Abstract
Environmental enrichment can dramatically influence the development and function of neural circuits. Further, enrichment has been shown to successfully delay the onset of symptoms in models of Huntington’s disease 1-4, suggesting environmental factors can evoke a neuroprotective effect against the progressive, cellular level damage observed in neurodegenerative disorders. The ways in which an animal can be environmentally enriched, however, can vary considerably. Further, there is no straightforward manner in which the effects of environmental enrichment can be assessed: most methods require either fairly complicated behavioral paradigms and/or postmortem anatomical/physiological analyses. This protocol describes the use of a simple and inexpensive behavioral assay, the Puzzle Box 5-7 as a robust means of determining the efficacy of increased social, sensory and motor stimulation on mice compared to cohorts raised in standard laboratory conditions. This simple problem solving task takes advantage of a rodent’s innate desire to avoid open enclosures by seeking shelter. Cognitive ability is assessed by adding increasingly complex impediments to the shelter’s entrance. The time a given subject takes to successfully remove the obstructions and enter the shelter serves as the primary metric for task performance. This method could provide a reliable means of rapidly assessing the efficacy of different enrichment protocols on cognitive function, thus paving the way for systematically determining the role specific environmental factors play in delaying the onset of neurodevelopmental and neurodegenerative disease.
Keywords: Behavior, Issue 94, Neuroscience, Mouse, Environmental Enrichment, Puzzle Box, Cognitive Behavior, Behavioral Task, Neuroprotection
Introduction
Environmental enrichment (EE) may be defined as surroundings that provide animals with increased opportunity for social interaction, motor activity, and greater sensory stimulation than usually experienced in a standard laboratory environment.EE has been shown to consistently affect the behavior of animals, bringing about changes such as reduction of stress and anxiety-related activity 8-10, improved performance in learning and memory tasks 8,11, early onset of motor coordination and exploratory activity 11, changes in maternal care 8 as well as resistance to addictive substances 12-15. Further, EE has been revealed to ameliorate the effects of neurodegenerative disorders, delaying the onset and decreasing the severity of symptoms in animal models of Huntington’s 1-4,16, Parkinson’s 17 and Alzheimer’s disease 18.
These changes correlate with the anatomical and molecular alterations EE is known to induce throughout the brain. Animals raised in enriched environments from early stages of development show a myriad of neural changes, including increased brain weight and cortical thickness, dendritic branching 9,2-22 and synaptic density 23. EE can alter both the level and timing of growth factor expression 9,24-30, which has been shown to contribute to accelerated development of sensory 25,26,28,29, mnemonic 30, as well as motor circuits 31,32.
Previous work has revealed at times contradictory findings when investigating the impact of EE, without taking into account the different types of animals and environments used within individual studies 9,24,27,30. Currently, there is no consistent and simple behavioral task that can be used to measure the effectiveness of various EE paradigms in different strains and species of animals.
The Puzzle Box task was designed as a simple test to determine an animal’s native problem solving ability 7. Animals placed in the open area are required to remove obstructing materials situated within a small opening in order to access a covered region/shelter. Each subject is given three trials with the same obstruction in order to assess three different cognitive attributes. The first trial yields a baseline indication of inherent or native problem solving ability. The second trial, run on the same day, gives some indication of the animal’s ability to improve upon and thus reinforce strategies for removing the specific obstruction. The third trial, conducted on the following day, provides insight into the ability of the subject to retain and recall the learned solution to the task.
The motivation for solving these “obstruction puzzles” by the animals can be varied, potentially evoking an innate desire to avoid open fields and seek shelter, as well as an inherent drive to explore their surroundings 6,7. The multitude of potential behavioral drivers underlying the desire to solve the Puzzle Box suggests that various areas of the brain are involved in mediating task performance. Previous work has shown that in murine models of schizophrenia, the prefrontal cortex as well as the hippocampus are involved in the acquisition of this task 5. A lesion study in rats has also revealed a large number of brain regions involved in Puzzle Box performance, including various thalamic nuclei, the hypothalamus, the cerebellum, and limbic structures. Together, these findings indicate that engaging in this problem solving task involves a host of neural structures associated with cognitive function.
The Puzzle Box has been used successfully to assess the problem solving ability of mice, as well as cognitive deficits exhibited by murine models of schizophrenia 5-7. Performance on the task has been shown to be highly consistent, and correlate well with outcomes of other cognitive behavioral tests 6. The goal of this work was thus to adapt the Puzzle Box task to become a simple and reliable means of determining the effectiveness of EE.
Protocol
Ethics statement: All procedures were approved by the Animal Ethics Committee of the University of Sydney and conformed to National Health and Medical Research Council of Australia guidelines. Procedures were performed on C57/BL6J mice which were reared at the University of Sydney Bosch Rodent Facility. All mice were housed in a single adequately-ventilated room in 21 °C ambient temperature on a 12 hr light-dark cycle with lights on at 0600 hr in individually ventilated cages with ad libitum access to dry food and water. Late-pregnancy females were randomly assigned to standard or environmentally enriched housing conditions.
1. Housing (Enrichment Levels):
Obtain 4 late-pregnancy adult female mice. Randomly assign 2 to the standard condition and place each of them into a clean standard mouse cage (overall dimensions 391 x 199 x 160 mm) containing one red mouse igloo. For the enriched condition, place the remaining 2 mice into a single clean rat-sized cage (overall dimensions 462 x 403 x 404 mm).
Into the enrichment cage, place a variety of objects designed to increase sensory and motor stimulation (e.g. running wheels, visual stimuli, scented cotton balls, Velcro strips).
Every 2 to 3 days move these objects about within the cage; refresh any that have been destroyed.
Upon weaning at 21 days postnatal, sex animals and place into male-female segregated housing consistent with the environmental condition they were raised. For the enriched condition ensure that there are between 3 and 10 mice per cage with 2 and 5 mice for standard. Commence behavioral testing once animals reach adulthood (12–14 weeks of age).
2. Construction of the Puzzle Box
Obtain 6 pieces of white acrylic (or other non-porous material): one 750 x 280 mm, two 280 x 250 mm, two 750 x 250 mm, and one 150 x 280 mm (see Figure 1).
Obtain one piece of black acrylic 280 x 250 mm, with a 40 x 40 mm square opening cut into one side of this piece (see Figure 1).
Assemble the Puzzle Box as follows: use the 750 x 280 mm piece as the bottom of the box, use 280 x 250 mm pieces as the ends of the box, and the 750 x 250 mm pieces as the sides of the box.
Measure 150 mm into the box from one end, and place the black piece of acrylic across the box so that it splits it into two compartments (one large, one small) with the opening flush with the bottom of the box.
Take the 150 x 280 mm piece of white acrylic and place it atop the smaller compartment of the box making sure it covers this area completely, providing a dark “goal-box” chamber. Affix this piece of acrylic to the body of the main box by hinges, or leave free to be completely removed during behavioral testing.
Take 3 pieces of acrylic (three 4 x 120 mm) and join to make a “u-shaped” channel.
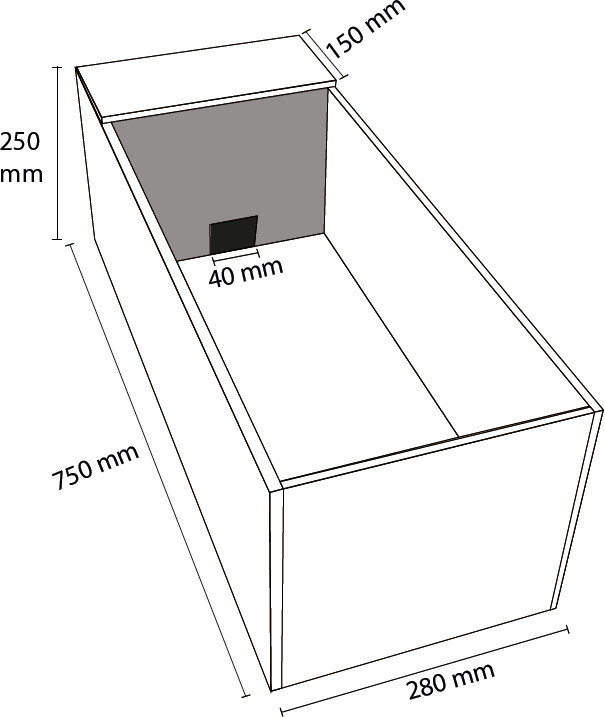 Figure 1: Schematic diagram of the Puzzle Box task. The Puzzle Box is an acrylic box consisting of an open-field area (600 x 280 mm) and a sheltered goal-box area (150 x 280 mm), measuring 750 x 280 mm in all. A 40 x 40 mm opening within the partition (grey) separating the two areas allows animals to access to the covered goal-box area from the open field. This opening is blocked with obstructions that are increasingly difficult to remove as testing progresses. Animals undergo a five-day protocol, consisting of four obstruction conditions with three trials for each condition.
Figure 1: Schematic diagram of the Puzzle Box task. The Puzzle Box is an acrylic box consisting of an open-field area (600 x 280 mm) and a sheltered goal-box area (150 x 280 mm), measuring 750 x 280 mm in all. A 40 x 40 mm opening within the partition (grey) separating the two areas allows animals to access to the covered goal-box area from the open field. This opening is blocked with obstructions that are increasingly difficult to remove as testing progresses. Animals undergo a five-day protocol, consisting of four obstruction conditions with three trials for each condition.
3. Running of the Puzzle Box Task
Thoroughly clean the Puzzle Box with 70% alcohol. Repeat this step between each animal tested.
Place a clean red mouse igloo within the goal-box area of the Puzzle Box, and replace the lid on the goal-box.
If there is an obstruction condition being tested, place the obstruction within the doorway of the goal-box (see Figure 2).
Place the mouse being tested into the open-field section of the Puzzle Box, oriented towards the goal-box, and at the end furthest from the goal-box.
Record the time taken for all four paws of the animal to enter the goal-box section of the Puzzle Box.
If an animal does not enter the goal-box, terminate the trial once the set time limit is reached (see Table 1).
Once a trial is finished, remove the animal from the Puzzle Box and place it into a separate holding cage until the next trial begins. Keep a 60 to 180 sec gap between trials for each animal.
For each animal, perform three trials per day for five consecutive days of testing, with four obstruction conditions and three trials of each condition. The third trial of a given obstruction condition should always administered on a subsequent day (see Table 1).
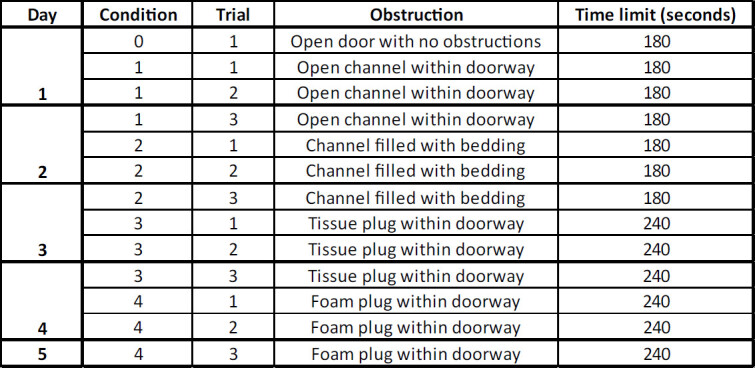 Table 1: Scheme of the Puzzle Box task. The Puzzle Box task is run for five days, and consists of four obstruction conditions. There are three trials on each of the first four days, and one on the fifth day. Each obstruction condition has three trials; the first two on one day, and the third the day immediately following. The first trial of an obstruction condition aims to test native problem-solving ability, the second trial examines task acquisition and reinforcement, and the third trial is used as an assay for solution retention and recall.
Table 1: Scheme of the Puzzle Box task. The Puzzle Box task is run for five days, and consists of four obstruction conditions. There are three trials on each of the first four days, and one on the fifth day. Each obstruction condition has three trials; the first two on one day, and the third the day immediately following. The first trial of an obstruction condition aims to test native problem-solving ability, the second trial examines task acquisition and reinforcement, and the third trial is used as an assay for solution retention and recall.
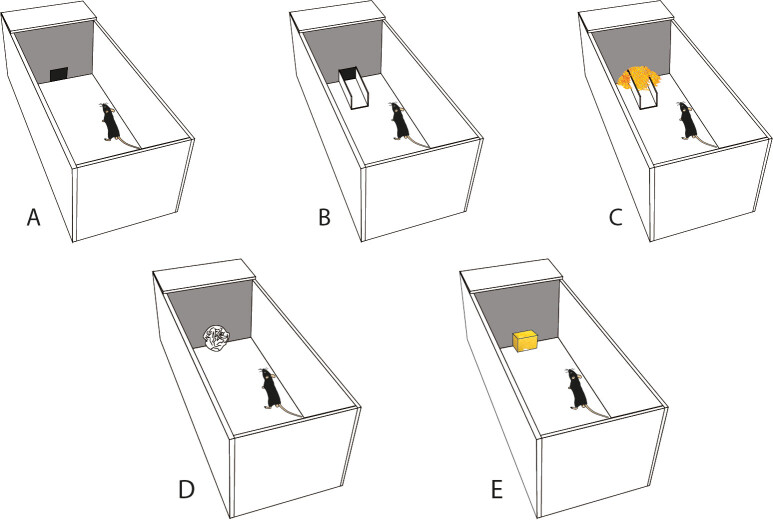 Figure 2: Obstruction conditions within the Puzzle Box task. Schematic diagrams of the Puzzle Box arena and the obstruction conditions used within this study. (A) Condition 0 (C0), with no obstruction present within the doorway between the open-field and goal-box areas. (B) Condition 1 (C1), with a “u shaped” channel present within the doorway between the open-field and goal-box areas. (C) Condition 2 (C2), where the channel is filled with clean bedding material. (D) Condition 3 (C3), with a “tissue plug” present within the doorway between the open-field and goal-box areas. (E) Condition 4 (C4) with a “foam plug” present within the doorway between the open-field and goal-box areas. Dimensions of the arena are as per listed in Methods and Figure 1.
Figure 2: Obstruction conditions within the Puzzle Box task. Schematic diagrams of the Puzzle Box arena and the obstruction conditions used within this study. (A) Condition 0 (C0), with no obstruction present within the doorway between the open-field and goal-box areas. (B) Condition 1 (C1), with a “u shaped” channel present within the doorway between the open-field and goal-box areas. (C) Condition 2 (C2), where the channel is filled with clean bedding material. (D) Condition 3 (C3), with a “tissue plug” present within the doorway between the open-field and goal-box areas. (E) Condition 4 (C4) with a “foam plug” present within the doorway between the open-field and goal-box areas. Dimensions of the arena are as per listed in Methods and Figure 1.
4. Interpretation of Data
Represent data as either time taken to complete the trial (including null trials where animals did not complete the task within the specified time limit) or as the number of null trials.
Use a repeated measures ANOVA to assess the effect of housing condition upon performance within the Puzzle Box, with obstruction type/condition (C) and task number (T) within-subjects factors and enrichment level (standard versus enriched) as between-subjects factor.
Representative Results
The results described here are a representative sample, with data taken from several cohorts consisting of different litters. All behavioral testing was conducted between 0700 and 1100 hr, with randomized testing order of animals within a cohort. Animals raised in an enriched environment (n = 14, 7 female and 7 male) took significantly less time to solve the obstruction tasks within the Puzzle Box than those raised within a standard environment (n = 15, 7 female and 8 male) (see Figure 3) (Repeated measures ANOVA with environment as between-subjects factor, F = 19.525, p <0.001). This effect of EE on performance was observed within individual trials during the Puzzle Box, where enriched mice required significantly less time to solve each of the individual obstruction puzzles, and was particularly marked during the first trial of each condition (Univariate ANOVA with environment as between-subjects factor: Condition 1-Trial 1 (C1T1), F = 4.308, p = 0.048; C1T3, F = 4.317, p = 0.047; C2T1, F = 9.466, p = 0.005; C2T2, F = 5.164, p = 0.031; C2T3, F = 7.031, p = 0.013; C3T1, F = 19.979, p = 0.000; C3T2, F = 5.788, p = 0.023; C3T3, F = 4.711, p = 0.039; C4T1, F = 5.094, p = 0.032). No effect of gender (repeated measures ANOVA with gender as between-subjects factor, F = 1.827, p = 0.188), nor any significant interaction between housing environment and gender was observed (repeated measures ANOVA with environment and gender as between-subjects factors, environment*sex, F = 0.395, p = 0.535).
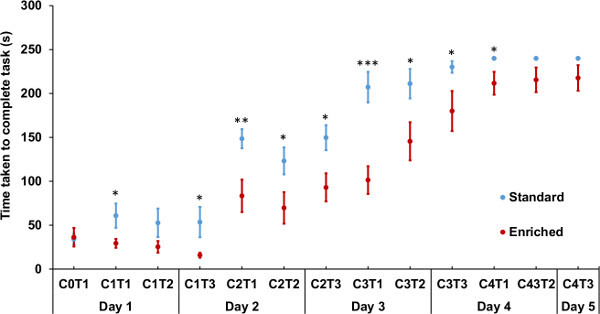 Figure 3: Environmentally enriched mice solve the Puzzle Box faster than standard housed animals. Puzzle Box task performance of adult animals raised from birth in either enriched or standard environments, as measured by the time taken for all four paws to enter the goal-box area in seconds. Animals raised in an enriched environment (red) solved the obstruction tasks within the Puzzle Box significantly faster than those raised in a standard environment (blue) (Repeated measures ANOVA with environment as between-subjects factor, F = 19.525, p <0.001). Improved performance within individual obstruction puzzle tasks was observed for enriched cohorts (Univariate ANOVA with environment as between-subjects factor: C1T1 F = 4.308, p = 0.048; C1T3, F = 4.317, p = 0.047; C2T1, F = 9.466, p = 0.005; C2T2, F = 5.164, p = 0.031; C2T3, F = 7.031, p = 0.013; C3T1, F = 19.979, p = 0.000; C3T2, F = 5.788, p = 0.023; C3T3, F = 4.711, p = 0.039; C4T1, F = 5.094, p = 0.032). C0: no obstruction; C1: U-shaped channel; C2: channel filled with bedding material; C3: tissue plug ; C4: foam plug. C1T1 refers to condition 1, trial 1 etc. (see text). Error bars: Standard error of the mean (SEM), enriched n = 14 (7 female, 7 male), standard n = 15 (7 female, 8 male). *: P <0.05, **: P <0.01, ***: P <0.001.
Figure 3: Environmentally enriched mice solve the Puzzle Box faster than standard housed animals. Puzzle Box task performance of adult animals raised from birth in either enriched or standard environments, as measured by the time taken for all four paws to enter the goal-box area in seconds. Animals raised in an enriched environment (red) solved the obstruction tasks within the Puzzle Box significantly faster than those raised in a standard environment (blue) (Repeated measures ANOVA with environment as between-subjects factor, F = 19.525, p <0.001). Improved performance within individual obstruction puzzle tasks was observed for enriched cohorts (Univariate ANOVA with environment as between-subjects factor: C1T1 F = 4.308, p = 0.048; C1T3, F = 4.317, p = 0.047; C2T1, F = 9.466, p = 0.005; C2T2, F = 5.164, p = 0.031; C2T3, F = 7.031, p = 0.013; C3T1, F = 19.979, p = 0.000; C3T2, F = 5.788, p = 0.023; C3T3, F = 4.711, p = 0.039; C4T1, F = 5.094, p = 0.032). C0: no obstruction; C1: U-shaped channel; C2: channel filled with bedding material; C3: tissue plug ; C4: foam plug. C1T1 refers to condition 1, trial 1 etc. (see text). Error bars: Standard error of the mean (SEM), enriched n = 14 (7 female, 7 male), standard n = 15 (7 female, 8 male). *: P <0.05, **: P <0.01, ***: P <0.001.
Discussion
The data presented demonstrate that the Puzzle Box can be used effectively to assess the impact of EE. Mice raised in enriched environments consistently took significantly less time to solve obstruction puzzles within this behavioral assay than did animals raised within standard laboratory conditions. Moreover, this difference was most prominent in the first trial for three of the four conditions tested, suggesting EE has a greater influence on an animal’s native problem-solving ability, relative to their capacity to reinforce or retain solutions to the problems presented by the task.
The major advantages of the Puzzle Box are its inexpensive material cost, simplicity in terms of construction and implementation, as well as a lack of need for prior training of the subjects to be tested. Further, the method can be adapted to utilize a variety of obstruction materials and conditions. For example, the protocol used here was adapted from previous studies that employed the Puzzle Box to assess cognitive ability in a variety of murine disease state models 5-7. Other studies have previously utilized variations of the Puzzle Box to assess the impact of cognitive-enhancing antipsychotics 33 and observational learning within this testing arena 34. The Puzzle Box therefore offers a behavioral task capable of assessing a wide variety of environmental, genetic and pharmacological manipulations, whilst being relatively time and cost effective.
This inherent flexibility, however, highlights the need for several key steps to successfully implement the task. As the method involves the physical removal of obstacles from a specific opening within the test arena, preliminary trials to determine which obstruction conditions are suitable and solvable within defined time limits by the animals to be assessed is critically important. This is particularly relevant when applying the task to determine the potential role of enrichment on animal models of neurodegenerative disorders whose motor abilities may be severely compromised 1-4,16,17. Moreover, multiple trials across different time intervals are required to thoroughly assess the cognitive abilities of the subjects being tested. Although task acquisition and retention are related, they can be considered as separate processes 35-37. As the findings in this study reveal, significant differences in performance can be greater within one of the factors assessed.
Although latency was the main metric used to monitor animal performance in this study, since the protocol includes video recording of all mice engaged in the task, it is also possible to perform a more detailed analysis of behavior within the testing apparatus. The way subjects from enriched and standard raised groups behave within the arena, including the manner in which they approach obstacles at each phase of the task may reveal further, more subtle differences in performance between the two cohorts 38-43. Combined with the capacity to adjust obstacles to accommodate the animals being tested, the Puzzle Box has the potential to provide a rapid and straightforward means of gaining insight into the influence of environmental factors on a range of cognitive behaviors.
Although simple and powerful, the Puzzle Box is not a substitute for a more thorough analysis of cognitive function. Instead, it provides a rapid and reliable first-pass assessment of problem solving, as well as task acquisition and recall that should then be examined more thoroughly using conventional learning tasks. The importance of such a method cannot be overstated. Traditional learning tasks can require a considerable amount of familiarizing and training of subjects before they can yield interpretable results, which may itself impact upon performance 44,45. Thus, an efficient and reliable method for obtaining a preliminary assessment of cognitive function that can be easily modified to the needs of individual experiments, such as the Puzzle Box, is highly advantageous.
Given that methods to alleviate and reverse the deleterious symptoms exhibited by transgenic models of disease states are being continually developed 46,47, a rapid and reliable means of assessing the effectiveness of the interventions from a behavioral perspective is critical. The data presented here suggests that this Puzzle Box is a useful tool that will enable such assessments.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgments
The authors have no acknowledgements.
References
- Hockly E, et al. Environmental enrichment slows disease progression in R6/2 Huntington's disease mice. Ann Neurol. 2002;51:235–242. doi: 10.1002/ana.10094. [DOI] [PubMed] [Google Scholar]
- Spires TL, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington's in mice. Nature. 2000;404:721–722. doi: 10.1038/35008142. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington's disease. BMC neuroscience. 2008;9:34. doi: 10.1186/1471-2202-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, et al. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol. 2011;227:42–52. doi: 10.1016/j.expneurol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, et al. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav Genet. 2005;35:675–692. doi: 10.1007/s10519-005-3423-9. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleon S, Plomin R. Evidence for general cognitive ability (g) in heterogeneous stock mice and an analysis of potential confounds. Genes Brain Behav. 2002;1:88–95. doi: 10.1034/j.1601-183x.2002.10204.x. [DOI] [PubMed] [Google Scholar]
- Sparling JE, Mahoney M, Baker S, Bielajew C. The effects of gestational and postpartum environmental enrichment on the mother rat: A preliminary investigation. Behav Brain Res. 2010;208:213–223. doi: 10.1016/j.bbr.2009.11.041. [DOI] [PubMed] [Google Scholar]
- Turner CA, Lewis MH. Environmental enrichment: effects on stereotyped behavior and neurotrophin levels. Physiol Behav. 2003;80:259–266. doi: 10.1016/j.physbeh.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Turner CA, Lewis MH, King MA. Environmental enrichment: effects on stereotyped behavior and dendritic morphology. Dev Psychobiol. 2003;43:20–27. doi: 10.1002/dev.10116. [DOI] [PubMed] [Google Scholar]
- Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- Simonetti T, Lee H, Bourke M, Leamey CA, Sawatari A. Enrichment from birth accelerates the functional and cellular development of a motor control area in the mouse. PLoS One. 2009;4:e6780. doi: 10.1371/journal.pone.0006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment) Proc Natl Acad Sci U S A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Progress in neurobiology. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Kondo M, et al. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome--Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008;27:3342–3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134:170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Gortz N, et al. Effects of environmental enrichment on exploration, anxiety, and memory in female TgCRND8 Alzheimer mice. Behav Brain Res. 2008;191:43–48. doi: 10.1016/j.bbr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and Anatomical Plasticity Brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. J Comp Physiol Psychol. 1960;53:509–519. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Li S, Tian X, Hartley DM, Feig LA. The environment versus genetics in controlling the contribution of MAP kinases to synaptic plasticity. Current biology : CB. 2006;16:2303–2313. doi: 10.1016/j.cub.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Angelucci F, et al. Increased concentrations of nerve growth factor and brain-derived neurotrophic factor in the rat cerebellum after exposure to environmental enrichment. Cerebellum. 2009;8:499–506. doi: 10.1007/s12311-009-0129-1. [DOI] [PubMed] [Google Scholar]
- Cancedda L, et al. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetta A, et al. Massage accelerates brain development and the maturation of visual function. J Neurosci. 2009;29:6042–6051. doi: 10.1523/JNEUROSCI.5548-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes BR, et al. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Landi S, Ciucci F, Maffei L, Berardi N, Cenni MC. Setting the pace for retinal development: environmental enrichment acts through insulin-like growth factor 1 and brain-derived neurotrophic factor. J Neurosci. 2009;29:10809–10819. doi: 10.1523/JNEUROSCI.1857-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S, et al. Retinal functional development is sensitive to environmental enrichment: a role for BDNF. FASEB J. 2007;21:130–139. doi: 10.1096/fj.06-6083com. [DOI] [PubMed] [Google Scholar]
- Pham TM, et al. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Pham TM, Soderstrom S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009;32:233–239. doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Sale A, et al. Maternal enrichment during pregnancy accelerates retinal development of the fetus. PLoS One. 2007;2:e1160. doi: 10.1371/journal.pone.0001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolansky MJ, Cabrera RJ, Ibarra GR, Mongiat L, Azcurra JM. Exogenous NGF alters a critical motor period in rat striatum. Neuroreport. 1999;10:2705–2709. doi: 10.1097/00001756-199909090-00003. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Paratcha GC, Ibarra GR, Azcurra JM. Nerve growth factor preserves a critical motor period in rat striatum. J Neurobiol. 1999;38:129–136. doi: 10.1002/(sici)1097-4695(199901)38:1<129::aid-neu10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Thompson R, Huestis PW, Crinella FM, Yu J. Brain mechanisms underlying motor skill learning in the rat. Am. J. Phys. Med. Rehabil. 1990;69(4):191–197. doi: 10.1097/00002060-199008000-00004. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Palomo V, Gil C, Martinez A, Roder JC. Dual inhibitor of PDE7 and GSK-3-VP1.15 acts as antipsychotic and cognitive enhancer in C57BL/6J mice. Neuropharmacology. 2013;64:205–214. doi: 10.1016/j.neuropharm.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Carlier P, Jamon M. Observational learning in C57BL/6j mice. Behav Brain Res. 2006;174:125–131. [Google Scholar]
- Cole BJ, Jones GH. Double dissociation between the effects of muscarinic antagonists and benzodiazepine receptor agonists on the acquisition and retention of passive avoidance. Psychopharmacology (Berl) 1995;118:37–41. doi: 10.1007/BF02245247. [DOI] [PubMed] [Google Scholar]
- Woodside BL, Borroni AM, Hammonds MD, Teyler TJ. NMDA receptors and voltage-dependent calcium channels mediate different aspects of acquisition and retention of a spatial memory task) Neurobiol Learn Mem. 2004;81:105–114. doi: 10.1016/j.nlm.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM, M N, et al. Impaired long-term memory retention: common denominator for acutely or genetically reduced hippocampal neurogenesis in adult mice. Behav Brain Res. 2013;252:275–286. doi: 10.1016/j.bbr.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Viola GG, et al. Influence of environmental enrichment on an object recognition task in CF1 mice. Physiol Behav. 2010;99:17–21. doi: 10.1016/j.physbeh.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Bahr NI, Weiss IC, Wurbel H. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73:209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Hattori S, et al. Enriched environments influence depression-related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav Brain Res. 2007;180:69–76. doi: 10.1016/j.bbr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Barbelivien A, et al. Environmental enrichment increases responding to contextual cues but decreases overall conditioned fear in the rat. Behav Brain Res. 2006;169:231–238. doi: 10.1016/j.bbr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sousa N, Almeida OF, Wotjak CT. A hitchhiker's guide to behavioral analysis in laboratory rodents. Genes Brain Behav. 2006;5 Suppl 2:5–24. doi: 10.1111/j.1601-183X.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, et al. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 2009;34:2601–2608. doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, et al. Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the RXR/RAR pathway. J Biol Chem. 2014. [DOI] [PMC free article] [PubMed]
- Perez HJ, et al. Neuroprotective effect of silymarin in a MPTP mouse model of Parkinson's disease. Toxicology. 2014;319C:38–43. doi: 10.1016/j.tox.2014.02.009. [DOI] [PubMed] [Google Scholar]


