Abstract
Certain classes of chemotherapies may exert acute vascular changes that may progress into long-term conditions that may predispose the patient to an increased risk of vascular morbidity. Yet, albeit the mounting clinical evidence, there is a paucity of clear studies of vascular toxicity and therefore the etiology of a heterogeneous group of vascular/cardiovascular disorders remains to be elucidated. Moreover, the mechanism that may underlie vascular toxicity can completely differ from the principles of chemotherapy-induced cardiotoxicity, which is related to direct myocyte injury. We have established a real-time, in vivo molecular imaging platform to evaluate the potential acute vascular toxicity of anti-cancer therapies.
We have set up a platform of in vivo, high-resolution molecular imaging in mice, suitable for visualizing vasculature within confined organs and reference blood vessels within the same individuals whereas each individual serve as its own control. Blood vessel walls were impaired after doxorubicin administration, representing a unique mechanism of vascular toxicity that may be the early event in end-organ injury. Herein, the method of fibered confocal fluorescent microscopy (FCFM) based imaging is described, which provides an innovative mode to understand physiological phenomena at the cellular and sub-cellular levels in animal subjects.
Keywords: Medicine, Issue 95, in-vivo imaging, fibered confocal endoscopic microscopy, real-time imaging, high-resolution animal imaging, vascular imaging, vascular impairment
Introduction
Clinical evidence indicates that several classes of chemotherapies elicit a variety of vascular pathologies manifested by Raynaud phenomenon, hypertension, myocardialinfarction, cerebrovascular attack, and hepatic veno-occlusivedisease1,2. “’Accidental’ anti-angiogenic drugs” is a fairly new term, which describes conventional chemotherapeutic agents that act as possible angiogenesis inhibitors, although they not originally developed for this purpose3-5 but designed to eliminate tumor cells by imposing as little “collateral damage” to normal cells as possible3. Several chemotherapies have been implied as vasculo-toxicants as observed in clinical studies using serum biomarkers. Among these are alkylating agents (such as cyclophosphamide), platinum compounds (such as cisplatin) and anthracyclines1,2,5-7.
Acute cardiovascular complications may occur as a result of vascular toxicity induced by chemotherapy. They may progress into chronic conditions like atherosclerosis and account for increased risk of late vascular morbidity. Yet, despite mounting clinical evidence, there is a paucity of designated studies emphasizing the mechanism of vascular toxicity and therefore, further elucidation of the exact pathogenesis they inflict is warranted.
A major challenge in revealing the mechanism of chemotherapy-induced vascular toxicity derives from the complexity of investigating vascular function in vivo. We describe herein a platform of high-resolution in vivo molecular imaging in mice that enables to capture blood flow and vessels’ characteristics. This platform facilitates the detection of direct treatment-induced vascular effects: in real-time, as well as following them over a period of time within the same individuals.
Protocol
Ethics statement: All experiments were approved by the Institutional Animal Care and Use Committee. Animal care was according to institutional guidelines. ICR female mice (7 - 8 weeks old; 25 - 30 g) were housed in air conditioned, light controlled animal facilities of the Sackler Faculty of Medicine in Tel-Aviv University. At term, animals were euthanized with anesthesia overdose.
1. Fibred Confocal Fluorescence Microscopy (FCFM) Calibration
Turn the device ON.
Connect the microprobe (mini0/30).
Calibrate the device according to manufacturer’s instructions.
2. Mice Preparation for Imaging
Anesthetize by a subcutaneous injection of both Ketaset (100 mg/kg) and XYL-M2 (6 mg/kg). Confirm proper anesthetization by unresponsiveness to toe-pinch.
Incise the skin below the groin in order to reveal the femoral arterial vessels. Keep the incision site moist with saline following incision.
Heat the tail by using a bag (or a glove) filled with warm water (not too hot to touch) for approximately 30 sec. Prepare an intravenous (IV) shunt for administration of FITC dextran (a contrast agent) and of either saline or chemotherapeutic agent, by inserting a needle (30 G, 1/2 inch) into the tail vein and attaching a 1 ml syringe to it. Ensure the vein is open by injecting saline. NOTE: An IV administration of FITC dextran (high molecular weight; 100 µl; 10 mg/ml; 2,000 kDa) facilitates visualizing the femoral microvasculature by FCFM. Doxorubicin (100 µl; 8 mg/kg, Adriamycin) or saline will also be later administered IV into the pre-heated tail vein.
Position the mouse supinely on a polystyrene stage. Secure the mouse to the pad and maintain position using surgical duct tape.
3. Imaging of Femoral Blood Vessels by FCFM During and After Administration of Doxorubicin or Saline
NOTE: The fibered confocal microscope used in this study is composed of two units: (1) microprobe (mini0/30). (2) Laser scanning unit (LSU-488; 488 nm wavelength).
Perform all time-laps analyses using the LSU 488 nm wavelength laser. NOTE: The main unit detector detects the filtered (500 - 650 nm) emitted fluorescence. The acquired images are reconstructed afterwards and displayed at a rate of 12 frames/sec.
Disconnect carefully the syringe from the needle and attach a new syringe containing FITC dextran. Administer (IV) 100 µl of FITC dextran.
Shift the microprobe (mini0/30) to a suitable field of view and fixate it, following adjustment to the z-axis, in order to obtain the corresponding image. Wait for the initial signal to fade until a clear and focused signal is visible.
Record a baseline blood flow for a short stabilization period (~30 sec). Then connect to the needle another syringe, containing either doxorubicin or saline. IV administer 100 µl doxorubicin or saline.
Monitor the flow of injected FITC-dextran continuously for 20 min. On the FCFM-associated software, use the diameter button on the upper ruler in order to measure the blood vessels and categorize them as small (<15 µm) or large (>15 µm).
Euthanize the animal with anesthesia overdose.
Representative Results
In vivo continuous imaging at real-time
The imaging apparatus used here is a high definition, fibered confocal microscope, equipped with a probe that enables visualization of vasculature and its response to various stimuli as chemotherapy. This method is minimally invasive since although it may facilitate imaging of deep vessels or organ, it requires a small incision for the probe. The probe bundles consist of tens of thousands of fibers, microscope optics and a proprietary precision connector. A schematic representation is shown in Figure 1.
Imaging of femoral microvasculature
We have classified the network of femoral blood vasculature according to vessels diameter (small < 15 µm; large > 15 µm) by FCFM in mice injected with FITC-dextran. A rapid vasoconstriction (2 - 5 min) of small vessels was induced by doxorubicin. A complete disappearance of the FITC-dextran fluorescent, 8 min post doxorubicin treatment (Figure 2A e-g, arrows; Video 1). In several mice, reduction of the fluorescent signal in the blood vessel and increase signal of its surrounding was observed in the perivascular region, a few seconds after doxorubicin administration (Figure 2B g, arrows). These results indicate that increase in vessel permeability and leakage of the high molecular weight dextran from the blood vessel to the surrounding tissues. No fluorescence signal was evident following administration of doxorubicin in mice that were not injected previously with FITC-dextran. Paclitaxel-treated mice demonstrated similar blood vessels structure and flow rate to those observed in saline-injected mice (not shown), throughout the measurement period.
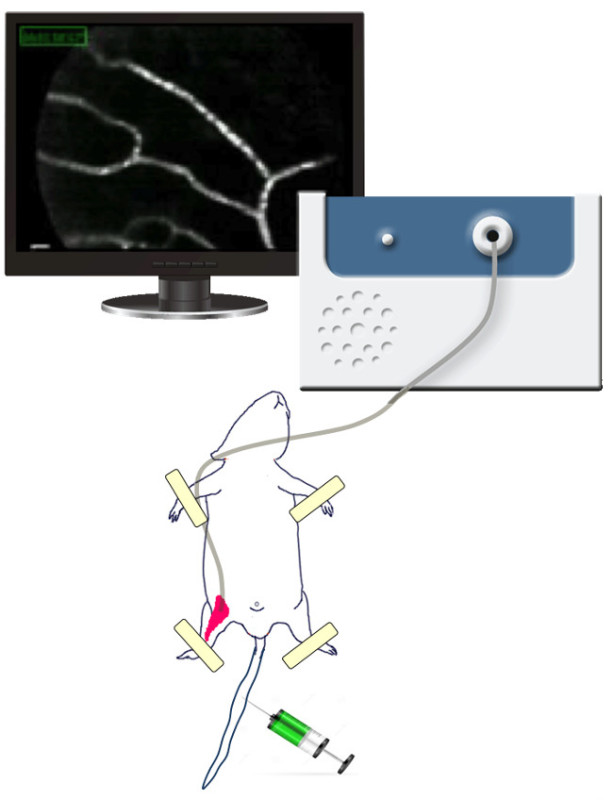 Figure 1. The FCFM. The confocal microscope used here is composed of two units: the microprobe (mini0/30) and the laser scanning unit (LSU-488; 488 nm wavelength).
Figure 1. The FCFM. The confocal microscope used here is composed of two units: the microprobe (mini0/30) and the laser scanning unit (LSU-488; 488 nm wavelength).
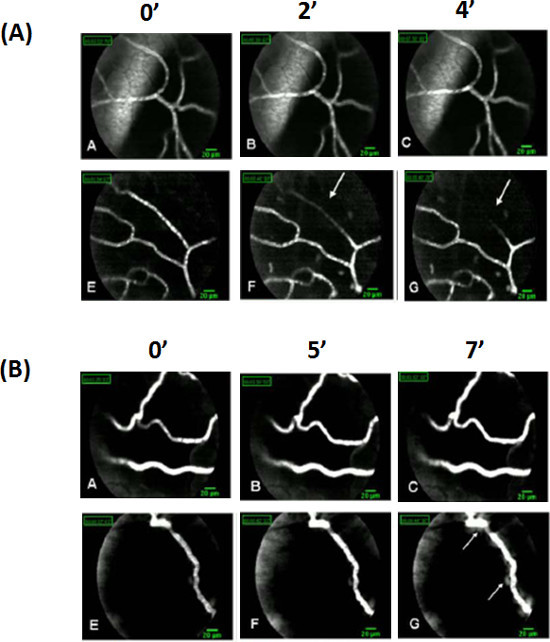 Figure 2. Images of FITC-dextran fluorescence signal in femoral microvasculature. Vessels were dichotomously classified to minor (<15 µm) or major (>15 µm) according to their caliber. Femoral microvasculature of FITC dextran (100 µl; 10 mg/ml) injected mice was imaged before and during IV administration of either doxorubicin or saline. (A) The minor vessels, imaged by FCFM, started vasoconstricting acutely 2 min after doxorubicin administration (f, arrow), showing a continuous narrowing of the fluorescent signal until its complete disappearance, with no recovery apparent recovery of the signal during the next eight minutes of the real-time imaging (g, arrow). (B) Appearance, in some snapshots, of a "hazy" area around the blood vessels walls of chemotherapy-injected mice (g arrow) immediately after injection, indicates potential Dextran-FITC leakage. Please click here to view a larger version of this figure.
Figure 2. Images of FITC-dextran fluorescence signal in femoral microvasculature. Vessels were dichotomously classified to minor (<15 µm) or major (>15 µm) according to their caliber. Femoral microvasculature of FITC dextran (100 µl; 10 mg/ml) injected mice was imaged before and during IV administration of either doxorubicin or saline. (A) The minor vessels, imaged by FCFM, started vasoconstricting acutely 2 min after doxorubicin administration (f, arrow), showing a continuous narrowing of the fluorescent signal until its complete disappearance, with no recovery apparent recovery of the signal during the next eight minutes of the real-time imaging (g, arrow). (B) Appearance, in some snapshots, of a "hazy" area around the blood vessels walls of chemotherapy-injected mice (g arrow) immediately after injection, indicates potential Dextran-FITC leakage. Please click here to view a larger version of this figure.
Video 1. Femoral microvasculature imaging. A representative photographed film of FITC-dextran fluorescent minor (< 15 µm in diameter) blood vessels. Ovarian and femoral microvasculature time laps photography: mice injected with 100 µl FITC-dextran (10 mg/ml) were imaged and photographed from the moment of IV administration of doxorubicin. 2 - 5 min after doxorubicin injection, minor vessels showed dramatic vasoconstriction followed by complete abolishment of the fluorescent signal, already 8 min after treatment. (AVI) Please click here to view this video.
Discussion
Evaluating chemotherapy-induced vascular toxicity is challenging due to the difficulty in visualizing the dynamics of vasculature in response to a stimuli in real-time. Numerous clinical studies have implicated that several chemotherapies cause direct vascular injury, yet the mechanism and characteristics of this toxicity remains to be elucidated. We have established a real-time, in vivo molecular imaging platform for evaluating the potential vascular toxicity of chemotherapy in mice comprising of fibered confocal fluorescent microscopy as described herein8-10. This high-resolution molecular imaging of mice is suitable for visualizing arterial blood flow and vessels’ architecture. It enables real-time detection of treatment-induced complications in the same animal over an extended period of time. We evaluated two classes of chemotherapies: doxorubicin which is known to be toxic to endothelial cells in vitro as well as in tissues obtained from animals treated with doxorubicin10-15, and paclitaxel as a control chemotherapy for which the former evidence for any vascular effect is very limited.
The laser scanning confocal technology of FCFM facilitates tracing fluorescence-dyed deep tissues at real-time and produces time-lapse video images of blood vessels in vivo9. In our study FCFM was used to observe the acute vascular effect of doxorubicin, as a prototype vasculotoxic agent. This effect, which began shortly after doxorubicin administration, was dependent upon blood vessels size: the smaller the vessels' diameter, the more prominent the damage. The fluorescence signal of small-diameter (<15 µm) vessels diminished gradually as a result of constant vessels constriction caused by doxorubicin. No apparent recovery was detected during the next 8 min of real-time imaging. Large diameter (>15 µm) vessels were less damaged; the integrity of their wall was compromised, exhibiting an irregular surface. These effects were unique to doxorubicin and were not evident when paclitaxel was utilized, indicating that this methodology delineates meticulously the specific effect of the drug.
Modifications and troubleshooting
Throughout the protocol, the laser power may be changed during the baseline recording in order to illustrate the vessels. However, laser power must not be changed during the experiment. In addition, a high laser power might bleach the fluorescence agent during time. Additionally, once recording is finished, it is possible to change the contrast of the movie and to edit its length and speed. Measurements of the vessels may then be made and changes that occur may be followed.
Limitations of the technique and critical steps
The FCMF device has few limitations. The region of interest (ROI) might change during the imaging time. Additionally, the imaged area might dry out; one should keep the area hydrated. The fluorescence agent might bleach and the signal will be lost. One critical step one should pay attention to is to keep the animal and the probe well fixed to avoid changes of the ROI.
Significance and future applications
The established experimental platform may serve as an immediate test for response to chemotherapy or alternatively as a potential biological marker to characterize the potential vascular toxicity profile. Based upon the studied mechanism of vascular impairment, the method can also be useful in the future for evaluating potential agents specified to reduce vascular toxicity induced by chemotherapy. The need to decrease the potential long term vascular complications in cancer survivors drives us to explore the mechanism behind chemotherapy-induced vascular toxicity.
Disclosures
None
Acknowledgments
None
References
- Chow AY, et al. Anthracyclines cause endothelial injury in pediatric cancer patients: a pilot study. J Clin Oncol. 2006;24(6):925–928. doi: 10.1200/JCO.2005.03.5956. [DOI] [PubMed] [Google Scholar]
- Nuver J, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005;23(36):9130–9137. doi: 10.1200/JCO.2005.01.4092. [DOI] [PubMed] [Google Scholar]
- Vos FY, et al. Endothelial cell effects of cytotoxics: balance between desired and unwanted effects. Cancer Treat Rev. 2004;30(6):495–513. doi: 10.1016/j.ctrv.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kerbel RS, et al. 'Accidental' anti-angiogenic drugs. anti-oncogene directed signal transduction inhibitors and conventional chemotherapeutic agents as examples.Eur. J Cancer. 2000;36(10):1248–1257. doi: 10.1016/s0959-8049(00)00092-7. [DOI] [PubMed] [Google Scholar]
- Soultati A, et al. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38(5):473–483. doi: 10.1016/j.ctrv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Tempelhoff GF, et al. Blood coagulation during adjuvant epirubicin/cyclophosphamide chemotherapy in patients with primary operable breast cancer. J Clin Oncol. 1996;14(9):2560–2568. doi: 10.1200/JCO.1996.14.9.2560. [DOI] [PubMed] [Google Scholar]
- Ben Aharon I, et al. Doxorubicin-induced vascular toxicity--targeting potential pathways may reduce procoagulant activity. PLoS One. 2013;8(9):e7515. doi: 10.1371/journal.pone.0075157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmel E, et al. Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy. J Vasc Res. 2004;41(5):400–411. doi: 10.1159/000081209. [DOI] [PubMed] [Google Scholar]
- Al-Gubory KH, Houdebine LM. In vivo imaging of green fluorescent protein-expressing cells in transgenic animals using fibred confocal fluorescence microscopy. Eur J Cell Biol. 2006;85(8):837–845. doi: 10.1016/j.ejcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph H, et al. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS One. 2011;6(9):e23492. doi: 10.1371/journal.pone.0023492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal V, Kaushal GP, Mehta P. Differential toxicity of anthracyclines on cultured endothelial cells. Endothelium. 2004;11(5-6):253–258. doi: 10.1080/10623320490904124. [DOI] [PubMed] [Google Scholar]
- Kim EJ, et al. Doxorubicin-induced platelet cytotoxicity: a new contributory factor for doxorubicin-mediated thrombocytopenia. J Thromb Haemost. 2009;7(7):1172–1183. doi: 10.1111/j.1538-7836.2009.03477.x. [DOI] [PubMed] [Google Scholar]
- Walsh J, Wheeler HR, Geczy CL. Modulation of tissue factor on human monocytes by cisplatin and adriamycin. Br J Haematol. 1992;81(4):480–488. doi: 10.1111/j.1365-2141.1992.tb02978.x. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, et al. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275(43):33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, et al. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36(38):11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]


