Abstract
Background
Post-stroke cognitive impairment is common and a decisive prognostic factor. However, few studies have reported on post-stroke cognition in young adults, especially long-term cognition. This study was designed to investigate the influence of baseline factors, treatments, and functional outcome on the long-term cognitive outcome in young adults with ischemic stroke.
Material/Methods
Consecutive patients aged 18–45 years between January 1, 2006 and December 31, 2010, with a first-ever ischemic stroke, were recruited for cognitive assessment by telephone from December 10 to December 31, 2013 using modified versions of “Telephone Instrument for Cognitive Status” (TICS-m) scale. The relationship of cognitive impairment with baseline factors, treatments, and functional outcome were evaluated.
Results
A total of 350 patients with an average age of 41.0±6.8 years (69.7% males and 30.3% females) were reviewed. The average follow-up period was 5.8±3.2 years, and cognitive impairment existed in 39.4% of patients at follow-up. Stroke severity on admission, functional outcome (modified Rankin Scale, mRS >2) at discharge, left anterior circulation syndrome, and stroke recurrence were markedly associated with post-stroke cognitive impairment (all P<0.01). Post-stroke cognition was also significantly related to mRS at follow-up (r=−0.563, P<0.001).
Conclusions
Post-stroke cognition was related to functional outcome: hence, treatment directed toward reducing functional disability might also reduce cognitive impairment.
MeSH Keywords: Adult; Hypoxia-Ischemia, Brain; Mild Cognitive Impairment
Background
The incidence of stroke in young adults is rising, and approximately 10–14% of all ischemic strokes occur in young adults aged 18–45 years [1–3]. Furthermore, post-stroke cognitive impairment, a decisive prognostic factor, is common [4] and may occur in up to 50% of young patients [5]. However, few studies address the cognition after stroke in young adults, especially the long term consequence [5–8].
Because of longer life expectancy, young stroke patients were informed about their cognitive prognosis, to recognize the risk factors of cognitive impairment may help to prevent the development of cognitive impairment. The present study aimed to investigate the long-term cognitive performance after ischemic stroke and the influence of baseline factors, treatments and functional outcome on this long-term cognitive outcome in young adults.
Material and Methods
Consecutive patients with a first-ever ischemic stroke of presumed arterial origin, (age: 18–45 years) were recruited from XuanWu Hospital from January 1, 2006 to December 31, 2010.
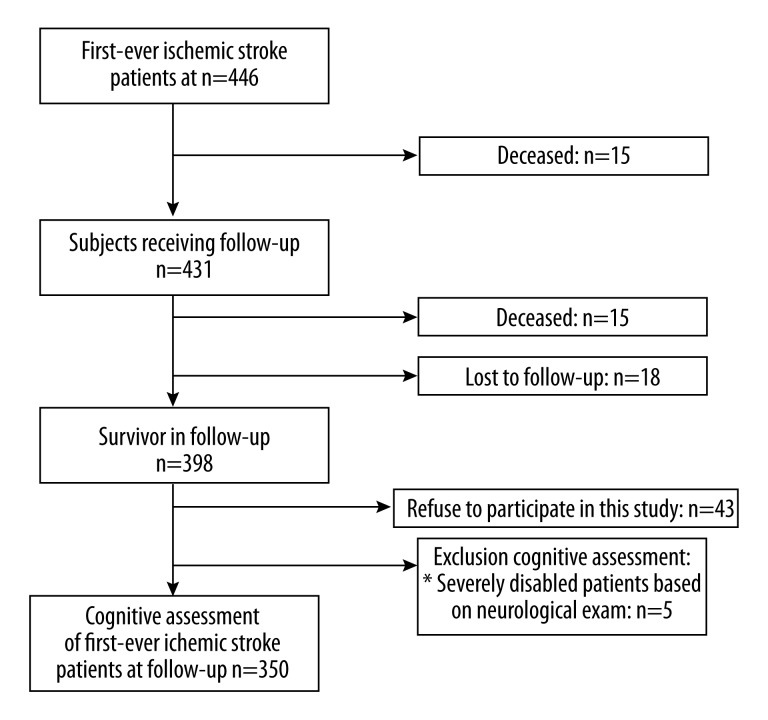
Ischemic stroke was defined as the loss of brain function due to either blockage of a blood vessel via thrombosis or arterial embolism, or by cerebral hypoperfusion, and confirmed by CT or MRI. Primary exclusion criteria included cerebral venous sinus thrombosis, retinal infarction, hearing or listening comprehension disorder and death. There were additional exclusion criteria for cognitive assessment on the basis of neurological examinations (Figure 1).
Figure 1.
Flowchart of patients’ recruitment. * Severe psychiatric disorder (n=1), inability to communicate in Chinese (n=1), blind and deaf (n=1) and severe aphasia (n=2).
Information of patients with stroke was collected from medical records prospectively. Categories of the etiologies of ischemic stroke were large artery atherosclerosis (LAA), cardioembolism (CE), small artery occlusion (SAO), other determined etiologies (OD) and undetermined etiologies (UND) according to the Adams TOAST criteria [9]. The involvement of lesion vessel was divided into right or left anterior and posterior circulation. Following variables were recorded: 1) baseline information: age, gender and education years; 2) post-stroke treatments: antiplatelets, anticoagulants, and statins; 3) vascular risk factors: arterial hypertension, current smoking, alcohol abuse, abuse of other drugs (cocaine, heroin, etc.), diabetes mellitus, hyperlipidemia, coronary artery disease, non-ischemic cardiopathy, peripheral arterial disease, chronic or paroxysmal atrial fibrillation (AF), valvular heart disease, previously diagnosed migraine, obesity; 4) stroke severity on admission according to the National Institute of Health Stroke Scale (NIHSS); 5) etiologies of stroke (see above); 6) mean hospital stay; 7) duration of follow-up and 8) outcome: mortality and functional outcome at discharge assessed by mRS score. Poor outcome was defined as an mRS score of 3–6.
This protocol was approved by the Institutional Review Board and written informed consent was obtained from each subject, either directly or from his/her guardians, before study.
Cognitive assessment
Cognitive assessment was performed between December 10, 2013 and the end of 2013 with the Modified Telephone Interview for Cognitive Status (TICS-m) by a trained interviewer over the telephone for each patient. The TICS-m includes following items: [10] 1) name, date, age, phone number (9 points); 2) counting backward (2 points); 3) first, a 10-word list learning exercise and then a delayed recall of that word list (10 points for each); 4) subtractions (5 points); 5) responsive naming (4 points); 6) repetition (2 points); 7) current President and Vice President (4 points); 8) finger tapping (2 points), and 9) word opposites (2 points), all of which are divided into four cognitive domains: 1) orientation; 2) registration, recent memory and delayed recall (memory); 3) attention/calculation; 4) semantic memory, comprehension and repetition (language). The total score is 50 points. Patients with a TICS-m score≤31 were considered a priori to have cognitive impairment [11].
In the Chinese version of the TICS-m, minor modifications are made for some items to make them more suitable for Chinese culture with the permission of the authors of original version [10]. For example, English words in verbal memory tests (item 5) and repetition task (item 8) were not translated into semantically equivalent Chinese ones. And the question for ‘Vice President’s name’ (item 9) was also replaced with ‘Prime Minister’ name.
Statistical analysis
All statistical analyses were performed using SPSS computerized statistics package (version 17.0). Data are expressed as means ± standard deviation (SD) or median or using descriptive statistics. Differences in all variables between the cognitive impairment group and normal cognition group were analyzed using the chi-square test for dichotomous outcome variables and one-way analysis of variance (ANOVA). Forward stepwise multiple logistic regression models for analysis all possible confounding factors in cognitive impairment in long-term follow-up. A value of P<0.05 was considered statistically significant.
Results
A total of 350 patients aged ≤45 years and having a first-ever cerebral infarction were studied. The demographic and clinical characteristics of these patients at baseline are described in Table 1. The mean age was 41.0±6.8 years at stroke onset, and 69.7% were men. The mean duration of follow-up was 5.8±3.2 years, whereas 53.4% had the duration of follow-up of ≥5 years.
Table 1.
Demographic and clinical characteristics and post-stroke treatments.
| Variables | Cognitive impairment (n=138) | Normal cognition (n=212) | P |
|---|---|---|---|
| Demographic data | |||
| Age (mean ±SD, years) | 42.1±7.6 | 40.6±6.8 | 0.038 |
| Age groups | 0.741 | ||
| 18–35 years | 33 (22.2) | 51 (24.1) | |
| 36–45 years | 105 (77.8) | 161 (75.9) | |
| Gender (Male) | 102 (73.9) | 142 (67.0) | 0.628 |
| Education years (mean ±SD) | 10.3±3.8 | 11.0±3.2 | 0.512 |
| NIHSS score on admission | 8.6±2.5 | 4.1±1.9 | <0.001 |
| Vascular risk factors | |||
| Obesity | 36 (26.1) | 37 (17.5) | 0.142 |
| Migraine | 3 (2.2) | 5 (2.4) | 1.0 |
| Arterial hypertension | 76 (55.1) | 79 (37.3) | 0.218 |
| Diabetes mellitus | 16 (11.6) | 15 (7.1) | 0.019 |
| Atrial fibrillation | 10 (7.2) | 8 (3.8) | <0.001 |
| Nonischemic cardiopathy | 2 (1.4) | 1 (0.5) | 0.817 |
| Coronary arterial disease | 7 (5.1) | 11 (5.2) | 1.000 |
| Mechanical/biological | 5 (3.6) | 3 (2.2) | 0.722 |
| Valvular replacement | |||
| Dyslipidemia | 7 (5.1) | 11 (5.2) | 1.000 |
| Peripheral arterial disease | 5 (3.6) | 7 (3.3) | 0.852 |
| Alcohol abuse | 60 (43.5) | 89 (42.0) | 0.894 |
| Current smoking | 77 (55.8) | 140 (66.0) | 0.615 |
| Other drug abuses | 3 (2.2) | 3 (1.4) | 0.461 |
| Active malignancy | 2 (1.4) | 1 (0.5) | 0.117 |
| Homocysteine | 3 (2.2) | 4 (1.9) | 0.855 |
| Current puerperium | 2 (1.4) | 2 (0.9) | 0.325 |
| Etiological stroke subtype | 0.011 | ||
| LAA | 63 (45.7) | 20 (9.4) | <0.001 |
| SAO | 15 (10.9) | 17 (8.0) | 0.251 |
| CE | 11 (8.0) | 18 (8.5) | 0.915 |
| OD | 24 (17.4) | 29 (13.7) | 0.323 |
| UND | 25 (18.1) | 128 (60.4) | <0.001 |
| Sites of stroke | |||
| Left anterior circulation | 71 (51.4) | 81 (38.2) | 0.010 |
| Right anterior circulation | 50 (36.2) | 98 (46.2) | 0.283 |
| Posterior circulation | 17 (12.3) | 33 (15.6) | 0.525 |
| Acute treatment | |||
| Intravenous alteplase | 37 (26.8) | 45 (21.2) | 0.211 |
| Follow-up duration | 0.183 | ||
| <5 years | 79 (57.2) | 84 (39.6) | |
| ≥5 years | 59 (42.8) | 128 (60.3) | |
| Post-stroke treatment | |||
| Statin treatment | 62 (45.0) | 74 (34.9) | 0.553 |
| Antiplatelet treatment | 75 (54.3) | 81 (38.2) | 0.069 |
| Anticoagulation treatment | 9 (6.5) | 12 (5.7) | 0.726 |
| In-hospital outcome | |||
| Hospital stay (days) | 10.2±2.5 | 8.3±2.4 | 0.124 |
| mRS >2 at discharge (%) | 87 (63.0) | 52 (24.5) | <0.001 |
| Stroke recurrence (%) | 49 (35.5) | 40 (18.9) | 0.006 |
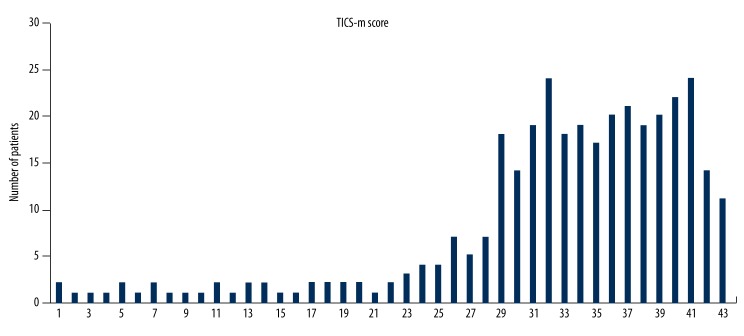
The distribution of TICS-m scores at follow-up is shown in Figure 2. About 39.4% of patients were diagnosed with cognitive impairment. The relationships between baseline prognostic factors and cognitive impairment were assessed in univariate analysis (Table 1). Advanced age, stroke severity on admission, a history of AF, and functional outcome at discharge (mRS >2), left anterior circulation syndrome, stroke recurrence and LAA, UND subtype of TOAST were significantly associated with the cognitive impairment at follow-up. In multiple variable analysis (Table 2), stroke severity on admission, functional outcome at discharge (mRS >2), left anterior circulation syndrome and stroke recurrence remained significant for subsequent cognitive impairment.
Figure 2.
Distribution of scores of the modified Telephone Instrument for Cognitive Status (TICS-m).
Table 2.
Logistic regression analysis of cognitive impairment in long-term follow-up: multivariate analysis of baseline characteristics, stroke subtype and neurological status.
| Variables | OR | P |
|---|---|---|
| NIHSS score on admission | 3.44 (1.47–8.03) | 0.004 |
| Stroke recurrence | 0.09 (0.01–0.63) | 0.016 |
| Left anterior circulation syndrome | 9.69 (2.56–36.72) | 0.001 |
| mRS >2 at discharge | 0.49 (0.41–0.60) | <0.001 |
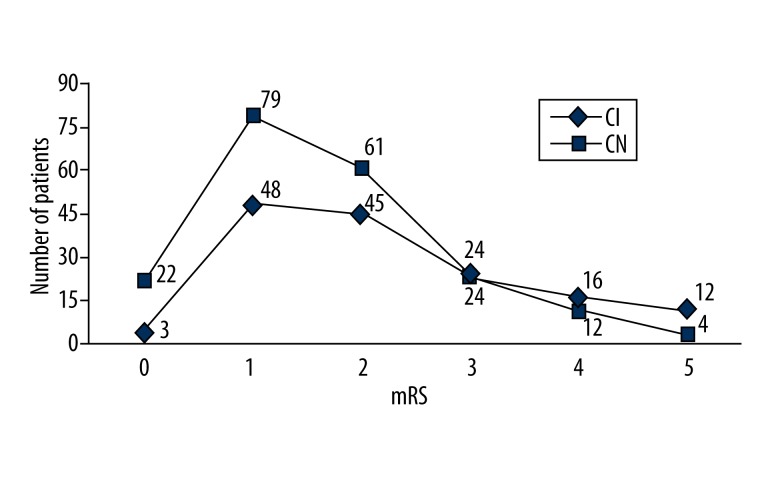
At follow-up, 92/350 subjects (26.3%) had an mRS >2. This represented a reduction from 39.7% (139/350) at the time of discharge. Post-stroke cognition was also related to mRS at follow-up (r=−0.563, P<0.001; Figure 3). Moreover, stroke severity at follow-up (mRS >2) was also significantly associated with post-stroke cognitive impairment (χ2=16.288, P<0.001).
Figure 3.
Relationship between post-stroke cognition and mRS at follow up. CI, cognitive impairment; CN, cognition normal.
Discussion
On the basis of a large hospital-based cohort, our study indicated that post-stroke cognitive impairment (PSCI) was related to the stroke severity on admission, left anterior circulation syndrome, stroke recurrence and the functional outcome, in both unadjusted and adjusted analyses.
Several studies, including a systematic review [4], have reported the associations of PSCI with functional outcome [6], stroke severity [12–15], and total anterior circulation syndrome [6], which were confirmed in the present study. Nevertheless, this study aimed to evaluate the long term cognition after stroke in young adults and the influence of mRS score on the long term cognition. Although available findings support a relationship between PSCI and functional outcome [14,16–19], limited information is available on the relationship of short term cognition with functional outcome at discharge or at 3 months after stroke and the information is particularly limited in young stroke. Notwithstanding, evaluation at discharge or at 3 months is a significant limitation for a midterm outcome evaluation, and long term cognition is a decisive prognostic factor. Our study confirmed the strong relationship between PSCI and mRS, suggesting that interventions attenuate dependency, both in acute stroke and post stroke, and reduce subsequent cognitive decline. Moreover, young post-stroke patients were recruited as a representative control group for neuropsychological examinations.
Several studies have demonstrated the association between subacute PSCI and age [12–14,16,20,21]. In our longer follow-up, age was not associated with a poor cognition state in young patients with ischemic stroke. Longer time interval is associated with an incident comorbidity that may interactively affect the cognitive performance [11,14,22]. Another explanation is the role of vascular lesions, and functional outcomes might be outstanding than neurodegenerative pathology in ischemic stroke patients.
The relationship of cognition with AF, TOAST and diabetes was less consistent across the TICS-m cognition measure. Several studies, albeit small, have reported the association of cognitive impairment with AF [13,16,20], diabetes [12,15], and hippocampal atrophy [23]. Others studies also reveal an association with female gender [16,22], prior stroke [14,15,24], hyperlipidemia [25,26], and alcohol intake [20]. These factors were not risk factors of cognitive impairment in this study, because the risk factors were controlled in the long-term follow-up. Although the subtype of TOAST of ischemic stroke is independently associated with the survival and functional outcome [27,28], an independent association between TOAST and subsequent cognitive impairment was not identified in the present study. In one study, the subtype of CE was found in up to 17% of patients and the subtype of UND was 36% [29]. However, in the present study, UND accounted for 43.7%, followed by LAA (23.8%), CE (8.3%), SAO (9.1%), and OD (15.1%). This may be ascribed to transesophageal echocardiography or transcranial Doppler not being performed which could have resulted in potential mechanisms, such as patent foramen ovale, not being identified.
Our study had several limitations. First, it was not a community-based study, and, therefore, our patients may not represent the young survivors with ischemic stroke. However, the age and sex standardized prevalence of stroke in our region is identical to that in China [14,20]. We, therefore, speculate that our cohort has a good external validity and our population is representative to the young Chinese population with stroke. Second, the cognition was screened by telephone, rather than extensive neuropsychological testing, and the telephone assessment of cognition will be affected by some factors such as hearing loss and whether the participant or a caregiver delivered the responses. However, TICS-m includes the assessment of multiple cognitive domains covering memory, orientation, attention and language. Importantly, the prevalence of cognitive impairment in our study is comparable to that in other similar studies. Third, our study did not include employability and quality of life as longer standing important factors of cognition in the young stroke. As a retrospective study, our data collection is not perfect. But, we will consider these two factors in the future prospective studies.
Conclusions
In young patients with ischemic stroke, long-term PSCI is common and associated with stroke severity on admission, left anterior circulation syndrome, stroke recurrence, and functional outcome. These factors are useful in clinical practice to identify subjects at a high risk for PSCI.
Footnotes
Source of support: This work was supported by Science and Technology Program of Beijing, China (D111107003111009)
References
- 1.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70:713–21. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 2.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195–203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, Kappelle LJ, Biller J, et al. Ischemic stroke in young adults. Experience in 329 patients enrolled in the Iowa Registry of stroke in young adults. Arch Neurol. 1995;52:491–95. doi: 10.1001/archneur.1995.00540290081021. [DOI] [PubMed] [Google Scholar]
- 4.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–18. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 5.Schaapsmeerders P, Maaijwee NA, van Dijk EJ, et al. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke. 2013;44:1621–28. doi: 10.1161/STROKEAHA.111.000792. [DOI] [PubMed] [Google Scholar]
- 6.Ankolekar S, Renton C, Sare G, et al. ENOS Trial Investigators: Relationship between poststroke cognition, baseline factors, and functional outcome: data from “efficacy of nitric oxide in stroke” trial. J Stroke Cerebrovasc Dis. 2014;23:1821–29. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Cao M, Ferrari M, Patella R, et al. Neuropsychological findings in young-adult stroke patients. Arch Clin Neuropsychol. 2007;22:133–42. doi: 10.1016/j.acn.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Malm J, Kristensen B, Karlsson T, et al. Cognitive impairment in young adults with infratentorial infarcts. Neurology. 1998;51:433–40. doi: 10.1212/wnl.51.2.433. [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Brandt J, Welsh KA, Breitner JC, et al. Hereditary influences on cognitive functioning in older men. A study of 4000 twin pairs. Arch Neurol. 1993;50:599–603. doi: 10.1001/archneur.1993.00540060039014. [DOI] [PubMed] [Google Scholar]
- 11.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18:318–24. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- 12.Censori B, Manara O, Agostinis C, et al. Dementia after first stroke. Stroke. 1996;27:1205–10. doi: 10.1161/01.str.27.7.1205. [DOI] [PubMed] [Google Scholar]
- 13.Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, et al. Poststroke dementia: clinical features and risk factors. Stroke. 2000;31:1494–501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- 14.Lin JH, Lin RT, Tai CT, et al. Prediction of poststroke dementia. Neurology. 2003;61:343–48. doi: 10.1212/01.wnl.0000078891.27052.10. [DOI] [PubMed] [Google Scholar]
- 15.Henon H, Durieu I, Guerouaou D, et al. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–22. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- 16.Tamam B, Tasdemir N, Tamam Y. The prevalence of dementia three months after stroke and its risk factors. Turk Psikiyatri Derg. 2008;19:46–56. [PubMed] [Google Scholar]
- 17.Kauhanen M, Korpelainen JT, Hiltunen P, et al. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30:1875–80. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 18.Tatemichi TK, Desmond DW, Stern Y, et al. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–7. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mok VC, Wong A, Lam WW, et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75:560–66. doi: 10.1136/jnnp.2003.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou DH, Wang JY, Li J, et al. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004;251:421–27. doi: 10.1007/s00415-004-0337-z. [DOI] [PubMed] [Google Scholar]
- 21.Rasquin SM, Verhey FR, van Oostenbrugge RJ, et al. Demographic and CT scan features related to cognitive impairment in the first year after stroke. J Neurol Neurosurg Psychiatry. 2004;75:1562–67. doi: 10.1136/jnnp.2003.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang WK, Chan SS, Chiu HF, et al. Frequency and determinants of poststroke dementia in Chinese. Stroke. 2004;35:930–35. doi: 10.1161/01.STR.0000119752.74880.5B. [DOI] [PubMed] [Google Scholar]
- 23.Zimny A, Bladowska J, Neska M. Quantitative MR evaluation of atrophy, as well as perfusion and diffusion alterations within hippocampi in patients with Alzheimer,s disease and mild cognitive impairment. Med Sci Monit. 2013;19:86–94. doi: 10.12659/MSM.883757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohjasvaara T, Erkinjuntti T, Ylikoski R, et al. Clinical determinants of poststroke dementia. Stroke. 1998;29:75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- 25.Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 26.Dufouil C, Richard F, Fievet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–38. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 27.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–66. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 28.Petty GW, Brown RD, Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–68. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 29.Varona JF, Guerra JM, Bermejo F, et al. Causes of ischemic stroke in young adults, and evolution of the etiological diagnosis over the long term. Eur Neurol. 2007;57:212–18. doi: 10.1159/000099161. [DOI] [PubMed] [Google Scholar]