Abstract
The retina is a part of the central nervous system that has organized architecture, with neurons in layers from the photoreceptors, both rods and cones in contact with the retinal pigmented epithelium in the most distant part on the retina considering the direction of light, and the ganglion cells in the most proximal distance. This architecture allows the isolation of the photoreceptor layer by vibratome sectioning. The dissected neural retina of a mouse aged 8 days is flat-embedded in 4% gelatin on top of a slice of 20% gelatin photoreceptor layer facing down. Using a vibratome and a double edged razor blade, the 100 µm thick inner retina is sectioned. This section contains the ganglion cells and the inner layer with notably the bipolar cells. An intermediary section of 15 µm is discarded before 200 µm of the outer retina containing the photoreceptors is recovered. The gelatin is removed by heating at 37 °C. Pieces of outer layer are incubated in 500 µl of Ringer's solution with 2 units of activated papain for 20 min at 37 °C. The reaction is stopped by adding 500 µl 10% fetal calf serum (FCS) in Dulbecco's Modified Eagle Medium (DMEM), then 25 units of DNAse I is added before centrifugation at RT, washed several times to remove serum and the cells are resuspended in 500 µl of DMEM and seeded at 1 x 105 cells/cm2. The cells are grown to 5 days in vitro and their viability scored using live/dead assay. The purity of the culture is first determined by microscopic observation during the experiment. The purity is then validated by seeding and fixing cells on a histological slide and analyzing using a rabbit polyclonal anti-SAG, a photoreceptor marker and mouse monoclonal anti-RHO, a rod photoreceptor specific marker. Alternatively, the photoreceptor layer (97% rods) can be used for gene or protein expression analysis and for transplantation.
Keywords: Neuroscience, Issue 94, Primary photoreceptor cell culture, Inherited retinal degeneration, Rod-derived Cone Viability Factor, S-Antigen, Flat-mounted mouse retina, Transplantation.
Introduction
The retina is an integral part of the central nervous system which has a conserved architecture among vertebrates. The neurons of the neural retina are organized in layers, with the most distant for the incident light, the photoreceptor layer in close contact with the retinal pigmented epithelium (RPE) at the back of the eye. Rod and cone photorecptors are light sensitive cells that rely on opsin sensitive molecules for photon capture. These molecules are enclosed on disk membranes of a cellular structure, located on the outer segment of the photoreceptor which points in the direction of the RPE1. This structure, which is most often affected very early in cases of photoreceptor degenerations, is renewed at the rate of 10% every day. The so called inner layer contains most of the other neurons that compute the signal received from the photoreceptors, the bipolar, amacrine and horizontal cells as well as the ganglion cells. These latter with their axons form a beam which is the optic nerve. This layering is so conserved that biologists have used the term displaced amacrine cells when the cells are found outside the inner plexiform layer2. Layers of neurons are distributed within an armature of radial Müller glial cells. Bipolar cells link photoreceptors to ganglion cells. They are located between the external plexiform layer and the inner plexiform layer. The ganglion cells form the inner plexiform layer in connection with the bipolar cells. The amacrin cells are named as association cells located in the inner plexiform layer between the bipolar cells and ganglion cells. The outer plexiform layer contains horizontal cells. This unique arrangement of neuronal layers of the central nervous system permits the isolation of the photoreceptor layer from the inner cell layer by slicing the flat-mounted retina using a vibratome.
Originally, this technique was used to isolate photoreceptors for transplantation in the eye of the rd1 mouse, a model of human retinitis pigmentosa (RP)3. The rd1 mouse carries a recessive mutation in the Pde6b gene which encodes for the rod-specific phosphodiesterase beta subunit. Recessive mutations of this gene result in RP in humans4. After rod photoreceptors have degenerated, the patient loses night vision, and surprisingly cone photoreceptors, which do not express the mutated gene, degenerate as a second step. Because the cones are required for color vision and visual acuity, the patients become progressively blind and an effective treatment for the disease has not yet developed. By grafting photoreceptor layer from a wild-type mouse the cone degeneration of the host mouse is delayed3,5. The rods lost in the rod-cone degenerative model could not be replaced by a transplant because the synaptic connection between rods and bipolar cells can only be obtained at a specific stage of retinal development, marked by the onset of Nrl expression6. The layer of photoreceptors is introduced by surgery in the sub-retinal space of the rod-less rd1 retina, between the RPE and the outer retina corresponding to only 3% of the remaining photoreceptors, the cones. Two weeks after surgery, 40% of the cones from the animal transplanted with a normal photoreceptor layer survived as compared to the animal transplanted with a normal layer of inner retinal cells, or to the sham-operated animal. The topography of cone survival, spread-out over the entire surface of the mutated retina located at the position of the grafted tissue indicates that the protective effect is due to a diffusible molecule7.
Next, we used a co-culture system as well as conditioned culture media to validate the fact that the protective effect relies on the secretion of a protein by rods8,9. We hypothesize that this protein will be expressed continuously and specifically by rods and that their death during the first phase of the disease will trigger secondary cone degeneration by the loss of a protective signal from rods in a non-cell autonomous manner10. Because of the importance of cone mediated central vision in primates this putative protein will be a highly relevant therapeutic tool for RP. Preserving the cones in RP would theoretically prevent a total of 1.5 million patients worldwide to become blind11. We have used a high content screening approach and a cone-enriched culture model to identify a cDNA encoding Rod-derived Cone Viability Factor (RdCVF) from a retinal cDNA library12. RdCVF is the spliced product of the NXNL1 gene which, interestingly is homologous to the gene encoding for thioredoxin proteins involved in redox homeostasis13. The second spliced product of the gene, RdCVFL is an enzyme that protects its target, the TAU protein against oxidative damage14. Administration of RdCVF prevents the secondary degeneration of cones and the loss of their visual function in a recessive, and dominant model of RP12,15. This demonstrates two important aspects of this innovative therapeutic strategy16. First, it can be applied in most of the RP cases in a gene-independent manner. Second, contrary to the competing factor CNTF, RdCVF survival is associated with the maintenance of visual function17. The absence of functional effect may explain the reason of the absence of clinical benefit of the administration of CNTF to RP patients18. RdCVF is most likely one of the important survival signal between rods and cones since cone rescue in vitro is inhibited by RdCVF immunodepletion12. In addition, the disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress19.
The use of photoreceptor layer is at the origin of identification of RdCVF and of a novel redox signal involved in neurodegenerative diseases20. This manuscript describes the protocol used to isolate and culture cells from the photoreceptor layer to characterize the activity of RdCVF. The photoreceptors can be maintained in culture for 5 to 7 days21. This technique can also be used to study the expression of photoreceptor specific genes.
Protocol
NOTE: The procedure was approved by the ethics committee Darwin from University Pierre and Marie Curie (Ce5/2009/048)
1. Preparation of Gelatin Solution, Instruments, Vibratome, Culture Media and Culture Plate
- Prepare a 20% gelatin solution at least one day before the experiment.
- Under the hood, add 500 µl of gentamycin [10 mg/ml] to a 500 ml bottle containing CO2 independent medium (CO2-i).
- In a separate beaker, add 20 ml of CO2-I (from step 1.1.1) and heat at 95 °C on a heating block with constant stirring for 15 min. Observe a color change to yellow, indicating that the solution is ready for further use.
- Weigh 4 g of gelatin and gradually poor to the solution prepared above (step 1.1.2) with increased stirring and heating at 95 °C for 45 min, to obtain a yellow color solution. Allow the gelatin solution to cool under the hood for approximately 5 min.
- Pour 4 ml of the gelatin solution into a 35 mm diameter culture dishes without introducing air bubbles. Keep the dish at 21 °C for 30 min. Cover the dishes with plastic film, and turn the dishes upside down. Optionally, store the dishes at 4 °C for up to 2 months before use.
- Prepare a 4% gelatin solution.
- Under the hood, add 25 ml of CO2-i medium in a sterile plastic container, and heat at 42 °C on a heating block. Remove the medium from the heating block and gradually add 1 g of gelatin to the hot medium and stir. Quickly return the container on the heating block (42 °C) and keep it warm during the process.
- Setting-up the vibratome apparatus:
- Remove the dishes containing 20% gelatin solution stored at 4 °C. Cut the gelatin with a scalpel. Flip the gelatin slice and stick it on the black support disc of the vibratome using a drop of super glue.
- Break a razor blade into two halves, and insert one half into the vibratome holding receptacle. Insert the black support disc onto the vibratome apparatus. Turn on the black knob to the right side. Fix the holding receptacle on the head of the vibratome. Add sufficient amount of CO2-i medium in the vibratome tank to cover the gelatin block.
- Switch on the vibratome and cut three 100 µm slices of gelatin block. Keep the gelatin block for further use.
- Prepare 40 ml of culture medium:
- Prepare Dulbecco's modified Eagle medium (DMEM) with 10% of fetal calf serum (FCS) under the hood. Prepare 1 mg/ml Poly-D-Lysine using phosphate buffer saline (PBS) pH 7.4.
- In a 96-well plate, add 2 µg/cm2 of Poly-D-Lysine to coat the bottom of the wells. Incubate the plate for 45 min at 37 °C within 5% CO2 incubator. Following incubation, remove PBS and replace with 200 µl / well DMEM and place again at 37 °C in 5% CO2 incubator before using.
2. Dissection of Entire Retina
- Enucleate the mouse:
- Sacrifice the mouse according to the European directive 2010/63: by cervical dislocation. Cut the head with curved-scissors and place it in a 100 mm diameter Petri dish after having disinfected the eye with disinfectant.
- Remove the eye after having detached the optic nerve by sectioning it very carefully using a curved grip. Then place both eyes in a 35 mm Petri dish containing sterile CO2-i medium at 21 °C.
- Removing the retina:
- Make a hole at the level of the eye-limb with the help of a 18 G needle to be able to introduce the scissors into the eye globe. Introduce straight scissors inside the hole and carefully cut the sclera below the iris on all the perimeter of the ocular globe to remove ciliary body.
- Remove the cornea and the lens and keep the posterior chamber of the eye with the retina contiguous to the sclera. With two fine grips, remove the vitreous located inside the posterior chamber without damaging the retina. Perform four radial cuts (retina/sclera) to permit flattening of the retina.
- Upturn the ocular globe and peel it as an “orange”. Detach the retina from the sclera and retinal pigmented epithelium (RPE). Detach the retina still attached at the level of the optic nerve very carefully with fine curved-scissors.
- Detach the remaining vitreous from the periphery toward the centre of the retina with two fine grips. Make sure that all the vitreous is completely removed to allow the flattening of the retina.
- Cut the extremity of a plastic pipette and transfer the retina to a 35 mm diameter Petri dish containing CO2-i medium with the plastic pipette.
3. Sealing of Flat-mounted Retina onto Gelatin Block
Remove 20 - 30 ml of CO2-i medium from the vibratome tank (the gelatin block is free) to permit the sealing of flat-mounted retina.
Transfer the retina to a glass slide using a plastic pipette and add a drop of CO2-i medium before transferring to the gelatin slice with the photoreceptor facing down on the gelatin slice.
Attach the retina to the gelatin block by gently injecting warmed 4% gelatin on one side of the block between the retina and the gelatin block and simultaneously expelling warm gelatin on the other side.
Aspirate 4% gelatin with a pasteur pipette. Wait for 10 min to allow total sealing. Add CO2-i medium to immerse the block and the blade completely.
4. Sectioning the Retina
NOTE: This step is critical to obtain a layer of photoreceptor cells without the other retinal cells (Figure 1).
Starting from the top on the gelatin block, cut 100 µm serial sections to reach the retina. Then cut 100 - 120 µm sections of the inner layer. Depending on the application, this layer may be discarded or stored in liquid nitrogen. Ensure that the speed of the vibratome is very slow (at 1 or 2) during the sectioning of the inner layer or photoreceptors layers.
Perform intermediate 15 µm sections of the retina corresponding to the interface between the inner and the outer retina. Observe this section under a microscope. The absence of blood vessels indicates that the outer retina has been reached.
Cut 200 µm of the outer retina (the photoreceptor layer) with gelatin and transfer it to a 35 mm diameter dish filled with 3 ml of CO2-i medium. Keep it on ice until all the photoreceptor layer sections of all retinas are obtained.
5. Dissociating Photoreceptor Cells
Take the dish(es) containing the photoreceptor layer(s) and put it(them) in an incubator at 37 °C for 10 min.
Using forceps gently separate the photoreceptor layer from the gelatin and transfer it in a new dish filled with 3 ml of Ringer’s solution. Repeat this step (5.2) for each photoreceptor layer preparation.
Incubate 2 units of papain with 25 µl of activator solution (1.1 mM EDTA; 5.5 mM L-cystein; 60 µM β-mercaptoethanol) in a 5 ml polypropylene sterile tube and incubate it 30 min at 37 °C within 5% CO2 incubator. The activation of papain is achieved at 37 °C. During this incubation, cut the photoreceptor layer into 2 mm2 pieces (not too small) and transfer these pieces into a 5 ml tubes.
Rinse the retina twice with 1.5 ml of solution Ringer’s solution followed by gravity sedimentation. Remove all remaining Ringer’s solution from the tube with the specimen.
Add 475 µl of Ringer’s solution to the tube containing activated papain and mix. Add this solution to the tube containing the retina.
Incubate the tube with the retina for 20 min at 37°C within 5% CO2 incubator. Stop the reaction by adding 1 ml of 10% FCS in DMEM. Add 25 U of deoxyribonuclease I (DNAse I) to digested DNA from death cells. Carefully homogenize the cell suspension using a 1 ml-pipette. Spin at 50 x g for 6 min at RT.
Discard the supernatant to remove traces of serum and add DMEM medium with supplements (see table of specific reagents_equipment) to the cell-pellet and resuspend carefully the cell suspension with a 1 ml pipette. Spin at 50 x g for 6 min at RT. Repeat this step (5.7) once more.
6 Culturing Photoreceptor Cells
The photoreceptor layer of a mouse at PN8 contains about 1 to 1.5 million cells using this technique. Seed the photoreceptor cells at 1 x 105 cells/cm2 in a culture plate with culture medium and culture the cells for 5 days at 37 °C within 5% CO2 incubator. On day 5, proceed to immunochemistry and western blotting study following methods provided by the antibody suppliers. NOTE: Steps to stop the culture and preliminary tests are described in reference 21.
Representative Results
Apart from transplantation, the photoreceptor layers have also been used to study cell signaling by seeding cells for making photoreceptor cultures12,22. Additionally, they are used to study gene expression and circadian rhythm23,24. We have used the cell photoreceptor layer from a wild-type mouse at post-natal day 8 to prepare photoreceptor cultures in the absence of FCS and maintained the cells for 5 days at 37 °C in an incubator with 5% CO2 (Figure 1E). The cells were then fixed using 4% paraformadehyde and then proceeded for immunocytochemical analysis using methods provided by the antibody suppliers either with mouse monoclonal anti-RHO (1:250, Millipore Mab5316) or rabbit polyclonal anti-SAG (1:200, a generous gift of Igal Gery and David Hicks). We used anti-RHO and anti-SAG (although anti-SAG label cones and rods) due to the fact that anti-SAG is an earlier marker, than anti-RHO. The proportion of cells positive for the anti-RHO antibody is lower than those positive for the anti-SAG antibodies (Figure 2). This can be explained by the fact that SAG, the rod arrestin is also expressed by cones which make 3% of photoreceptors in the isolated layer, or alternatively by the fact that during post-natal maturation of the retina, the expression of SAG precedes that of RHO25,26.
The photoreceptor layer was also used to monitor the specific expression of genes by photoreceptors of the wild-type retina and brain of animals aged 35 days RNA were prepared using CsCl ultracentrifugation 27 and hybridized to mouse DNA chip Array. The messengers for rhodopsin (Rho), S-arrestin (Sag) and cone-transducin (Gnat2) are expressed specifically in the retina as compared to the brain (Figure 3A). The expression is prominent in the photoreceptor layer (PR) than in the whole retina which encompasses the inner retinal layer showing that these genes are expressed indeed by photoreceptors. For Gnat2, the relative expression as compared to Rho and Sag shows that it is expressed by cones contained within the isolated photoreceptor layer. The expression of recoverin (Rcvrn) in the photoreceptor layer as compared to the whole retina is increased (Figure 3B).
The photoreceptor layer was also used to monitor the expression of RHO and GNAT2 using western blotting following methods provided by the antibody suppliers and to compare to the inner retinal layer (Figure 4). Notice the absence of RHO and GNAT228, the markers of rods and cones respectively in the whole retina and in the retina of rd1 mouse at post-natal day 35.
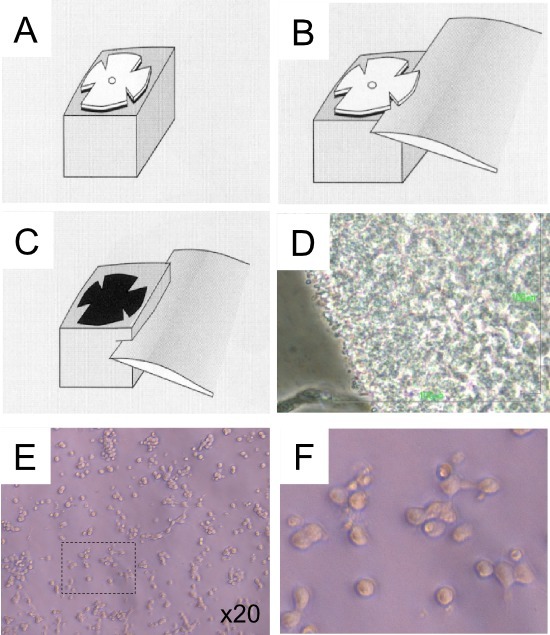 Figure 1. Schematic view of the vibratome sectioning of mouse retina. (A) Installation of the flat-mounted retina with the photoreceptor facing down on the gelatin slice. (B) Section of the inner retina with the vibratome blade. (C) Section of the outer retina added of gelatin. (D) Control of the presence of photoreceptor at the edge of the fragment of retina after the isolation of photoreceptor layer. Scale bar used for green fluorescence is used of a white light microscope. The photoreceptors are located in the edge of the picture. (E) Cultured photoreceptor cells (post-five days). (F) Higher magnification of panel E.
Figure 1. Schematic view of the vibratome sectioning of mouse retina. (A) Installation of the flat-mounted retina with the photoreceptor facing down on the gelatin slice. (B) Section of the inner retina with the vibratome blade. (C) Section of the outer retina added of gelatin. (D) Control of the presence of photoreceptor at the edge of the fragment of retina after the isolation of photoreceptor layer. Scale bar used for green fluorescence is used of a white light microscope. The photoreceptors are located in the edge of the picture. (E) Cultured photoreceptor cells (post-five days). (F) Higher magnification of panel E.
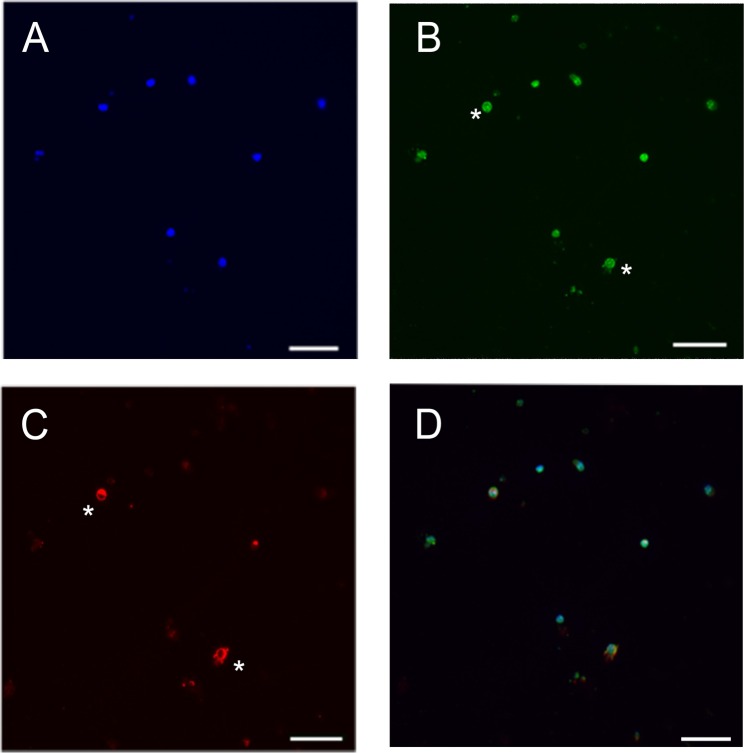 Figure 2. Differential expression of RHO and SAG in mouse photoreceptor culture. (A) Staining of nuclei (blue), (B) SAG staining (green), (C) RHO staining (red). (D) Merged image. Because staining of SAG is expressed earlier than RHO staining, only two cells are double labeled SAG and RHO (see *). Scale bar: 23 µm. Please click here to view a larger version of this figure.
Figure 2. Differential expression of RHO and SAG in mouse photoreceptor culture. (A) Staining of nuclei (blue), (B) SAG staining (green), (C) RHO staining (red). (D) Merged image. Because staining of SAG is expressed earlier than RHO staining, only two cells are double labeled SAG and RHO (see *). Scale bar: 23 µm. Please click here to view a larger version of this figure.
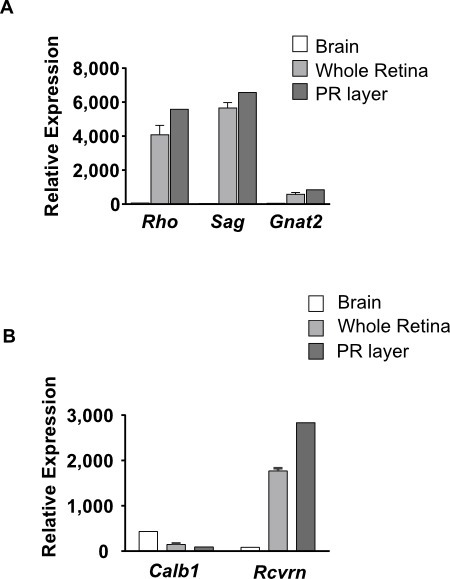 Figure 3. Comparison of the expression of three genes in brain and whole retina and photoreceptors of the wild-type animals aged thirty five days. (A) Rhodopsin (Rho), S-arrestin (Sag) and cone-transducin (Gnat2). (B) Calbindin (Calb1) and Recoverin (Rcvrn). The data are displayed as relative expression. The relative expression is an expression value obtained from microarray data after normaliszation with the software Robust Multi-array Average (RMA) available in the knowledge database KBaSS (http://kbass.institut-vision.org/KBaSS/).
Figure 3. Comparison of the expression of three genes in brain and whole retina and photoreceptors of the wild-type animals aged thirty five days. (A) Rhodopsin (Rho), S-arrestin (Sag) and cone-transducin (Gnat2). (B) Calbindin (Calb1) and Recoverin (Rcvrn). The data are displayed as relative expression. The relative expression is an expression value obtained from microarray data after normaliszation with the software Robust Multi-array Average (RMA) available in the knowledge database KBaSS (http://kbass.institut-vision.org/KBaSS/).
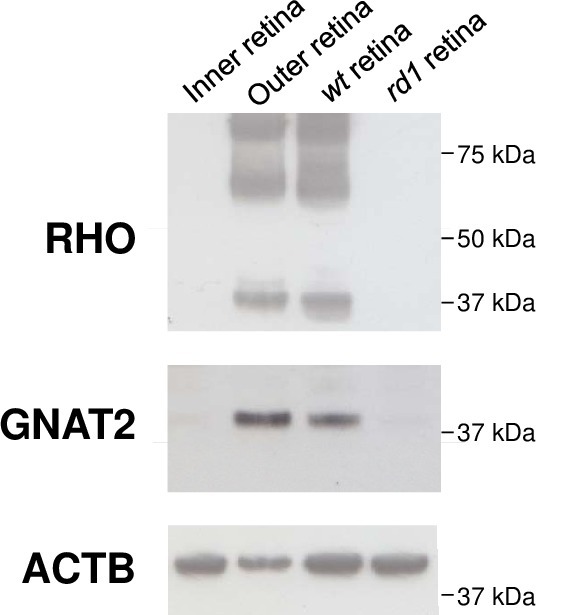 Figure 4. Expression of RHO and GNAT2 in outer retina from a wild-type retina at post-natal day 35. Absence of their expression in the inner retina and in the retina of the rd1 mouse at post-natal day 35. ACTB, beta-actin. Please click here to view a larger version of this figure.
Figure 4. Expression of RHO and GNAT2 in outer retina from a wild-type retina at post-natal day 35. Absence of their expression in the inner retina and in the retina of the rd1 mouse at post-natal day 35. ACTB, beta-actin. Please click here to view a larger version of this figure.
Discussion
The retina is a model organ in biology. Study of the retina led to 6 major discoveries in biology. It is at the origin of the first tumor suppressor gene RB1. It reveals the molecular link between the receptor tyrosine kinases and the MAP kinases through the interaction with Son of sevenless. It was involved in the discovery of PAX6, the first master control gene for organ morphogenesis. It is at the center of the genetic association of Complement factor H (CFH) with age-related macular degeneration (AMD), the first disease susceptibility gene identified by genome wide association screen (GWAS). Finally, it led to the first successful gene therapy for Leber congenital amaurosis, the first corrective gene therapy trial in human of any inherited disease. The structure of this organ is conserved in most vertebrate species. Its accessibility for manipulation in vivo has encouraged early on functional genomics investigations on this integral part of the central nervous system.
We show here how to separate the photoreceptor layer from the inner layer of the retina by vibratome sectioning. This step is critical to obtain pure photoreceptor cultures. Our dissection protocol makes the contamination by RPE cells very unlikely.
One of the major challenges is the flattening of the retina that is necessary to section properly the inner and the outer retina. The sectioning of the retina is best on retina with small diameter as those from rodents and this diameter is a limitation of the technique with current available material.
It is advisable before starting a biologically meaningful experiment, to practice on a sample of the retina. We illustrate the method by representative results obtained from cultured photoreceptor cells, using the material to perform expression study of both mRNAs and proteins. Expression studies can also be performed alternatively on sections obtained by laser capture microdissection, but the cultures are best performed using vibratome sectioning. We could have used the technique of microdissection laser with a complete different strategy. But, to collect the photoreceptor layer with the microdissection apparatus for culture, it would be necessary to avoid fixative and this would complicate the current methodology significantly.
We are developing a protocol aimed at studying the kinetics of gene and protein expression in vibratome sections during the degeneration of photoreceptors in models of retinitis pigmentosa. We believe that the detailed description of the protocol will be useful to researchers in the field of retinal biology, and most particularly for proteomic and metabolomic studies.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Igal Gery and David Hicks for anti-SAG antibodies. Ram Fridlich for reading the manuscript.
References
- Kolb H, Nelson R, Fernandez E, Jones B, editors. The Organization of the Retina and Visual System. Webvision. 2014. [PubMed]
- Perez De Sevilla Muller L, Shelley J, Weiler R. Displaced amacrine cells of the mouse retina. J Comp Neurol. 2007;505(2):177–189. doi: 10.1002/cne.21487. [DOI] [PubMed] [Google Scholar]
- Mohand-Said S, et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29(5):290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- Daiger S, Sullivan L, Bowne S. RetNet, the Retinal Information Network. Houston, TX: The University of Texas Health Science Center; 2014. Available from: https://sph.uth.edu/retnet. [Google Scholar]
- Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118(6):807–811. doi: 10.1001/archopht.118.6.807. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Transplantation of photoreceptor and total neural retina preserves cone function in P23H rhodopsin transgenic rat. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohand-Said S, et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci USA. 1998;95(14):8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fintz AC, et al. Partial characterization of retina-derived cone neuroprotection in two culture models of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2003;44(2):818–825. doi: 10.1167/iovs.01-1144. [DOI] [PubMed] [Google Scholar]
- Cronin T, Leveillard T, Sahel JA. Retinal degenerations: from cell signaling to cell therapy; pre-clinical and clinical issues. Curr Gene Ther. 2007;7(2):121–129. doi: 10.2174/156652307780363143. [DOI] [PubMed] [Google Scholar]
- Wright AF. A searchlight through the fog. Nat Genet. 1997;17(2):132–134. doi: 10.1038/ng1097-132. [DOI] [PubMed] [Google Scholar]
- Leveillard T, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36(7):755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid Redox Signal. 2007;9(1):25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- Fridlich R, et al. The thioredoxin-like protein rod-derived cone viability factor (RdCVFL) interacts with TAU and inhibits its phosphorylation in the retina. Mol Cell Proteomics. 2009;8(6):1206–1218. doi: 10.1074/mcp.M800406-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17(5):787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Strategies for delivery of rod-derived cone viability factor. Retina. 2005;25(8 Suppl):S47. doi: 10.1097/00006982-200512001-00020. [DOI] [PubMed] [Google Scholar]
- Komaromy AM, et al. Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol Ther. 2013;21(6):1131–1141. doi: 10.1038/mt.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol. 2013;156(2):283–292. doi: 10.1016/j.ajo.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T, et al. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010;17(7):1199–1210. doi: 10.1038/cdd.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveillard T, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci Transl Med. 2010;2(26):26ps16. doi: 10.1126/scitranslmed.3000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V, Hicks D, Dreyfus H. Changes in ganglioside composition of photoreceptors during postnatal maturation of the rat retina. Glycobiology. 1998;8(2):183–190. doi: 10.1093/glycob/8.2.183. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Kinkl N, Sahel J, Dreyfus H, Hicks D. Survival of purified rat photoreceptors in vitro is stimulated directly by fibroblast growth factor-2. J Neurosci. 1998;18(23):9662–9672. doi: 10.1523/JNEUROSCI.18-23-09662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman S, et al. The homeobox gene CHX10/VSX2 regulates RdCVF promoter activity in the inner retina. Hum Mol Genet. 2010;19(2):250–261. doi: 10.1093/hmg/ddp484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu C, Hicks D, Felder-Schmittbuhl MP. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011;34(3):507–516. doi: 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Weber C, Friedlander M. Global gene expression analysis of the developing postnatal mouse retina. Invest Ophthalmol Vis Sci. 2004;45(3):1009–1019. doi: 10.1167/iovs.03-0806. [DOI] [PubMed] [Google Scholar]
- Brooks MJ, Rajasimha HK, Roger JE, Swaroop A. Next-generation sequencing facilitates quantitative analysis of wild-type and Nrl(-/-) retinal transcriptomes. Mol Vis. 2011;17:3034–3054. [PMC free article] [PubMed] [Google Scholar]
- Delyfer MN, et al. Transcriptomic analysis of human retinal surgical specimens using jouRNAI. J Vis Exp. 2013. [DOI] [PMC free article] [PubMed]
- Ying S, et al. A CAT reporter construct containing 277bp GNAT2 promoter and 214bp IRBP enhancer is specifically expressed by cone photoreceptor cells in transgenic mice. Curr Eye Res. 1998;17(8):777–782. [PubMed] [Google Scholar]


