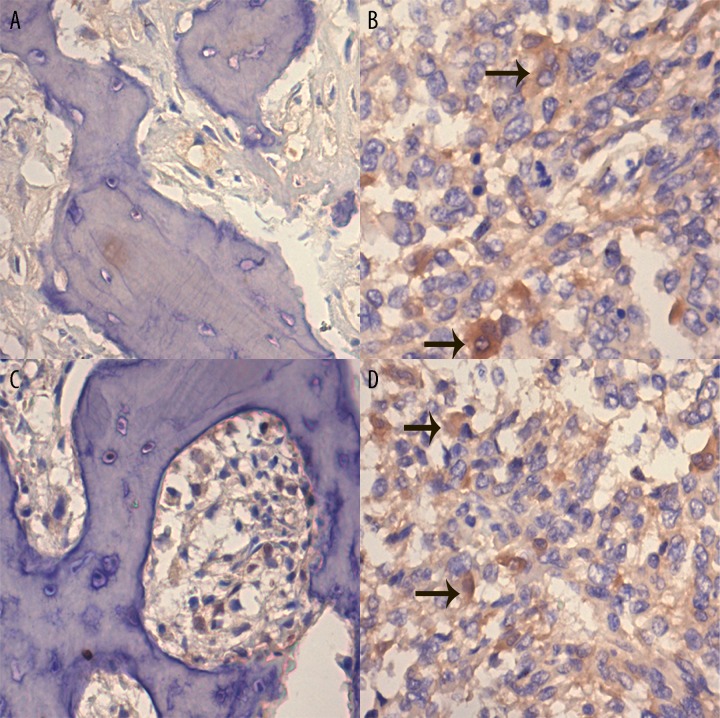
The expression levels of Src and p-Src in osteosarcoma and osteochondroma tested by immunohistochemistry are shown in Table 1. The expression of Src was observed in 4 (20.0%) of the total osteochondroma cases, and p-Src in 2 of the total number of cases (10.0%). In the total number of osteosarcoma patients, there was expression of Src and p-Src in 73 cases (62.9%) and 75 cases (64.7%), respectively. The chi-square test confirmed that the difference in the expression level of both markers between osteosarcoma and osteochondroma was statistically significant (p<0.05, Table 1). Typical immunohistochemistry images of Src and p-Src in osteosarcoma and osteochondroma specimens are shown in Figure 1. The positive immunohistochemistry results were the tan particles observed in the cytoplasm. The chi-square test showed that the expression level of Src and p-Src was associated with metastasis and clinical stage (p<0.05), while the expression level of both markers showed no association with age, gender, or histological subtype (Tables 2 and 3).
Table 1.
Expression of Src and p-Src in osteosarcomas and osteochondromas.
| Cases | Src expression | p-Src expression | |||||
|---|---|---|---|---|---|---|---|
| Expression | Low expression | P | Expression | Low expression | P | ||
| OS | 116 | 73 (62.9%) | 43 (37.1%) | 0.0003* | 75 (64.7%) | 41 (35.3%) | 0.0005* |
| OC | 20 | 4 (20.0%) | 16 (80.0%) | 2 (10.0%) | 18 (90.0%) | ||
OS – osteosarcoma; OC – osteochondroma.
Significant p<0.05.
Figure 1.
Representative expression of Src and p-Src in osteosarcoma and osteochondroma samples. The sections were counterstained with hematoxylin and the positive staining is brown (indicated by black arrow). (A) immunohistochemistry of Src in osteochondroma specimen (×200). (B) immunohistochemistry of Src in osteosarcoma specimen (×200). (C) immunohistochemistry of p-Src in osteochondroma specimen (×200). (D) immunohistochemistry of p-Src in osteosarcoma specimen (×200).
Table 2.
Correlation between the expression of Src and p-Src and clinicopathological data.
| Clinical feature | Src expression | p-Src expression | ||||
|---|---|---|---|---|---|---|
| Expression | Low expression | P | Expression | Low expression | P | |
| Age (years) | 0.268 | 0.310 | ||||
| ≤50 | 42 | 25 | 44 | 23 | ||
| >50 | 31 | 18 | 31 | 18 | ||
| Gender | 0.949 | 0.789 | ||||
| Male | 41 | 30 | 42 | 29 | ||
| Female | 32 | 13 | 33 | 12 | ||
| Histological type | 0.146 | 0.120 | ||||
| Osteoblastic | 35 | 15 | 37 | 13 | ||
| Chondroblastic | 13 | 11 | 14 | 10 | ||
| Fibroblastic | 12 | 8 | 12 | 8 | ||
| Telangiectatic | 7 | 8 | 7 | 8 | ||
| Mixed | 6 | 1 | 5 | 2 | ||
| Primary site | 0.889 | 0.584 | ||||
| Femur | 28 | 15 | 30 | 13 | ||
| Tibia | 19 | 15 | 20 | 14 | ||
| Humerus | 13 | 8 | 13 | 8 | ||
| Fibula | 4 | 1 | 2 | 3 | ||
| Ilium | 5 | 2 | 6 | 1 | ||
| Other | 4 | 2 | 4 | 2 | ||
| Metastasis | 0.044* | 0.020* | ||||
| Yes | 51 | 22 | 53 | 20 | ||
| No | 22 | 21 | 22 | 21 | ||
| Clinical stage | 0.0004* | 0.0005* | ||||
| Stage I B | 2 | 3 | 2 | 3 | ||
| Stage II A | 3 | 14 | 4 | 13 | ||
| Stage II B | 40 | 24 | 40 | 24 | ||
| Stage III | 28 | 2 | 29 | 1 | ||
P<0.05.
Table 3.
Patients characteristic.
| Patients characteristic | Src expreossion | P-Src expression | Age | Gender | Metastasis | Clinical stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | ≤50 | >50 | Male | Female | Yes | No | I B | II A | II B | III | |
| Src expreossion | ||||||||||||||
| High | 72 | 1 | 42 | 31 | 42 | 32 | 51 | 22 | 2 | 3 | 40 | 28 | ||
| Low | 3 | 40 | 25 | 18 | 30 | 13 | 22 | 21 | 3 | 14 | 24 | 2 | ||
| P-Src expression | ||||||||||||||
| High | 72 | 3 | 44 | 31 | 42 | 33 | 53 | 22 | 2 | 4 | 40 | 29 | ||
| Low | 1 | 40 | 23 | 18 | 29 | 12 | 20 | 21 | 3 | 13 | 24 | 1 | ||
| Age | ||||||||||||||
| ≤50 | 42 | 25 | 44 | 23 | 36 | 31 | 44 | 23 | 3 | 7 | 38 | 19 | ||
| >50 | 31 | 18 | 31 | 18 | 35 | 14 | 29 | 30 | 2 | 10 | 26 | 11 | ||
| Gender | ||||||||||||||
| Male | 40 | 30 | 42 | 29 | 36 | 35 | 45 | 26 | 2 | 9 | 37 | 23 | ||
| Female | 32 | 13 | 33 | 12 | 31 | 14 | 28 | 17 | 3 | 8 | 27 | 7 | ||
| Metastasis | ||||||||||||||
| Yes | 51 | 22 | 53 | 20 | 44 | 29 | 45 | 48 | 2 | 7 | 34 | 30 | ||
| No | 22 | 21 | 22 | 21 | 23 | 20 | 26 | 17 | 3 | 10 | 30 | 0 | ||
| Clinical stage | ||||||||||||||
| I B | 2 | 3 | 2 | 3 | 3 | 2 | 2 | 3 | 2 | 3 | ||||
| II A | 3 | 14 | 4 | 13 | 7 | 10 | 9 | 8 | 7 | 10 | ||||
| II B | 40 | 24 | 40 | 24 | 38 | 26 | 37 | 27 | 24 | 30 | ||||
| III | 28 | 2 | 29 | 1 | 19 | 11 | 23 | 7 | 30 | 0 | ||||
Prognostic value of Src and p-Src expression
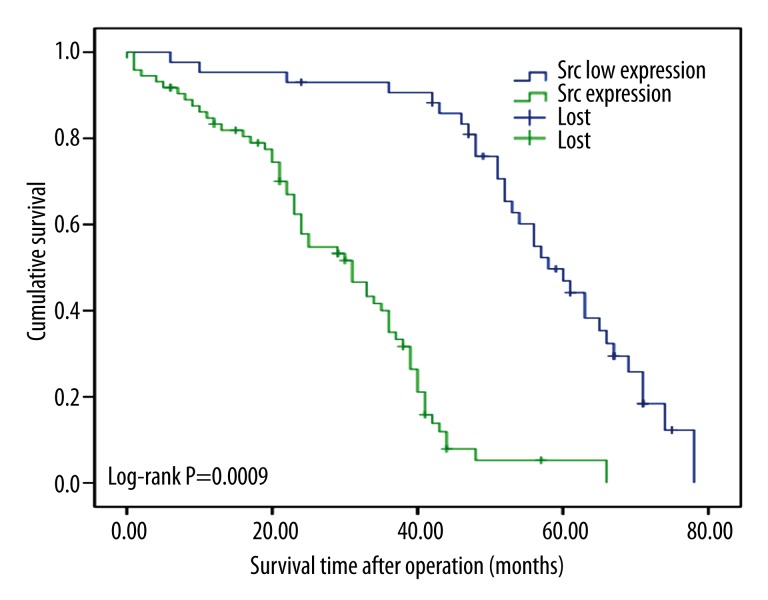
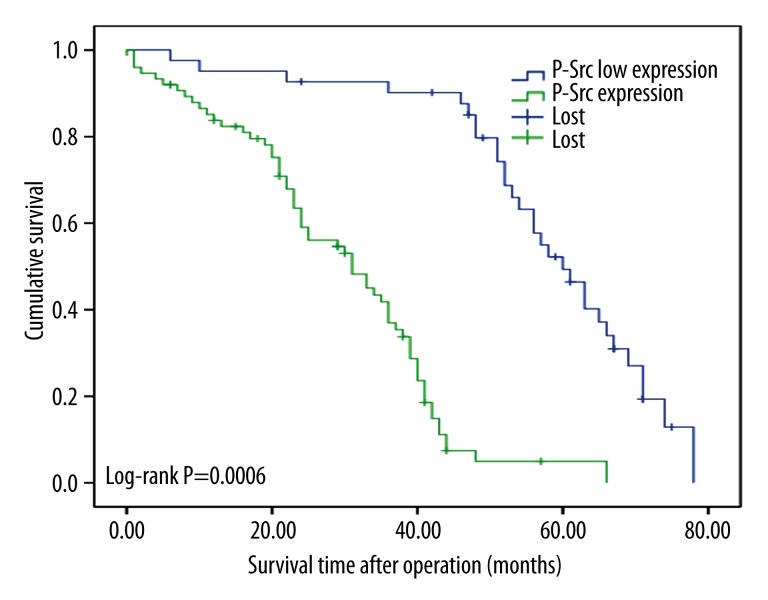
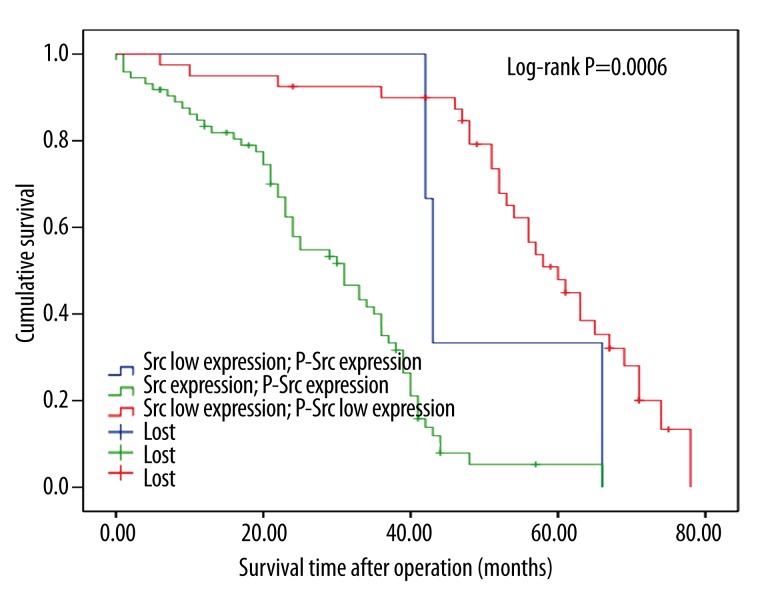
The Kaplan-Meier survival curves show that the expression of the 2 proteins is correlated with shorter survival time (p<0.05, log-rank test, Figures 2 and 3). Based on the expression levels of the 2 proteins, all patients were divided into 3 groups and survival analysis showed that patients that demonstrated expression of p-Src and Src expression were associated with the minimum survival time, whereas patients with low expression of both markers had the longest survival time (p<0.05, Figure 4).
Figure 2.
Postoperative survival curves for patients according to the expression of Src.
Figure 3.
Postoperative survival curves for patients according to the expression of p-Src.
Figure 4.
Postoperative survival curves for patients according to the conjoint analysis of Src and p-Src expression.
Cox multivariate analysis showed that the expression levels of the 2 proteins, clinical stage, and tumor metastasis were significantly associated with survival, while there was no correlation between age or gender and survival (Table 4).
Table 4.
Multivariate survival analysis of overall survival in patients with osteosarcoma.
| Variables | P | RR | 95% CI for RR |
|---|---|---|---|
| Age | 0.065 | ||
| Gender | 0.820 | ||
| Metastasis status | 0.014 | 1.853 | 1.133–3.032 |
| Clinical stage | 0.000 | 1.852 | 1.325–2.587 |
| Src | 0.029 | 2.472 | 1.095–5.582 |
| p-Src | 0.005 | 3.584 | 1.457–8.816 |
RR – relative risk; CI – confidence interval.
Correlation of Src and p-Src in osteosarcoma
We used multivariate analysis to determine whether expression of Src and p-Src in osteosarcoma were related. The result showed that the expression of Src and p-Src was positively correlated (r=0.926, p=0.0005).
Discussion
Due to the poor survival rate of osteosarcoma patients, information on the mechanism of osteosarcoma is needed to evaluate the prognosis, so that the physician can decide which treatment will best help the patient. Because the occurrence and evolution of osteosarcoma is the result of oncogene activation or inactivation of tumor suppressor genes, the prognosis of osteosarcoma as judged by clinical features only is not accurate, which was confirmed by modern molecular genetics. The molecular mechanisms leading to the development and progression of osteosarcomas is not yet understood. We notice that the proliferation, invasion, and metastasis of osteosarcoma involve multiple signaling pathways [23–25]. More importantly, these pathways can affect each other by Src through crosstalk, but the clinical significance remains debatable. To confirm our hypothesis that Src and p-Src are potential tumor markers, their protein level and relationship with prognosis were analyzed using immunochemistry.
A number of animal and cell experiments have confirmed that Src and p-Src can be detected in osteosarcoma cells [20–22], and in other malignant tumors such as squamous cell carcinoma of the tongue, breast cancer, rhabdomyosarcoma, and Ewing’s sarcoma, Src and p-Src also showed high expression [17,18,26–28]. In this study, we used immunohistochemistry to show that the expression percentage of Src and p-Src was 20% and 10% of the 20 osteochondroma cases, respectively, while in 116 cases of osteosarcoma, the expression rate of both proteins was significantly increased, with 62.9% and 64.7% of cases showing high expression of Src and p-Src, respectively. Table 2 shows that the minimum theoretical frequency in osteosarcoma and osteochondroma group was greater than 5, so we chose to use the Pearson chi-square test and these findings are consistent with previous research. In addition, we confirmed that the expression of Src and p-Src in osteosarcoma patients were significantly higher than in the osteochondroma group, but the result may be biased because the 20 cases in the osteochondroma group is obviously far less than the 116 cases in the osteosarcoma group. It was difficult to collect more osteochondroma specimens from the few patients who underwent surgery, and we need more cases for a more reliable result in the next study. Another interesting result is that there is a big mean difference in age between the osteosarcoma (19.1 years) and osteochondroma (60.4 years) group. Although osteosarcoma and osteochondroma both mainly occur in young people, the patients with osteosarcoma seek treatment earlier for the rapid progression, compared to the patients with osteochondroma who seek treatment later for the slow or stationary progression, so the patients with osteochondroma are older than the patients with osteosarcoma in the study.
Src and p-Src promote osteosarcoma invasion, migration, metastasis, proliferation, and other biological effects [20–22]. Therefore, Src and p-Src may be of great relevance to osteosarcoma patients’ clinical and pathological features and prognosis. We compared the expression levels of Src and p-Src with patient survival time, pathology, metastasis, and clinical stage, and the results showed that high expression of Src and p-Src was positively correlated with metastasis and clinical stage, and was negatively correlated with postoperative survival time. This phenomenon has been confirmed in other malignancies. Li et al. [29] found that breast cancer patients with increased Src expression had a high probability of tumor metastasis. Maślikowski et al. [30] detected the expression of Src and another 42 kinds of tumor factors in breast cancer and lung cancer through immunohistochemistry and confirmed that Src has a negative correlation with prognosis. Singh et al. [31] showed that Src and p-Src could promote colon cancer invasion and metastasis. Donahue et al. [32] found that p-Src was associated with shorter survival time and lower tumor tissue differentiation in pancreatic cancer patients. Tsao et al. [33] demonstrated that, in malignant pleural mesothelioma, higher levels of clinical stage correlated with higher expression of p-Src. In the study, the survival curves showed throughout an “offset” behavior after a lag phase in Figures 2 and 3. The reasons for these phenomena might be the following aspects: although the statistical analysis methods were appropriate, the result could not fully deduce the real situation due to the small sample size; the expression of Src/p-Src in a single slice might not sufficiently reflect the expression of the 2 proteins in the tumor because the tumor cells were nonuniform distribution in tumor; and the immunohistochemistry results judged by humans might limit the detection accuracy.
The Src inhibitor dasatinib had been demonstrated to inhibit the development of osteosarcoma in vitro and in vivo. Davis et al. [34] treated a canine osteosarcoma with dasatinib for 26 weeks, and 24 months of follow-up demonstrated no recurrence. Shor et al. [35] confirmed that dasatinib could suppress the expression of Src in osteosarcoma cells, induce osteosarcoma cell apoptosis, and could prevent cell migration and invasion in vitro. Moreover, Shor et al. demonstrated that dasatinib may provide a benefit by controlling the growth and metastasis of sarcomas in patients. However, some of the research findings are not so optimistic. Hingorani et al. [36] demonstrated that dasatinib could completely inhibit Src phosphorylation in primary osteosarcomas in the nude mouse, but could not stop the progression of lung metastases. Therefore, further data on the relationship between Src and lung metastasis of osteosarcoma are required.
Immunohistochemistry is a semi-quantitative technique; hence, we need to be cautious about its results. In addition, osteochondromas were used as a control in this study due to limited conditions, so the results may not be as forceful as a comparison with normal bone specimens. Therefore, this experiment is a preliminary study and requires a larger number of cases for long-term follow-up. In order to provide more valuable experimental data, a multi-unit collaboration and the use of accurate quantitative experimental indicators for statistical analysis are needed.
Conclusions
In this study we have preliminarily identified Src/p-Src as potential biomarkers of osteosarcoma in clinic patients, which could promote osteosarcoma progression or metastasis and may indicate a poorer prognosis.
Acknowledgments
We thank Uni-edit Co. Ltd. for editing and proofreading this manuscript.
Footnotes
Source of support: The study was funded by the key grant from Hubei Provincial Natural Science (NO. 2014CFA063)
References
- 1.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–41. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 2.Bielack SS, Marina N, Ferrari S, et al. Osteosarcoma: the same old drugs or more? J Clin Oncol. 2008;26:3102–13. doi: 10.1200/JCO.2008.17.1108. [DOI] [PubMed] [Google Scholar]
- 3.Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Harting MT, Blakely ML. Management of osteosarcoma pulmonary metastases. Semin Pediatr Surg. 2006;15:25–29. doi: 10.1053/j.sempedsurg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26:1–18. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- 6.Hayden JB, Hoang BH. Osteosarcoma: basic science and clinical implications. Orthop Clin North Am. 2006;37:1–7. doi: 10.1016/j.ocl.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 8.Meyers PA. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9:1035–49. doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- 9.Hattinger CM, Pasello M, Ferrari S, et al. Emerging drugs for high-grade osteosarcoma. Expert Opin Emerg Drugs. 2010;15:615–34. doi: 10.1517/14728214.2010.505603. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Xu J, Zhou L, et al. Hepatitis B virus large surface antigen promotes liver carcinogenesis by activating the Src/PI3K/Akt pathway. Cancer Res. 2011;71:7547–57. doi: 10.1158/0008-5472.CAN-11-2260. [DOI] [PubMed] [Google Scholar]
- 11.Bai KJ, Chen BC, Pai HC, et al. Thrombin-induced CCN2 expression in human lung fibroblasts requires the c-Src/JAK2/STAT3 pathway. J Leukoc Biol. 2013;93:101–12. doi: 10.1189/jlb.0911449. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XT, Ding L, Kang LG, Wang ZY. Involvement of ER-α36, Src, EGFR and STAT5 in the biphasic estrogen signaling of ER-negative breast cancer cells. Oncol Rep. 2012;27:2057–65. doi: 10.3892/or.2012.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan P, McDaniel RE, Kim HR, et al. Modulating therapeutic effects of the c-Src inhibitor via oestrogen receptor and human epidermal growth factor receptor 2 in breast cancer cell lines. Eur J Cancer. 2012;48:3488–98. doi: 10.1016/j.ejca.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri MK, Koch P, Stenzinger A, et al. PTPIP51, a positive modulator of the MAPK/Erk pathway,is upregulated in glioblastoma and interacts with 14-3-3β and PTP1B in situ. Histol Histopathol. 2011;26:1531–43. doi: 10.14670/HH-26.1531. [DOI] [PubMed] [Google Scholar]
- 15.Michels S, Trautmann M, Sievers E, et al. SRC signaling is crucial in the growth of synovial sarcoma cells. Cancer Res. 2013;73:2518–28. doi: 10.1158/0008-5472.CAN-12-3023. [DOI] [PubMed] [Google Scholar]
- 16.Arai R, Tsuda M, Watanabe T, et al. Simultaneous inhibition of Src and Aurora kinases by SU6656 induces therapeutic synergy in human synovial sarcoma growth, invasion and angiogenesis in vivo. Eur J Cancer. 2012;48:2417–30. doi: 10.1016/j.ejca.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Abraham J, Chua YX, Glover JM, et al. An adaptive Src-PDGFRA-Raf axis in rhabdomyo- sarcoma. Biochem Biophys Res Commun. 2012;426:363–68. doi: 10.1016/j.bbrc.2012.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung CL, Ngo VN, Grohar PJ, et al. Loss-of-function screen in rhabdomyosarcoma identifies CRKL-YES as a critical signal for tumor growth. Oncogene. 2013;32:5429–38. doi: 10.1038/onc.2012.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan H, Zhou Z, Gallick GE, et al. Targeting Lyn inhibits tumor growth and metastasis in Ewing’s sarcom. Mol Cancer The. 2008;7:1807–16. doi: 10.1158/1535-7163.MCT-08-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zucchini C, Manara MC, Pinca RS, et al. CD99 suppresses osteosarcoma cell migration through inhibition of ROCK2 activity. Oncogene. 2014;33:1912–21. doi: 10.1038/onc.2013.152. [DOI] [PubMed] [Google Scholar]
- 21.Windischhofer W, Huber E, Rossmann C, et al. LPA-induced suppression of periostin in human osteosarcoma cells is mediated by the LPA(1)/Egr-1 axis. Biochimie. 2012;94:1997–2005. doi: 10.1016/j.biochi.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibuya H, Hamamura K, Hotta H, et al. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012;103:1656–64. doi: 10.1111/j.1349-7006.2012.02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Zhong D, Lin B, et al. P38 MAPK regulates the expression of ether à go-go potassium channel in human osteosarcoma cells. Radiol Oncol. 2013;47:42–49. doi: 10.2478/v10019-012-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Wang L, Wu Y, et al. Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway. Toxicology. 2013;304:120–31. doi: 10.1016/j.tox.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Peng L, Liu A, Shen Y, et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol Rep. 2013;29:571–78. doi: 10.3892/or.2012.2165. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Izhak O, Cohen-Kaplan V, Nagler RM. The prognostic role of phospho-Src family kinase analysis in tongue cancer. J Cancer Res Clin Oncol. 2010;136:27–34. doi: 10.1007/s00432-009-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai R, Tsuda M, Watanabe T, et al. Simultaneous inhibition of Src and Aurora kinases by SU6656 induces therapeutic synergy in human synovial sarcoma growth, invasion and angiogenesis in vivo. Eur J Cancer. 2012;48:2417–30. doi: 10.1016/j.ejca.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Huang F, Fan L, et al. Phosphatidylethanolamine-binding protein 4 is associated with breast cancer metastasis through Src-mediated Akt tyrosine phosphorylation. Oncogene. 2014;33:4589–98. doi: 10.1038/onc.2013.408. [DOI] [PubMed] [Google Scholar]
- 30.Maślikowski BM, Néel BD, Wu Y, et al. Cellular processes of v-Src transformation revealed by gene profiling of primary cells – implications for human cancer. BMC Cancer. 2010;10:41. doi: 10.1186/1471-2407-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh AB, Sharma A, Dhawan P. Claudin-1 expression confers resistance to anoikis in colon cancer cells in a Src-dependent manner. Carcinogenesis. 2012;33:2538–47. doi: 10.1093/carcin/bgs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donahue TR, Tran LM, Hill R, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18:1352–63. doi: 10.1158/1078-0432.CCR-11-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsao AS, He D, Saigal B, et al. Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion. Mol Cancer Ther. 2007;6:1962–72. doi: 10.1158/1535-7163.MCT-07-0052. [DOI] [PubMed] [Google Scholar]
- 34.Davis LE, Hofmann NE, Li G, et al. A case study of personalized therapy for osteosarcoma. Pediatr Blood Cancer. 2013;60:1313–19. doi: 10.1002/pbc.24512. [DOI] [PubMed] [Google Scholar]
- 35.Shor AC, Keschman EA, Lee FY, et al. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007;67:2800–8. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- 36.Hingorani P, Zhang W, Gorlick R, Kolb EA. Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin Cancer Res. 2009;15:3416–22. doi: 10.1158/1078-0432.CCR-08-1657. [DOI] [PubMed] [Google Scholar]