Abstract
Objective:
Carotid–femoral pulse-wave velocity (PWV) is a measure of aortic stiffness that is strongly associated with increased risk of cardiovascular morbidity and mortality. The aim of the current study was to identify the molecular markers and the pathways involved in differences in PWV in women, in order to further understand the regulation of arterial stiffening.
Methods:
A total of 280 known metabolites were measured in 1797 female twins (age range: 18–84 years) not on any antihypertensive medication. Metabolites associated with PWV (after adjustment for age, BMI, metabolite batch, and family relatedness) were entered into a backward linear regression. Transcriptomic analyses were further performed on the top compounds identified.
Results:
Twelve metabolites were associated with PWV (P < 1.8 × 10−4). One of the most strongly associated metabolites was uridine, which was not associated with blood pressure (BP) and traditional risk factors but correlated significantly with the gene-expression levels of the purinergic receptor P2RY2 (Beta = −0.010, SE = 0.003, P = 0.007), suggesting that it may play a role in regulating endothelial nitric oxide synthase phosphorylation. On the other hand, phenylacetylglutamine was strongly associated with both PWV and BP.
Conclusion:
Circulating levels of uridine, phenylacetylglutamine, and serine appear strongly correlated with PWV in women.
Keywords: biomarkers, metabolomics, pulse-wave velocity
INTRODUCTION
Carotid–femoral pulse-wave velocity (PWV), a measure of large artery stiffness, is a well known independent predictor of cardiovascular morbidity and mortality [1–4], and it is considered an integrative measure of the impact of cardiovascular risk factors [5]. Though arterial stiffening has been associated with ageing [6], hypertension [7], diabetes mellitus [8], hypercholesterolaemia [9,10], and chronic kidney diseases [11], its physiopathology is still not fully understood.
Recent advances in metabolomics have allowed for high-throughput assay of an extensive set of small molecules in a number of biological fluids. Low-molecular weight metabolites represent the intermediates and end-products of metabolic pathways that reflect physiological functions and, thus, may mirror the early stages of a pathological state [12].
A small study followed longitudinally 174 individuals and found that plasma fatty acid composition (assessing levels of 10 fatty acids) correlates with both PWV and mortality [13]. Full metabolomic profiling regarding PWV has not yet been attempted.
In this study, we performed metabolomic screening in a large cohort of women from TwinsUK to identify the novel metabolites that associate with PWV. We further explored the relationship between metabolites associated with PWV and gene-expression data to further understand the molecular mechanisms underlying arterial stiffening. We also explored the association of the identified metabolites with the Framingham 10-year cardiovascular risk assessment [14].
MATERIALS AND METHODS
Study population
The study participants were twins enrolled in the TwinsUK Registry, a national register of adult twins recruited as volunteers without selecting for any particular disease or trait [15]. All recruited twins were of the same sex. In this study, we analysed data from 1797 female twins who had complete data for body composition and metabolomics profiling.
The study was approved by the St. Thomas’ Hospital Research Ethics Committee, and all twins provided informed written consent.
Pulse-wave velocity measurement
Carotid–femoral PWV was calculated from the sequential recordings of carotid and femoral artery pressure waveforms using the same SphygmoCor device and applanation tonometry. Difference in the time of pulse arrival from the R-wave of the electrocardiogram between the two sites was taken as the transit time, and the difference in path length was estimated using surface measurements with a flexible tape measure between the sternal notch and the point of applanation at the femoral artery as previously described. PWV is determined by dividing the distance by transit time. Coefficient of variation between operators was less than 10% [5]. Measurements were made in triplicate, and mean values were used for analysis. PWV measurements were available in 1797 women.
Metabolomics measurements
Nontargeted metabolite detection and quantification was conducted by the metabolomics provider Metabolon, Inc. (Durham, North Carolina, USA) on fasting blood samples, as described previously [16]. In this study, we analysed 280 structurally named biochemicals (known metabolites) categorized into the following broad categories – amino acids, acylcarnitines, lysophospholipids, carbohydrates, vitamins, lipids, nucleotides, peptides, xenobiotics, and steroids.
Muther expression data
The Muther gene-expression dataset consists of 825 abdominal fat samples. Gene expression was analysed with the Illumina Human HT-12 V3 (Illumina Inc., San Diego, California, USA) [17], 586 individuals entered the metabolite association analysis.
Statistical analysis
Statistical analysis was carried out using Stata version 11 (Stata Corp., College Station, Texas, USA). We inverse normalized the metabolite data, as the metabolite concentrations were not normally distributed. To avoid spurious false-positive associations because of small sample size, we excluded metabolic traits with more than 20% missing values. We imputed the missing values using the minimum run day measures.
We looked for the metabolites associated with PWV by running random intercept linear regression adjusting for age, BMI, metabolite batch, and family relatedness. We corrected for multiple comparisons using Bonferroni correction, thus giving a significant threshold of P = 1.8 × 10−4 (0.05/280 metabolites). We then used a stepwise backward regression model to identify a set of metabolites that were significantly associated with each phenotype using P less than 0.01 as cut-off threshold. As metabolites in their nature can be affected by many factors, in particular dietary factors [18], we run random intercept linear regressions to test the effect of fruit and vegetable intake and alcohol intake on the association between the metabolites and PWV.
Associations of metabolites with gene-expression levels in fat were tested using random intercept linear regression after adjusting for age, BMI, metabolite batch, expression batch, and family relatedness.
Finally, we explored the association of selected metabolites with Framingham 10-year cardiovascular risk [14] by running random intercept logistic regression adjusting for age, BMI, metabolite batch, and family relatedness.
RESULTS
The descriptive characteristics of the study participants are shown in Table 1. After adjustment for covariates, we identified 12 metabolites, whose levels significantly correlated with PWV (Table 2). We then proceeded to analyse, in a multivariate model, which of these metabolites contributed independently and identified only three metabolites: phenylacetylglutamine, serine, and uridine (Table 3). The proportion of the variance explained by the circulating levels of these three compounds is R2 = 30%. Of the three metabolites, only phenylacetylglutamine was associated with both SBP and DBP [SBP: −1.6 (−2.36; −0.84), P = 4.1 × 10−5; DBP: −0.88 (−1.33; −0.44), P = 8.8 × 10−5]; however, the metabolite–PWV association remained significant even after adjusting for mean arterial pressure (MAP) in the linear model and after adjusting for dietary factors (fruit and vegetable intake and alcohol intake). Published studies have shown that PWV can predict cardiovascular risk that is not accounted by the traditional factors included in the Framingham risk score [19]. We therefore proceeded to assess whether these three metabolites were associated with the Framingham risk score. We find that phenylacetylglutamine is associated with the Framingham cardiovascular risk score [Beta = −0.04, standard error (SE) = 0.01, P = 0.004]. However, there was no association with both uridine and serine and the Framingham cardiovascular risk score (uridine: Beta = −0.003, SE = 0.01, P = 0.8; serine: Beta = −0.002, SE = 0.01, P = 0.85). Also, none of the three metabolites were associated with either total or HDL cholesterol. This suggests that some of the molecular pathways contributing to PWV are independent of the traditional cardiovascular disease risk factors measured by the Framingham score (Fig. 1).
TABLE 1.
Demographic characteristics of the study population
| Phenotype | TwinsUK |
| n | 1797 |
| Male: female | 0 : 1797 |
| Monozygotic twin:dizygotic twin:singletons | 812 : 860 : 125 |
| Age (years) | 57.93 (9.17) |
| BMI (kg/m2) | 26.33 (4.80) |
| DBP (mmHg) | 78.39 (9.48) |
| PWV (m/s) | 9.33 (1.95) |
| SBP (mmHg) | 127.03 (16.13) |
Values are given as mean (SD). PWV, pulse-wave velocity.
TABLE 2.
List of metabolites significantly associated with pulse-wave velocity after adjusting for age, BMI, experimental batch, family relatedness, and multiple testing
| Metabolite | Super-p | Sub-p | Beta (95% CI) | P |
| Methionine | a-a | Cysteine, methionine, SAM, taurine metabolism | −0.24 (−0.34; −0.15) | 1.33 × 10−6 |
| Glutamine | a-a | Glutamate metabolism | −0.22 (−0.32; −0.11) | 6.47 × 10−5 |
| Glycine | a-a | Glycine, serine and threonine metabolism | −0.23 (−0.33; −0.14) | 2.62 × 10−6 |
| Serine | a-a | Glycine, serine and threonine metabolism | −0.26 (−0.36; −0.16) | 1.86 × 10−7 |
| 3-Phenylpropionate (hydrocinnamate) | a-a | Phenylalanine and tyrosine metabolism | −0.18 (−0.27; −0.09) | 6.69 × 10−5 |
| Phenylacetylglutamine | a-a | Phenylalanine and tyrosine metabolism | −0.17 (−0.26; −0.08) | 1.37 × 10−4 |
| Indolepropionate | a-a | Tryptophan metabolism | −0.18 (−0.27; −0.09) | 1.64 × 10−4 |
| Trans-4-hydroxyproline | a-a | Urea cycle; arginine and proline metabolism | −0.19 (−0.28; −0.1) | 6.66 × 10−5 |
| Urea | a-a | Urea cycle; arginine and proline metabolism | −0.21 (−0.31; −0.11) | 8.47 × 10−5 |
| Glycerate | ch | Glycolysis, gluconeogenesis, pyruvate metabolism | −0.19 (−0.29; −0.09) | 1.28 × 10−4 |
| Threonate | c and v | Ascorbate and aldarate metabolism | −0.26 (−0.35; −0.16) | 1.17 × 10−7 |
| Uridine | n | Pyrimidine metabolism, uracil containing | −0.26 (−0.34; −0.17) | 9.03 × 10−9 |
a-a, amino-acid; c and v, cofactor and vitamin; ch, carbohydrate; CI, confidence interval; n, nucleotide; sub-p, sub pathway; super-p, super pathway.
TABLE 3.
List of metabolites significantly associated with pulse-wave velocity in the stepwise backward regression
| Metabolite | Super-p | Sub-p | Beta (95% CI) | P |
| Phenylacetylglutamine | a-a | Phenylalanine and tyrosine metabolism | −0.13 (−0.22–0.05) | 2.20 × 10−3 |
| Serine | a-a | Glycine, serine and threonine metabolism | −0.17 (−0.28–0.07) | 1.20 × 10−3 |
| Uridine | n | Pyrimidine metabolism, uracil containing | −0.18 (−0.28–0.09) | 1.00 × 10−4 |
a-a, amino-acid; CI, confidence interval; n, nucleotide; sub-p, sub pathway; super-p, super pathway.
FIGURE 1.
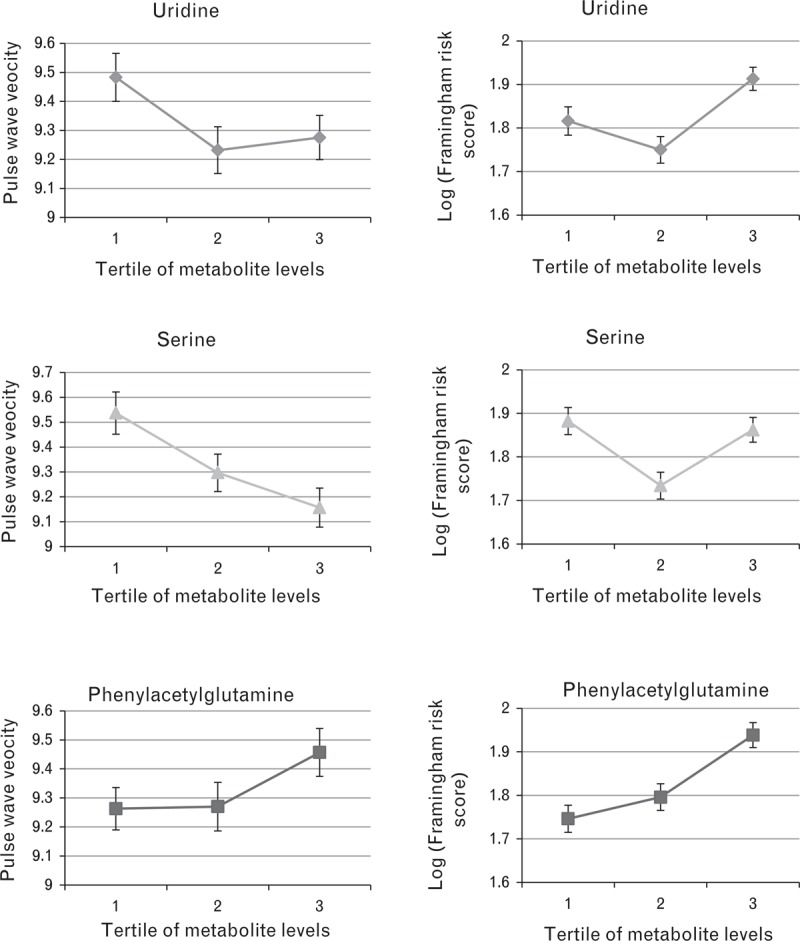
Metabolite associations with PWV and cardiovascular risk as measured by the Framingham risk scores. Mean PWV and log Framingham risk scores (adjusted for age, BMI and batch) are shown by tertiles of selected metabolites. PWV, pulse-wave velocity.
Uridine
Uridine is associated with lower PWV in our data (Table 2). When we tested for correlations between uridine and gene expression, we found that none of the expression probes passed Bonferroni correction for uridine. However, circulating uridine levels were nominally associated with the gene-expression levels in fat of P2RY2 (Beta = −0.010, SE = 0.003, P = 0.007), suggesting that it may play a role in regulating endothelial nitric oxide synthase (eNOS) phosphorylation which may in turn influence arterial stiffness. This association may be mediated through an effect on endothelial function independent of the classical risk factors. Uridine is known to be an agonist of P2 receptors, particularly the P2Y subclass which consists of eight known human P2Y receptors (1, 2, 4, 6, and 11–14). This is relevant as P2Y1, P2Y2, and possibly P2Y4 are the purinergic receptors involved in eNOS phosphorylation during endothelial activation [20].
Uridine is a nucleotide base used as a dietary supplement for increasing the synthesis of cellular membranes and for other neurological properties. Uridine is able to exert an acute cardioprotective effect against myocardial ischaemia when preloaded, which is abolished by blocking potassium channels on the mitochondria (with 5-hydroxydecanoate); it appears that uridine preloading preserves the levels of energy metabolites (ATP, creatine phosphate, and uridine) and subsequently reduced lipid peroxidation [21]. The nominal association with expression levels of a purinergic receptor suggests that uridine may exert its role on PWV via endothelial dysfunction [22].
Serine
Serine is one of the naturally occurring amino acids and it is synthesized in the body from other metabolites. It participates in the biosynthesis of purines and pyrimidines, and is also the precursor to numerous other metabolites, including sphingolipids and folate, the principal donor of one-carbon fragments in biosynthesis. In our data, we find that circulating levels of serine are significantly associated with lower PWV and with expression levels of a probe on the cystatin-like 1 (CSTL1) gene on chromosome 20 (Beta = 0.09, SE = 0.02, P = 4.26 × 10−8). The cystatin locus on chromosome 20 contains the majority of the type 2 cystatin genes and pseudogenes, and has been associated with cerebral haemorrhage and cerebritis [23].
Phenylacetylglutamine
Phenylacetylglutamine is a major nitrogenous metabolite that accumulates in uraemia [24]. It is the glutamine conjugate of phenylacetic acid produced in humans and is also a well known gut microbial cometabolite whose levels are significantly different between Asian and North American individuals [25]. Although Holmes et al.[25] did not measure the correlation between phenylacetylglutamine and blood pressure (BP), they did test that between BP and hippurate (another microbial cometabolite significantly different between Asians and Caucasians, positively correlated with phenylacetylglutamine) and reported a negative correlation with DBP. Such published results are consistent with our findings, that is, a negative correlation between phenylacetylglutamine and SBP, cardiovascular risk and PWV. In our data, we also find a weak negative correlation between hippurate and SBP (Beta = −0.97, SE = 0.39, P < 0.012).
Phenylacetylglutamine levels in our data are correlated with adipocyte gene-expression levels of the cell death activator CIDE (CIDEC: Beta = 0.05, SE = 0.01, P = 6.97 × 10−8). This gene is regulated by insulin and its expression is positively correlated with insulin sensitivity [26]. Mutations in this gene may contribute to insulin-resistant diabetes [27]. CIDEC plays an important role in energy metabolism and lipid droplet formation [28], and its hepatic expression is increased in obese humans and is downregulated by marked weight loss [29].
DISCUSSION
Using metabolomic profiling, we searched for the molecular markers and the mechanisms involved in differences in PWV in women in order to investigate the regulation of arterial stiffening. We identified 12 blood metabolites, mainly amino acids, with high statistical significance associated with PWV. We also report three metabolites amongst those identified to be independently associated with PWV: uridine, serine, and phenylacetylglutamine achieving an R2 of 30%. Of the three metabolites identified, the one showing the strongest association is uridine. Uridine triphosphate (UTP, which unfortunately is not measured by the current metabolomic panel) stimulates vasodilatation, automaticity in ventricular myocytes, and release of tissue-plasminogen activator, indicating that UTP may be important in cardiac regulation [30]. Uridine levels may be reflecting lower UTP levels or they may be cardioprotective via some other mechanism. Interestingly, however, uridine levels are not correlated with Framingham risk. We also report that circulating levels of serine are associated with PWV but not with Framingham risk. Our data indicate that the mechanisms underlying the association of PWV with uridine and serine are likely to be independent of the traditional CVD risk factors. Recent studies have shown that PWV improves cardiovascular event prediction [31,32]. Our data are, therefore, consistent and suggest that there are molecular mechanisms related to arterial stiffening and cardiovascular mortality that are not fully encompassed by the traditional cardiovascular risk factors.
We also report a novel association between phenylacetylglutamine and both Framingham risk scores and PWV. The negative correlation with phenylacetylglutamine is consistent with the previous reports on gut-microbiome-derived metabolites [25] and BP. We find that this metabolite is strongly associated with the gene-expression levels of CIDEC, a gene related to insulin resistance, suggesting that this metabolite may be related to this pathway. Phenylacetylglutamine is related to the gut microbiome composition and a number of reports have linked the function of gut bacteria to insulin resistance. Therefore, it is possible that CIDEC may be linking these two pathways and suggests a new mechanism linking insulin resistance and gut microbiome in BP regulation.
Our study, therefore, on one hand is consistent with the current knowledge of insulin resistance and endothelial activation mechanisms in determining arterial stiffening. On the other hand, our data suggest that some of these mechanisms may be related to the mechanisms that deserve further exploration, for example, those that link with the gut microbiome. These data also suggest that serine and uridine levels are linked to arterial stiffening, possibly via endothelial dysfunction but in a way that is not reflected directly on the traditional CVD risk factors.
The current study has several strengths. It used a nontargeted metabolomic approach that identifies a wide range of biochemicals in addition to lipids. TwinsUK is a very large and accurately phenotyped population, and this allowed us to explore the potential confounders (e.g. diet and MAP). The availability of expression and genetic data enabled us to explore some of the biological implications of the three metabolites identified.
We note some study limitations. Our study sample consisted of women only, and some metabolites could be influenced by sex-specific hormones. In addition, previous studies suggested that traditional risk factors [33] are less reliable in predicting risk in women than in men. We have only tested individuals of European descent, and the levels of one of the metabolites identified (phenylacetylglutamine) are known to vary between Asians and Caucasians. More importantly, because of the novelty of the phenotypes, we could not validate our results in an independent cohort. The cross-sectional nature of our data does not allow us to draw conclusions as to whether the metabolites identified are causative of arterial stiffness or merely correlated with it. However, our results highlight the relevance of investigating the molecular pathways related to PWV as this may lead to the identification of molecular mechanisms involved in cardiovascular diseases, in particular linked to endothelial activation, that act through other pathways. The identification of key metabolites related to PWV should encourage further research into this field.
ACKNOWLEDGEMENTS
The authors wish to express their appreciation to all the study participants of the TwinsUK study.
Sources of funding This work was supported by the EU Framework Programme 7 small-scale focused research collaborative project EurHEALTHAging 277849; Metabolomic analysis was funded by Pfizer; TwinsUK was funded by the Wellcome Trust; European Community's Seventh Framework Programme (FP7/2007–2013). This study also receives support from the National Institute for Health Research (NIHR) Clinical Research Facility at Guy's and St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. BHF Special Project Grant SP/08/005/25115 was given to S.P.
Conflicts of interest
R.P.M. is an employee of Metabolon, Inc.
M.J.B. is a full-time employee of Pfizer; J.T. and M.J.B. are share holders of Pfizer.
Reviewer's Summary Evaluation
Reviewer 1
This original study proposed by Menni et al. points to molecular pathways and biological processes involved in arterial stiffness in women. The identification of biological mechanisms affecting arterial viscoelastic properties paves the way for eventual specific treatment.
Footnotes
Abbreviations: BP, blood pressure; CIDEC, cell death activator CIDE; CSTL1, cystatin-like 1; eNOS, endothelial nitric oxide synthase; MAP, mean arterial pressure; PWV, pulse-wave velocity; SE, standard error; UTP, uridine triphosphate
REFERENCES
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 2.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006; 113:664–670. [DOI] [PubMed] [Google Scholar]
- 3.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension 2010; 55:9–14. [DOI] [PubMed] [Google Scholar]
- 4.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012; 60:1455–1469. [DOI] [PubMed] [Google Scholar]
- 5.Cecelja M, Jiang B, McNeill K, Kato B, Ritter J, Spector T, et al. Increased wave reflection rather than central arterial stiffness is the main determinant of raised pulse pressure in women and relates to mismatch in arterial dimensions: a twin study. J Am Coll Cardiol 2009; 54:695–703. [DOI] [PubMed] [Google Scholar]
- 6.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005; 46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 8.Giannattasio C, Failla M, Capra A, Scanziani E, Amigoni M, Boffi L, et al. Increased arterial stiffness in normoglycemic normotensive offspring of type 2 diabetic parents. Hypertension 2008; 51:182–187. [DOI] [PubMed] [Google Scholar]
- 9.Riggio S, Mandraffino G, Sardo MA, Iudicello R, Camarda N, Imbalzano E, et al. Pulse wave velocity and augmentation index, but not intima-media thickness, are early indicators of vascular damage in hypercholesterolemic children. Eur J Clin Invest 2010; 40:250–257. [DOI] [PubMed] [Google Scholar]
- 10.Giannattasio C, Mangoni AA, Failla M, Carugo S, Stella ML, Stefanoni P, et al. Impaired radial artery compliance in normotensive subjects with familial hypercholesterolemia. Atherosclerosis 1996; 124:249–260. [DOI] [PubMed] [Google Scholar]
- 11.Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 2012; 60:1451–1457. [DOI] [PubMed] [Google Scholar]
- 12.Hollywood K, Brison DR, Goodacre R. Metabolomics: current technologies and future trends. Proteomics 2006; 6:4716–4723. [DOI] [PubMed] [Google Scholar]
- 13.Anderson SG, Sanders TA, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009; 53:839–845. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–753. [DOI] [PubMed] [Google Scholar]
- 15.Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: TwinsUK and Healthy Ageing Twin Study. Int J Epidemiol 2013; 42:76–85doi: 10.1093/ije/dyr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C, Kastenmuller G, Petersen AK, Bell JT, Psatha M, Tsai PC, et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol 2013; 42:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet 2012; 44:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menni C, Zhai G, Macgregor A, Prehn C, Romisch-Margl W, Suhre K, et al. Targeted metabolomics profiles are strongly correlated with nutritional patterns in women. Metabolomics 2013; 9:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunder M, Janic M, Kejzar N, Sabovic M. Associations among different functional and structural arterial wall properties and their relations to traditional cardiovascular risk factors in healthy subjects: a cross-sectional study. BMC Cardiovasc Disord 2012; 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 2009; 119:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krylova IB, Bulion VV, Selina EN, Mironova GD, Sapronov NS. Effect of uridine on energy metabolism, LPO, and antioxidant system in the myocardium under conditions of acute coronary insufficiency. Bull Exp Biol Med 2012; 153:644–646. [DOI] [PubMed] [Google Scholar]
- 22.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006; 48:602–608. [DOI] [PubMed] [Google Scholar]
- 23.Levy E, Jaskolski M, Grubb A. The role of cystatin C in cerebral amyloid angiopathy and stroke: cell biology and animal models. Brain Pathol 2006; 16:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman L, Egestad B, Jornvall H, Bergstrom J. Identification and determination of phenylacetylglutamine, a major nitrogenous metabolite in plasma of uremic patients. Clin Nephrol 1989; 32:124–128. [PubMed] [Google Scholar]
- 25.Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008; 453:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M, Nagasawa M, Omae N, Ide T, Akasaka Y, Murakami K. Differential regulation of CIDEA and CIDEC expression by insulin via Akt1/2- and JNK2-dependent pathways in human adipocytes. J Lipid Res 2011; 52:1450–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med 2009; 1:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonezawa T, Kurata R, Kimura M, Inoko H. Which CIDE are you on? Apoptosis and energy metabolism. Mol Biosyst 2011; 7:91–100. [DOI] [PubMed] [Google Scholar]
- 29.Hall AM, Brunt EM, Klein S, Finck BN. Hepatic expression of cell death-inducing DFFA-like effector C in obese subjects is reduced by marked weight loss. Obesity (Silver Spring) 2010; 18:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erlinge D, Harnek J, van Heusden C, Olivecrona G, Jern S, Lazarowski E. Uridine triphosphate (UTP) is released during cardiac ischemia. Int J Cardiol 2005; 100:427–433. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kones R. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des Dev Ther 2011; 5:325–380. [DOI] [PMC free article] [PubMed] [Google Scholar]


