Abstract
We present a protocol for measuring in vivo aortic stiffness in mice using high-resolution ultrasound imaging. Aortic diameter is measured by ultrasound and aortic blood pressure is measured invasively with a solid-state pressure catheter. Blood pressure is raised then lowered incrementally by intravenous infusion of vasoactive drugs phenylephrine and sodium nitroprusside. Aortic diameter is measured for each pressure step to characterize the pressure-diameter relationship of the ascending aorta. Stiffness indices derived from the pressure-diameter relationship can be calculated from the data collected. Calculation of arterial compliance is described in this protocol.
This technique can be used to investigate mechanisms underlying increased aortic stiffness associated with cardiovascular disease and aging. The technique produces a physiologically relevant measure of stiffness compared to ex vivo approaches because physiological influences on aortic stiffness are incorporated in the measurement. The primary limitation of this technique is the measurement error introduced from the movement of the aorta during the cardiac cycle. This motion can be compensated by adjusting the location of the probe with the aortic movement as well as making multiple measurements of the aortic pressure-diameter relationship and expanding the experimental group size.
Keywords: Medicine, Issue 94, Aortic stiffness, ultrasound, in vivo, aortic compliance, elastic modulus, mouse model, cardiovascular disease
Introduction
Increased aortic stiffness is a hallmark of cardiovascular disease. Aging1, smoking2, diabetes3, hyperlipidemia4, and other risk factors of cardiovascular disease have been shown to increase aortic stiffness. Epidemiological studies have further demonstrated aortic stiffness as a powerful independent predictor of the onset of coronary heart disease and stroke, as well as the occurrence of cardiovascular events and mortality5-8. Because of the clinical and public health significance of increased aortic stiffness, current research is focused on understanding the mechanisms underlying the development and progression of vascular stiffness. Great interest therefore exists in developing accurate measures of vascular stiffness in experimental models of cardiovascular disease.
A material’s stiffness can be characterized by its stress-strain relationship and quantified as elastic modulus. A linear elastic material deforms reversibly and its stress increases proportionally to strain. The aorta and the large arteries are nonlinear elastic bodies: when stretched, the stiffness of the artery does not remain constant but increases with the degree of distension. This nonlinearity in the mechanical properties of large arteries is due to the different stiffness properties of the load bearing elements, namely elastin and collagen, which constitute the vessel wall. Elastin is highly extensible with an elastic modulus of 0.6 MPa. In comparison, collagen is very stiff with an elastic modulus of 1 GPa9. The initial stiffness exhibited by the aorta at lower strain values is attributed to elastin while the high stiffness exhibited at high strain values is due to collagen. Load is transferred from elastin to collagen as the vessel distends and this region of load transferring is where the vascular system operates. Therefore, at physiologic pressures, arterial stiffness depends on the contribution of both elastin and collagen10.
The distribution and orientation of elastin and collagen vary by layer within the arterial wall. In the media, the elastin, collagen, and smooth muscle cells are bundled into tight helices that are layered concentrically. This arrangement allows the artery to resist high loads in the circumferential direction. The adventitia is predominantly collagen with little elastin and the collagen fibers are organized in a net-like fashion. These collagen fibers are wavy in an unstressed state and straighten out as load increases. Stiffness increases as the collagen fibers straighten out, thereby preventing the artery from overstretching and rupturing. Because of the structural organization and varying orientation of the collagen fibers, arteries are anisotropic: the stiffness exhibited depends on if the vessel is stretched longitudinally or circumferentially11. In vivo stiffness is therefore a composite of the aorta’s longitudinal and circumferential stiffness.
Arterial stiffness is generally quantified in vivo as compliance or pulse wave velocity (PWV). Arterial compliance is defined as C = ∆D / ∆P where ∆D is change in diameter and ∆P is the corresponding change in pressure. Lower values of compliance indicate stiffer vessels. Compliance is calculated from the pressure-dimension relationship of the artery and is therefore a direct measure of stiffness. As stiffness is disseminated non uniformly in the vasculature12, compliance should be measured at the same/similar location in each subject to make meaningful comparisons between experimental groups.
The difference between compliance and elastic modulus is that elastic modulus is normalized to the material’s dimensions. Compliance therefore reflects structural stiffness, whereas elastic modulus reflects material stiffness. With aging, arterial wall thickness increases and elastin/collagen ratio decreases, so both structural stiffness and material stiffness are greater.
Compared to compliance, PWV is an indirect measure of arterial stiffness. PWV is the speed at which a pressure pulse travels along a length of artery and is influenced by the properties of the vessel wall. The Moens-Korteweg equation is used to model the relationship between PWV and elastic modulus: PWV2 = E h / (2 ρ r) where E is incremental elastic modulus, h is wall thickness, ρ is blood viscosity, and r is vessel radius. A higher PWV value therefore suggests a stiffer vessel.
Compliance and elastic modulus can be measured experimentally ex vivo on an excised segment of vessel. To determine compliance, the vessel segment is mounted on a pressure myograph13,14. Pressure within the vessel is increased step-wise and the resulting change in diameter is tracked using video microscopy. Compliance is determined from the pressure-diameter data. Incremental elastic modulus can be measured by tensile testing. In these experiments, the vessel is pulled apart step-wise and force-displacement data is collected until the vessel ring breaks. Stress and strain values can be calculated and plotted to determine incremental elastic modulus. These ex vivo approaches can be used to evaluate changes in the passive properties that influence stiffness.
In vivo, in addition to wall content, vascular stiffness is influenced dynamically by smooth muscle tone and blood pressure13,15,16. PWV is the most widely used method for measuring in vivo aortic stiffness in experimental models. PWV can be determined noninvasively using Doppler ultrasound or applanation tonometry17. Pressure pulse is measured at two separate locations and the time required for the pulse to traverse the distance is the pulse wave velocity. Because PWV is measured over a length of aorta, it is an averaged value of stiffness. Large arteries are nonlinear elastic, so stiffness and therefore PWV will vary with arterial pressure. A higher PWV value could therefore arise from increased stiffness or elevated pressure. PWV values therefore must be normalized to blood pressure to derive conclusions about the vessel’s stiffness. Measurement methods that incorporate the influence of blood pressure with the passive properties of the vascular wall and the effects of vasoactive mediators that alter tone would yield a physiologically relevant index of arterial stiffness. This approach is implemented by measuring PWV invasively using a catheter with two pressure sensors separated at a fixed distance13. This dual pressure catheter is inserted into the aorta and vasoactive drugs, such as phenylephrine or sodium nitroprusside, are infused intravenously through a venous catheter to raise and lower arterial pressure.
This protocol describes a method to determine aortic stiffness in vivo from its pressure-dimension relationship in a mouse model. This approach offers several advantages over the invasive PWV measurement. Stiffness indices, such as compliance, can be calculated from the pressure-dimension data collected by this procedure. Moreover, this technique allows for measurement of local aortic stiffness because stiffness is measured from a single location. This approach is particularly useful in measuring ascending aortic stiffness as the short length of this region makes a PWV measurement difficult to obtain. Research interest exists specifically in the ascending aorta because its mechanical properties influence the perfusion of the coronary circulation and the cardiac response to vascular dysfunction.
To measure the pressure-diameter relationship of the aorta in vivo, the ascending aorta is visualized and its diameter is measured by ultrasound imaging. Aortic blood pressure is measured invasively with a pressure catheter. Blood pressure is changed incrementally by intravenous infusion of vasoactive drugs. Phenylephrine constricts blood vessels and is used to raise aortic pressure. Sodium nitroprusside dilates blood vessels and is used to lower aortic pressure. Systolic and diastolic aortic diameters and corresponding aortic pressures are measured for each pressure increment. Compliance can be calculated from the pressure-diameter data collected.
Protocol
This protocol has been approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
1. Preparation of Solutions, Materials, and Animal
Prepare a 300 µg/ml solution of phenylephrine (PE) and 300 µg/ml solution of sodium nitroprusside (SNP) in 0.9% saline. Prepare a separate heparin-saline solution by mixing 1 ml of 1,000 U/ml heparin into 10 ml of 0.9% saline. NOTE: Drugs should be at room temperature before use.
Make the catheter for intravenous drug infusion from two 30 G x ½” hypodermic needles and PE 10 polyethylene tubing. To make the catheter, insert one needle into one end of the tubing. Remove the needle portion of the other hypodermic needle and insert the blunt end into the other end of the tubing. Attach the catheter to a 1 ml syringe and fill the catheter with the heparin-saline solution.
Place mouse in the anesthesia induction chamber containing 2-2.5% isoflurane in 100% oxygen. Leave the mouse in the induction chamber until it is unresponsive to external stimuli.
Remove the mouse from the induction chamber and place it on the heated electrocardiogram (ECG) pad. Maintain the animal at 2% isoflurane.
Apply vet ointment or saline solution to the animal’s eyes to prevent dryness during the procedure.
2. Insertion of Catheter into Tail Vein
Since the tail veins are located laterally on both sides of the tail, place the animal on its side for better access. Secure the mouse onto the ECG pad with tape. Make sure the animal is kept warm to promote vasodilation of the tail veins.
Using a piece of silastic tubing as a tourniquet, tie the tourniquet around the base of the tail. Tie the tourniquet tight enough to collapse the veins but not enough to cut off the arterial circulation. After 2-3 min, the vein should bulge out and become more visible.
Gently pull the tail taut. Bend the tail at an angle with one hand and hold the needle parallel to the tail with the other. Pierce the needle where the tail is bent through the skin into the vein. Blood will push back into the catheter if the needle is inserted into the vein.
Place one drop of tissue glue where the needle is inserted to secure the catheter. Remove the tourniquet and confirm patency by injecting saline with little resistance.
3. Insertion of Blood Pressure Catheter through Femoral Artery
Place the pressure catheter into a 30 ml syringe filled with distilled water and connect the catheter to the pressure control unit. Soak the catheter in water, plugged in, for 30-45 min during the set-up and surgery procedures.
- Place the animal supine and tape its paws onto the ECG pad. Apply depilatory cream on the chest and area over the femoral artery.
- Wait 3-5 min and remove cream and hair. Thoroughly remove hair from the chest to prevent artifacts during the ultrasound. Wipe both the chest and hind limb regions with a moistened pad to remove excess depilatory cream.
Using fine scissors, make an incision in the skin above the location of the femoral artery. Dissect through the subcutaneous fat tissue to reveal the femoral artery. The femoral artery is partially covered by the abdomen. Use hemostats to move the abdomen away. Keep the tissues moist by covering it with moist gauze or alternatively dripping saline over it periodically to prevent tissues from drying out.
Using fine forceps, separate the nerve away from the artery-vein bundle. Gently pierce through the sheath around the artery-vein bundle to separate the artery from the vein. Pass one suture around the artery at the proximal end and place two sutures at the distal end.
Securely knot the most distal suture to stop distal blood flow. Use hemostats to pull the proximal suture to temporarily stop blood flow into the femoral artery. Use microscissors to make a small incision into the femoral artery. Make the incision near the distal knot.
Calibrate the data acquisition software to the catheter using the calibration settings on the pressure control unit. Switch the pressure control unit back to reading the transducer and balance the pressure catheter so that the catheter outputs 0 mm Hg in the water filled syringe.
- Insert the catheter into the femoral artery. Open the incision with fine forceps with one hand and insert the catheter head into the artery with the other hand.
- Knot the middle suture around the catheter wire to secure the catheter into the artery. Relax the proximal suture and advance the catheter forward into the abdominal aorta. Knot the proximal suture to further secure the catheter and to prevent bleeding.
Carefully move the ECG pad with mouse, pressure catheter and saline syringe to the ultrasound imaging stage. Connect the blood pressure catheter to the pressure control unit. Place the saline syringe in the syringe pump. Allow the animal and the catheter to equilibrate for 20 min.
4. Measuring Aortic Diameter Over a Range of Blood Pressures
Reduce isoflurane to 1.5%. Visualize the ascending aorta longitudinally on B-mode using a long axis view. Mount the transducer onto the rail system so that the same view is maintained for the duration of the experiment.
On the ultrasound mainframe, place the M-mode cursor over the section of aorta to be tracked. Track the aortic diameter change over the cardiac cycle using M-mode.
- Change the saline in the syringe to the PE solution and place the syringe into the syringe pump.
- Record M-mode at baseline aortic pressure. Begin infusion at 360 µg/kg/min and infuse for 1 min for aortic pressure to reach a plateau. For a 25 g mouse, this dose equates to 30 µl/min.
- Record the M-mode, then stop the infusion, and wait 2 min for blood pressure to return to baseline.
Lower infusion rate to 240 µg/kg/min. For a 25 g mouse, this dose equates to 20 µl/min. Start infusion, infuse for 1 min for blood pressure to plateau, and record M-mode. Stop the infusion, and wait 2 min for blood pressure to return to baseline.
Repeat step 4.4 for 120 µg/kg/min PE (10 µl/min for a 25 g mouse).
Replace PE with saline and infuse the saline at the rate used for the 360 µg/kg/min infusion (30 µl/min for a 25 g mouse). Infuse for 2-3 min, until further infusion does not produce an increase in aortic pressure and pressure is returning to baseline. Wait 5 min for the blood pressure to stabilize at baseline.
- Replace saline with SNP.
- Record M-mode at baseline aortic pressure. Begin infusion at 240 µg/kg/min (20 µl/min for 25 g mouse)and infuse for 1 min. When aortic pressure reaches a plateau, record the M-mode. Stop the infusion and wait 2 min for blood pressure to return to baseline.
Lower infusion rate to 120 µg/kg/min (10 µl/min for 25 g mouse). Start infusion, infuse for 1 min for blood pressure to plateau, and record M-mode. Stop infusion and wait 2 min for blood pressure to return to baseline.
Repeat step 4.8 for 60 µg/kg/min SNP (5 µl/min for 25 g mouse).
5. Terminating the Experiment
To euthanize the animal, increase isoflurane to 4%. When breathing has slowed, usually in 1-2 min, cut through the sternum with scissors to open the thoracic cavity and expose the heart.
Grasp the heart with medium forceps and excise it from the body by cutting at the ascending aorta with scissors.
Representative Results
A longitudinal image of the left ventricle and ascending aorta is captured on B-mode, as shown in Figure 1. Alternatively, a longitudinal image of only the aorta can be obtained, as in Figure 2. The movement of the aortic wall during the cardiac cycle appears as two white lines on the M-mode, as shown in Figure 3. The aortic lumen is the area in between the lines. Aortic pressure is modulated by infusion of vasoactive drugs. PE raises the aortic pressure, as shown in Figure 4A, and SNP lowers pressure, as shown in Figure 4B. M-mode is recorded when blood pressure plateaus, 1 min after the start of infusion. Aortic pressure is changed incrementally through changing the dose of the drug administered, as shown in Figure 5. Dose of drug is controlled through the rate of infusion. All drug doses are in µg/kg/min. Maximum and minimum diameters are measured from the M-mode, shown in Figure 3. These diameters correspond to the systolic and diastolic aortic pressures recorded by the pressure catheter.
Systolic and diastolic diameter and pressure values of three cardiac cycles are measured at baseline and for each PE and SNP dose. The standard deviation between three diameter measurements at one drug dose ranges from 0.01 mm to 0.04 mm. Aortic diameter can be plotted against its corresponding aortic pressure to illustrate the pressure-diameter relationship, as shown in Figure 6A.
These pressure-diameter values are used to calculate aortic compliance. Arterial compliance is calculated by
C = (Dsys– Ddia) / (Psys– Pdia)(1)
where Dsys and Ddia are systolic and diastolic diameters and Psys and Pdia are systolic and diastolic pressures. Compliance and mean aortic pressure (MAP) are calculated at baseline and for each PE and SNP dose. Compliance is plotted against MAP to demonstrate the pressure-dependency of stiffness. Because of the nonlinear elastic behavior of the aorta, compliance decreases with increasing MAP, as seen in Figure 6B.
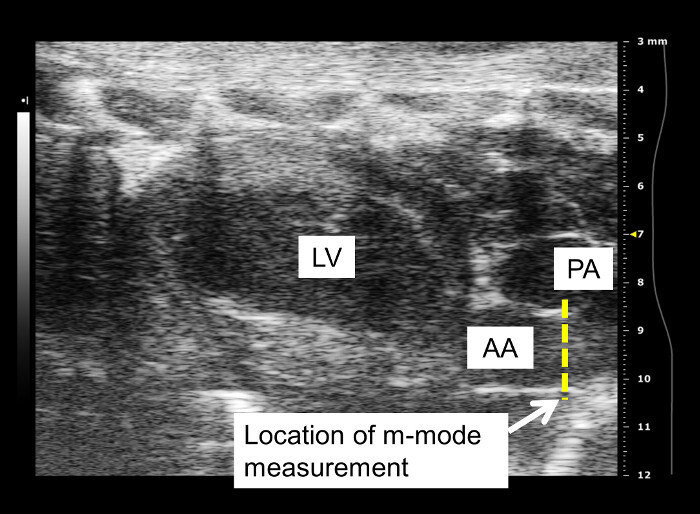 Figure 1: Longitudinal view of ascending aorta on B-mode. Diameter measurements are taken from a longitudinal image of the ascending aorta leaving the left ventricle. LV: left ventricle; PA: pulmonary artery; AA: ascending aorta. Visualization of the pulmonary artery depends on the probe placement. Aortic diameter is measured distal to the aortic valve. Frequency of the probe used to capture this image is 40 MHz.
Figure 1: Longitudinal view of ascending aorta on B-mode. Diameter measurements are taken from a longitudinal image of the ascending aorta leaving the left ventricle. LV: left ventricle; PA: pulmonary artery; AA: ascending aorta. Visualization of the pulmonary artery depends on the probe placement. Aortic diameter is measured distal to the aortic valve. Frequency of the probe used to capture this image is 40 MHz.
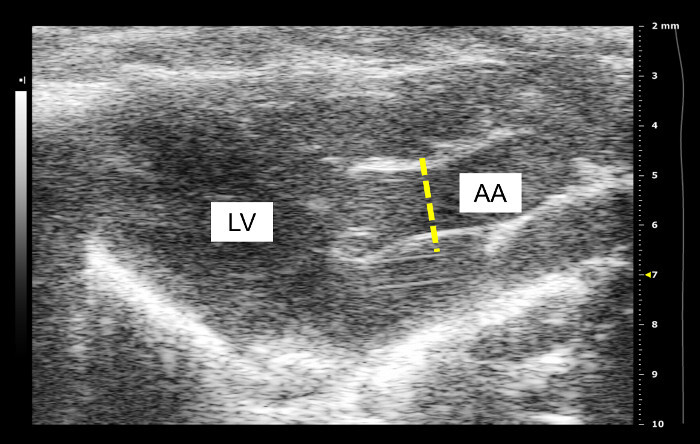 Figure 2: Alternate view of ascending aorta on B-mode. The ascending aorta is featured more prominently and the left ventricle and heart walls are less distinct. AA: ascending aorta; LV: left ventricle. Frequency of probe used to record this image is 40 MHz.
Figure 2: Alternate view of ascending aorta on B-mode. The ascending aorta is featured more prominently and the left ventricle and heart walls are less distinct. AA: ascending aorta; LV: left ventricle. Frequency of probe used to record this image is 40 MHz.
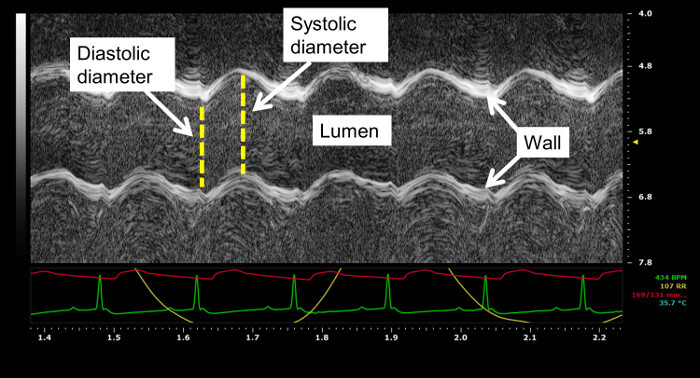 Figure 3: Aorta visualized on M-mode. Aortic diameter is measured from the M-mode image. The movement of the aortic wall appears as two wavy lines. The space in between the two lines is the aortic lumen. Systolic and diastolic aortic diameters of three cardiac cycles are measured from the M-mode. In this image, aortic pressure recorded by the pressure catheter, ECG signal, and respiratory cycle are displayed in red, green, and yellow on the M-mode. Probe frequency used to record this image is 40 MHz and the acquisition sweep speed is 1,200 Hz.
Figure 3: Aorta visualized on M-mode. Aortic diameter is measured from the M-mode image. The movement of the aortic wall appears as two wavy lines. The space in between the two lines is the aortic lumen. Systolic and diastolic aortic diameters of three cardiac cycles are measured from the M-mode. In this image, aortic pressure recorded by the pressure catheter, ECG signal, and respiratory cycle are displayed in red, green, and yellow on the M-mode. Probe frequency used to record this image is 40 MHz and the acquisition sweep speed is 1,200 Hz.
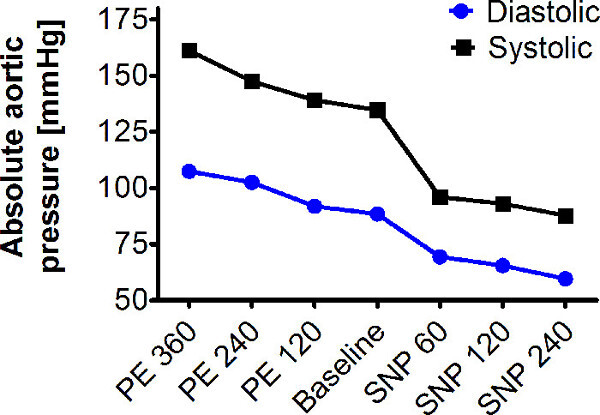 Figure 4: Changing aortic pressure incrementally. Aortic pressure is changed incrementally by the dose of drug infused. Drug dose is modulated by the infusion rate. All doses are in µg/kg/min.
Figure 4: Changing aortic pressure incrementally. Aortic pressure is changed incrementally by the dose of drug infused. Drug dose is modulated by the infusion rate. All doses are in µg/kg/min.
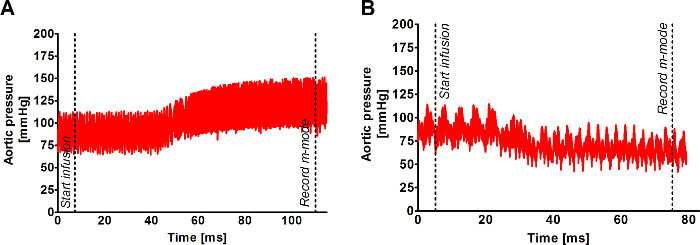 Figure 5: Modulating aortic pressure with vasoactive drugs. Aortic pressure is increased with infusion of vasoconstrictor phenylephrine (PE) and decreased with infusion of vasodilator sodium nitroprusside (SNP). Aortic pressure plateaus 1 min after the start of the drug infusion. M-mode of the aortic diameter is recorded at the plateau. (A) shows the rise in aortic pressure with 360 µg/kg/min PE infusion. (B) shows the decrease in aortic pressure with 240 µg/kg/min SNP infusion. The time the infusion is begun and the time the M-mode is recorded are labeled on the traces. Aortic pressure is changed incrementally by the dose of drug infused. Drug dose is modulated by the infusion rate. All doses are in µg/kg/min.
Figure 5: Modulating aortic pressure with vasoactive drugs. Aortic pressure is increased with infusion of vasoconstrictor phenylephrine (PE) and decreased with infusion of vasodilator sodium nitroprusside (SNP). Aortic pressure plateaus 1 min after the start of the drug infusion. M-mode of the aortic diameter is recorded at the plateau. (A) shows the rise in aortic pressure with 360 µg/kg/min PE infusion. (B) shows the decrease in aortic pressure with 240 µg/kg/min SNP infusion. The time the infusion is begun and the time the M-mode is recorded are labeled on the traces. Aortic pressure is changed incrementally by the dose of drug infused. Drug dose is modulated by the infusion rate. All doses are in µg/kg/min.
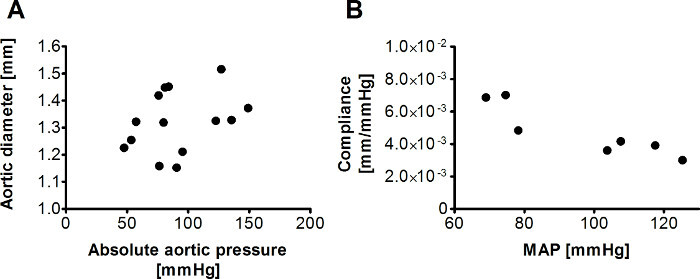 Figure 6: Diameter vs. pressure and compliance vs. mean aortic pressure plots. Aortic diameter can be plotted against its corresponding aortic pressure to show the pressure-diameter relationship (A). Compliance can be calculated for each pressure increment and plotted against the mean aortic pressure (MAP) to show the pressure dependency of aortic stiffness (B).
Figure 6: Diameter vs. pressure and compliance vs. mean aortic pressure plots. Aortic diameter can be plotted against its corresponding aortic pressure to show the pressure-diameter relationship (A). Compliance can be calculated for each pressure increment and plotted against the mean aortic pressure (MAP) to show the pressure dependency of aortic stiffness (B).
Discussion
Taking diameter measurements at several pressure increments over a wide range of pressure values is necessary for accurate characterization of the pressure-diameter relationship. The upper and lower pressure limits that can be pharmacologically induced may vary by the experimental group but the ideal range is around 25 mm Hg to 125 mm Hg diastolic and 50 mm Hg to 200 mm Hg systolic. Doses of 360 µg/kg/min PE and 240 µg/kg/min SNP generally elicit the limits of the pressure range. However, doses of PE can be increased to 480 µg/kg/min and SNP to 360 µg/kg/min to verify that the limits have been reached. Working concentrations of PE and SNP can be decreased to achieve finer pressure increments. As the diameter will change with aortic pressure, inducing the same pressure values between animals and experimental groups is not important.
Venous and arterial cannulation can be performed at other locations with the same outcomes. Tail vein cannulation can be challenging because of the small size of the tail vein. Moreover, the tail vein is not readily visible in dark colored mice. The femoral vein can be cannulated as an alternative. This route may be easier since the femoral vein is more accessible. For pressure catheter insertion, besides the femoral artery, the catheter can be inserted through the carotid. The femoral artery is preferable over the carotid artery, however, because the chest region remains intact for the ultrasound imaging. Femoral artery cannulation can be more difficult because the femoral artery is smaller. Using a 1.2 F catheter and introducing the catheter in the proximal femoral artery beneath the abdominal cavity will facilitate the cannulation process. Placing a few drops of a vasodilating agent like lidocaine onto the femoral artery or using a catheter introducer can also help enlarge the vessel to facilitate catheter insertion. The pressure catheter should be handled and used according to the manufacturer’s instructions.
Location of the catheter within the aorta does not need to be consistent between animals as the pressure drop within the aorta is insignificant. However, placing the catheter in the abdominal aorta may be better to minimize interference with the ultrasound imaging of the thoracic aorta. Some ultrasound mainframes can record pressure real-time with the M-mode trace, thereby giving a pressure measurement for every diameter measured on the M-mode. Unfortunately, because the location where the diameter is measured is not the same location as where pressure is recorded, a lag exists between the pressure recorded at the catheter and the actual pressure in the ascending aorta. As a result, only maximum and minimum diameter measurements can be used for the data analysis.
The primary limitation of this method is the uncertainty in measurement introduced by the aorta shifting in and out of the ultrasound plane during the cardiac cycle. Motion-introduced error is common to all imaging based studies, including MRI and CT. Compensation strategies include using anatomical features to shift the frame of reference with the movement18 and are implemented during data processing. As motion compensation software is not readily available, the investigator has to be vigilant about adjusting the location of the probe to track the shift in location of the aorta as blood pressure rises and decreases. Diameter measurements should also be taken through the center of the aorta. However, determining whether the M-mode recording location is passing through the center can be difficult to judge on the ultrasound image, especially with the aorta shifting positions. The uncertainty introduced by these limitations manifest in the degree of scatter in the data, as evident in Figure 6. Obtaining an image of the cross-section instead of longitudinal axis of the ascending aorta could be a solution. However, obtaining this view can sometimes be more challenging and the resulting M-mode trace can be less clear. The cross-sectional circumference from the B-mode image could be measured instead of the diameter from the M-mode image. However, determining when maximum and minimum circumference has been achieved will be limited by the B-mode frame rate and may be more difficult to judge than on the M-mode.
Making multiple measurements of the pressure-diameter plot and increasing experimental group size can improve accuracy of the data. The pressure-diameter data can be collected from several locations along the chest. This protocol would first be carried out with the probe placed on one location on the chest. The aorta would then be visualized with the probe placed on another location and the protocol repeated.
Vasoactive agents used to modulate blood pressure could potentially affect aortic smooth muscle tone, which in turn would affect stiffness. However, manipulation of aortic pressure by venous return has been shown to produce similar changes in invasively measured PWV as pharmacologic manipulation in rats. These findings demonstrate that infusion of vasoactive drugs act primarily on the peripheral resistance arteries and do not significantly affect aortic smooth muscle tone19.
This protocol can be performed in rats with a few minor modifications. The chest is shaved prior to applying depilatory cream. A commercially available 27 G x ½” catheter is used for drug infusion. The drug doses used to modulate aortic pressure are 40, 80, and 120 µg/kg/min of PE and 40, 80, and 120 µg/kg/min of SNP.
Besides the ascending aorta, regional differences in aortic stiffness can be determined with this protocol. Regional stiffness measured by this approach would be more precise than by PWV as measurements are taken from one location as oppose to two locations for PWV. However, regions along the aorta that can be measured with this technique are limited to those that can be visualized by ultrasound.
Elastic modulus can also be calculated from the data collected by this method if a wall thickness measurement can be obtained. Accurate in vivo measurement of the mouse aorta is limited by the resolution limits of current ultrasound technology. Future improvement of ultrasound technology could make in vivo wall thickness measurement more feasible. As an alternative, thickness measurements can be performed ex vivo. Pressure myography would provide the most accurate measurements because thickness can be measured at each pressure increment.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by a National Heart, Lung, and Blood Institute grant 1RO1-HL-105296-01 (to D.E. Berkowitz) and an Australian Research Council Grant DP110101134 (to A. Avolio).
References
- Mitchell GF, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women - The Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41:183–187. doi: 10.1161/01.hyp.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- Lehmann ED, Gosling RG, Sonksen PH. Arterial wall compliance in diabetes. Diabet Med. 1992;9:114–119. doi: 10.1111/j.1464-5491.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Wang Y-X, et al. Reduction of cardiac functional reserve and elevation of aortic stiffness in hyperlipidemic Yucatan minipigs with systemic and coronary atherosclerosis. Vasc. Pharmacol. 2002;39:69–76. doi: 10.1016/s1537-1891(02)00247-1. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction: An Individual Participant Meta-Analysis of Prospective Data From 17,635 Subjects. J. Am. Coll. Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FU, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Laurent S, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2nd edn. New York, NY: Springer; 1993. [Google Scholar]
- Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- Gasser TC, Ogden RW, Holzapfel GA. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. Journal of The Royal Society Interface. 2006;3:15–35. doi: 10.1098/rsif.2005.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- Jung SM, et al. Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice. Am. J. Physiol.-Heart Circul. Physiol. 2013;305:803–810. doi: 10.1152/ajpheart.00103.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam L, et al. Decreased S-Nitrosylation of Tissue Transglutaminase Contributes to Age-Related Increases in Vascular Stiffness. Circ. Res. 2010;107:117–243. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- Fitch RM, Vergona R, Sullivan ME, Wang YX. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc. Res. 2001;51:351–358. doi: 10.1016/s0008-6363(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Bergel DH. The static elastic properties of the arterial wall. The Journal of Physiology. 1961;156:445–457. doi: 10.1113/jphysiol.1961.sp006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup AJ, et al. Applanation Tonometry in Mice: A Novel Noninvasive Technique to Assess Pulse Wave Velocity and Arterial Stiffness. Hypertension. 2014;21:21. doi: 10.1161/HYPERTENSIONAHA.114.03312. [DOI] [PubMed] [Google Scholar]
- Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: age-related changes. J Vasc Surg. 2009;49:1029–1036. doi: 10.1016/j.jvs.2008.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin M, Hammond A, Lindesay G, Viegas K, Avolio AP. In vitro and in vivo use of vasoactive agents in characterising aortic stiffness in rats: testing the assumptions. Hypertens. 2012;30:42. [Google Scholar]


