Abstract
Background
In cosmetic science, noninvasive sampling of the upper part of the stratum corneum is conveniently performed using strippings with adhesive-coated discs (SACD) and cyanoacrylate skin surface strippings (CSSSs).
Methods
Under controlled conditions, it is possible to scrutinize SACD and CSSS with objectivity using appropriate methods of analytical morphology. These procedures apply to a series of clinical conditions including xerosis grading, comedometry, corneodynamics, corneomelametry, corneosurfametry, corneoxenometry, and dandruff assessment.
Results
With any of the analytical evaluations, SACD and CSSS provide specific salient information that is useful in the field of cosmetology. In particular, both methods appear valuable and complementary in assessing the human skin compatibility of personal skincare products.
Conclusion
A set of quantitative analytical methods applicable to the minimally invasive and low-cost SACD and CSSS procedures allow for a sound assessment of cosmetic effects on the stratum corneum. Under regular conditions, both methods are painless and do not induce adverse events. Globally, CSSS appears more precise and informative than the regular SACD stripping.
Keywords: irritation, morphometry, quantitative morphology, stripping
Introduction
The human stratum corneum (SC) is a superficial continuous membrane made of tightly stacked corneocytes parted by multilamellar lipid sheets. Its deep cohesive portion is called the stratum compactum, contrasting with the loose superficial part referred to as the stratum disjunctum. Such a structure undergoes a maturation process while it moves upwardly to the surface of the skin. There is a progressive reduction in intercorneocyte cohesion, ending with physiologically imperceptible desquamation.1 Considering the typical continuous renewal of the epidermis, and the structure of the SC as its end product, the SC corresponds to a recollection of the past history of the recent life of the epidermis.1 Alterations in the organization of corneocytes, the presence of parakeratosis, and various other aspects are therefore clues for the intervention of some previous physiopathological disturbances.
At most locations on the body surface, the SC is typically composed of 15 or so layers of ordered flattened corneocytes. At the skin surface, these cells are approximately 1 μm thick, and their mean area reaches approximately 1,000 μm2. The corneocyte area is influenced by the anatomic location, and a variety of environmental conditions including chemical irritation and ultraviolet light (UVL) action modulate the epidermal renewal.1 In addition, the average corneocyte size is assumed to increase with aging. This feature is probably linked to a prolonged corneocyte transit time during its progression throughout the SC.
Some cosmetic compounds act on the SC, and modulate its structure and the functions that it serves. Other compounds target the SC by an indirect route through modulations in the physiology of the underlying stratum spinosum. No proper pharmacology in the SC exists without adequate and reliable techniques for objective analytical recordings of the effects of the test products. In this review, emphasis is placed on some of the methods using distinct skin strippings.
SC collection
Smears and skin scrapings are simple procedures for getting SC samples. Unfortunately, the collected material is obviously torn out and fragmented. The surface topography and the cell organizations are not appreciated in such samplings. As a consequence, quantification procedures are almost impossible to perform on such material in a reproducible way.
Basically, SC stripping is the regular way for collecting the stratum disjunctum.2 Using casual adhesive transparent tapes is poorly reliable for quantitative assessments because their adhesion to the SC is uncontrolled and appears variable among different brands. By contrast, SC stripping using adhesive-coated discs (SACD) is conveniently performed.3 An improved method using a liquid cyanoacrylate pressure-sensitive adhesive on a polyester slide was designed for getting a better sampling of the SC structure.4–10 Such a procedure corresponds to the cyanoacrylate skin surface stripping (CSSS), formerly called skin surface biopsy4 or follicular biopsy.11
SACD uses a crystal clear adhesive-coated disc (D-Squame®; Cuderm Corporation, Dallas, TX, USA; and Corneofix®; CK Electronic, Cologne, Germany). The device provides adequate rigidity and adhesion for uniformly sampling a defined area of the stratum disjunctum. After peeling off the protective seal, the disc is applied to the SC surface using a gauge spring dynamometer for ensuring a calibrated and reliable pressure.3 Frequently, the chosen pressure is in the range of 100–250 g/cm2. Both the pressure and the application time of the disc influence the amount of collected SC. A short-time (approximately 5 seconds) application of the disc removes less stratum disjunctum than an extended duration (approximately 30–60 minutes) of application. This is likely due to occlusion modifying both the intrinsic SC moisture and the intercorneocyte cohesion. Any greasy topical agent applied to the skin before sampling impairs the adhesion of the disc to the SC, thus yielding unreliable information. Such sampling failures are limited following delipidization of the skin surface using, for instance, ether:acetone (1:1).
For the CSSS method (3S-Biokit; CK Technology, Visé, Belgium), a droplet of cyanoacrylate glue is deposited onto a clear polyethylene strip. This material is pressed against the SC surface for approximately 15–30 seconds. In presence of discrete SC moisture, the cyanoacrylate polymerizes and firmly adheres to the SC. Ultimately, the material is gently lifted at one tip, and peeled off the skin. CSSS is adequately performed from any site on the body, with two main provisos. First, CSSS harvesting from a hairy area is commonly painful due to pulling out hairs. Furthermore, the CSSS quality is inadequate owing to the erratic contact of the cyanoacrylate bound to the SC. It is therefore advisable to shave such areas before CSSS harvesting. Second, a problem results from the natural strong intercorneocyte cohesion on the palms and soles. Such intercellular binding is commonly stronger than the glue bond on CSSS. This limitation impairs the collection of a uniform thin layer of corneocytes from these sites. However, such CSSS samplings become adequate in some instances when the SC cohesiveness is compromised.
Overall aspect of normal CSSS
CSSS from normal skin reveals a regular crisscross network of high-peaked crests corresponding to discrete skin surface creases called the first-, second-, and third-hollow order lines. Their patterns of distribution are distinct on various body regions. The first-order lines correspond to grooves in the latticework papillary relief at the dermo–epidermal interface.7 In young individuals, junctions of the shallow first- and second-order lines border contiguous polyhedral shaped SC plateaus (Figure 1A). On stretching the skin surface, a reorganization of these lines occurs. With aging, such a network progressively alters its original configuration, bringing the lattice along the skin tension lines spontaneously into alignment.8 The process ends with the vanishing of the shallow skin surface lines (Figure 1B). Thus, it is possible to assess the overall texture of the superficial dermis on CSSS. As a result, conditions such as dermal aging, dermal atrophy (dermatoporosis), striae distensae, sclerosis, scars, and many other skin connective tissue alterations are conveniently observed noninvasively using CSSS.7,8,10 Such morphological assessment of the skin microrelief is possibly quantified combining computerized image analysis and profilometry (Figure 1C).
Figure 1.
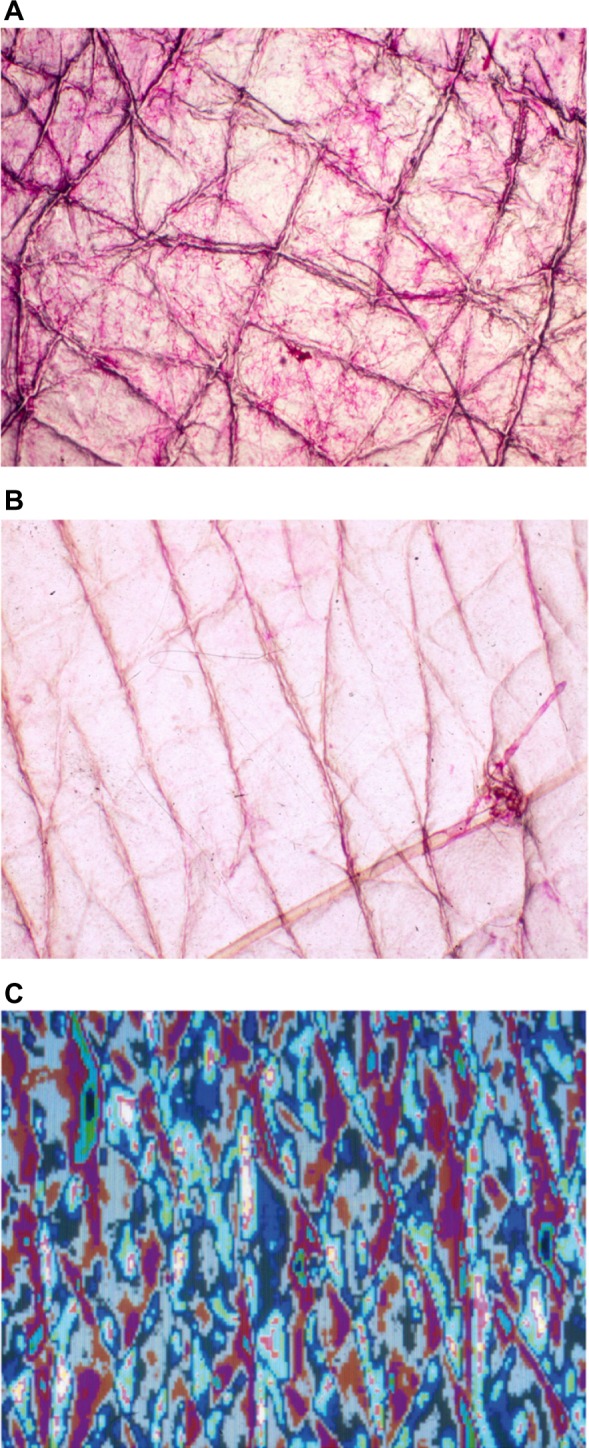
Regular aspects of CSSS from the forearm.
Notes: (A) Crisscross pattern of shallow lines in a young adult. (B) Alignment of shallow lines along Langer’s lines in an elderly subject. (C) Image analysis for profilometry of the skin surface pattern.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
Cytologic presentations of corneocytes are hardly visible on unstained CSSS. For improving their perceived aspect, several histochemical dyes are useful. A mixture of toluidine blue and basic fuchsin (TBBF) in 30% ethanol is a simple dye that is conveniently handled in an office setting.7,8,10 The regular SC exhibits a rather uniform cohesive pattern of adjacent corneocytes. Each single corneocyte is typically anucleated and contains a water-insoluble protein complex corresponding to a highly organized keratin microfibrillar matrix. Such cell structure is encapsulated in a protein- and lipid-enriched shell. The cell boundaries appear clearly stained by a thin polyhedral TBBF rim.10 The cornified cell envelope exhibits different stages of maturation among corneocytes.12,13 Basically, two distinct presentations of cornified cell envelopes are distinguished. They correspond to the so-called fragile immature envelopes and the rigid mature ones, respectively (Figure 2A). The former type is recognized on CSSS by a deep TBBF staining contrasting with the rimmed pattern of the mature corneocytes.10 Scanning electron microscopy of immature corneocytes commonly exhibits a paving of small protrusions of similar sizes.10
Figure 2.
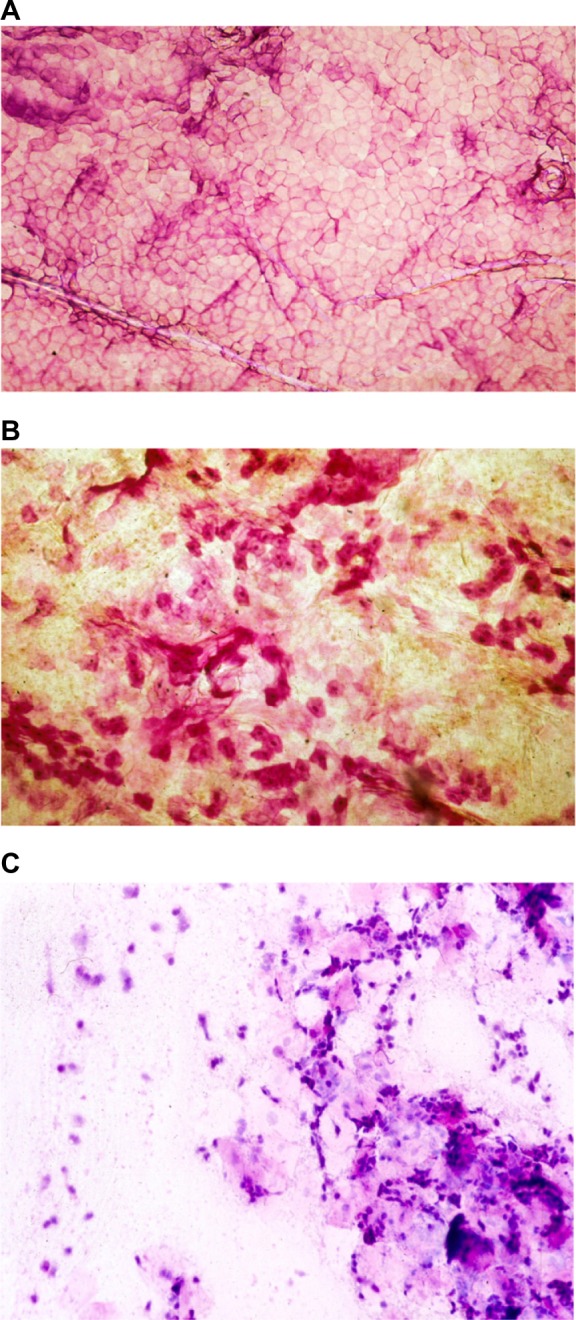
Cytologic aspects in CSSS.
Notes: (A) Uniform paving of corneocytes. (B) Dispersed parakeratotic cells. (C) Inflammatory cells.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
Lipid staining such as the Nile red stain conveniently reveals sebum-enriched follicular pores and follicular casts collected on CSSS. Any histochemical positivity at the follicular sites corresponds to two distinct phenomena. First, the visualized sebum closely represents the direct lipid production by each single follicle. Second, the lipids produced and poured out from other follicles in their vicinity run at the skin surface and are finally collected in the slope of other follicular openings.
Analytical morphology of skin strippings
Skin strippings, including SACD and CSSS, are used as nearly noninvasive diagnostic methods and are useful in many skin conditions.1,5,7,10 In addition, they are examples of tools that combine analytical morphology and other procedures for the evaluation of the kinetics and efficacy of various cosmetics and toiletries. Many cosmetic compounds and cosmeceuticals exert modulating effects, in part influencing the SC presentation. Analytical morphology applied to SACD and CSSS provides either quantitative, semiquantitative, or binary data. These procedures include various techniques, and they afford quantitative and statistically evaluable data following image processing. The challenge of analytical evaluations of skin strippings is to quantify some specific aspects of the structure and functions of the SC.14,15 Image analysis of the aspect of samples seen under the microscope, and optical profilometry of the surface of the samples are two classical methods of analytical morphology that are conveniently applied to skin strippings. Other biometrological approaches have been designed, including visual rating evaluations of sampled material placed on a black background. More precise evaluations are gained from reflectance colorimetry and light transmission assessments. The weight of collected SC samples on SACD are measured and can further be analyzed for the molecular identification of specific components. For that purpose, the samples are conveniently deposited in the well of microanalysis plates.
In these respects, skin strippings are used in four main ways:
Evaluation of the SC structure
Evaluation of the SC dynamics
In situ evaluation of the superficial skin biocene
Use of CSSS as a substrate for a set of ex vivo bioassays
Analytical morphology denotes a set of techniques that provide quantitative data and statistically evaluable information from morphological aspects. Of note, meaningful measurements are dependent upon careful thought given to the sampling process. Pressure and time of application of the clear disc in SACD, and the quality of the cyanoacrylate liquid bond in CSSS are of the utmost importance. The cleanness of the sampled SC without any residual greasy product is mandatory.
Some analytical measurements are conveniently performed on CSSS. They commonly rely on the combination of optical properties of the samples, reflectance colorimetry, and morphometry-based image analysis. Some aspects of skin conditions, including their severity and any cosmetic improvement, are conveniently assessed on CSSS following the disclosure of some typical features in the SC.
Because SACD and CSSS are very thin compared to the width of the sampling, both samplings are regarded as nearly two-dimensional structures. Under microscopic examination, the size of the field of vision is determined by the combination of the magnification and the microscope eyepiece. Usually, the eyepiece field diaphragm opening diameter determines the size of the field of vision. Morphometry concerns measurements made of shapes. Such measurements primarily rely on point and linear procedures. The proportion of points in an ocular graticule gives a relative value for its area. Such values are converted to absolute values when the area of the sample covered by the graticule is determined.
It is possible to measure the area of the exposed aspect of corneocytes, and to detect any presence of parakeratosis (Figure 2B) and other cells on such specimens (Figure 2C). On healthy skin, parakeratotic cells are rare, and those present are not clustered. By contrast, clumps of parakeratotic cells usually suggest a pathologic process. They are recognized by the presence of a nucleus central to the polyhedral cell. Optical profilometry is conveniently applied to CSSS. There is a clinical relevance for the skin roughness parameters (Ra and Rz) gained on CSSS.
Grading and analytical morphology of xerosis
Xerosis refers to a rough and dry-looking aspect of the skin. The relationship between xerosis and lack of water in outer layers of the SC is ambiguous.16 Most xerotic conditions are in fact a failure of the normal corneocyte shedding process. These cells remain attached to each other with unequal strength until rafts of cells detach partially from the skin surface (Figure 3). This process of corneocyte clumping is possibly assessed using visual and tactile scoring. Such crude evaluations suffer from variability by inconsistencies from grade to grade, and from poor reproducibility. Subtle variations in the environmental conditions jeopardize subjective grading systems since ambient hydration swells the outer SC and often camouflages low-grade scaling and dryness. Thus, in order to investigate any xerotic process, and to determine the efficacy of a moisturizer, an evaluation program should be encouraged to go beyond usual clinical observations.
Figure 3.
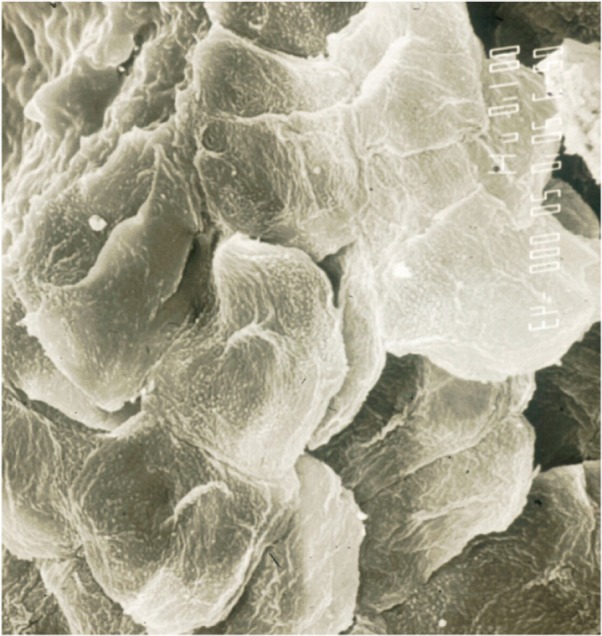
Corneocyte clumpiness collected on a CSSS (scanning electron microscopy).
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
Some cosmetics and cosmeceuticals reduce desquamation and scaling. Normal desquamation takes place imperceptibly as the release of single corneocytes at the skin surface. When the intracorneal cohesion fails to decline evenly in the stratum disjunctum, clumps of corneocytes remain stuck together, and they come off as scales. The resulting harsh feel by sensing fingers, and the altered appearance under light reflectance correspond to xerosis, often interpreted as dry skin by laypeople. In fact, the concept of dry skin remains a controversial matter.16
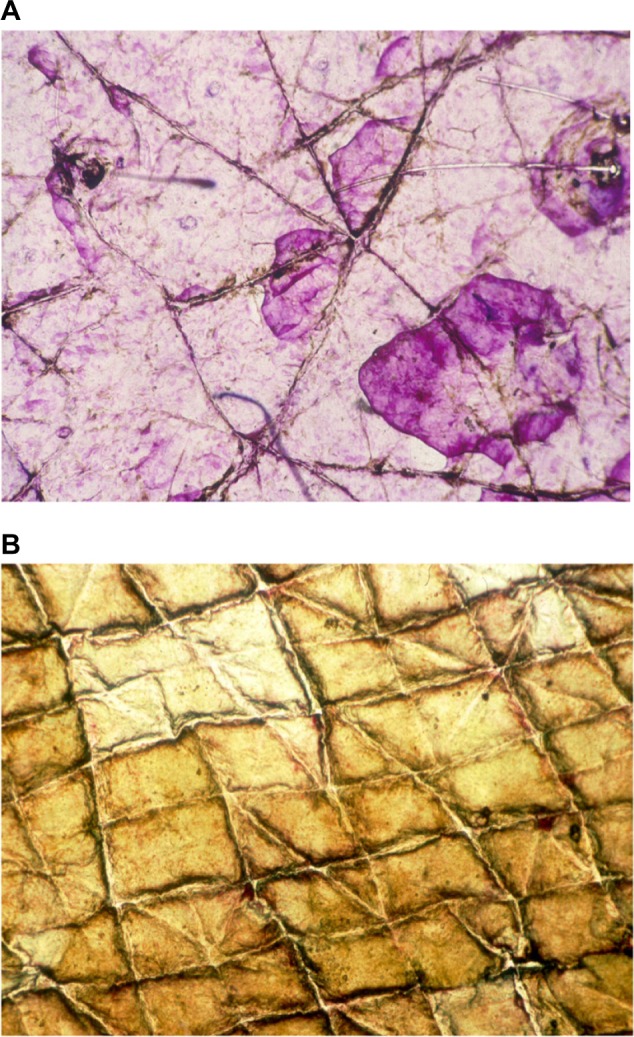
Xerosis refers to various forms of predominantly orthokeratotic hyperkeratosis. This definition and its limits are idiosyncratic, varying according to different local traditions among laboratories and cosmetic companies. A semiquantitative grading of orthokeratotic xeroses is conveniently achieved on CSSS stained for 3 minutes with the TBBF staining solution.7,10,16 The samples are graded7,10 as follows:
Type 0: normal SC without any evidence for hyperkeratosis
Type 1: hyperkeratosis of the first- and second-order lines and/or the adnexal openings
Type 2: focal hyperkeratosis covering less than 30% of the skin surface plateaus (Figure 4A)
Type 3: hyperkeratosis covering over 30% of the skin surface plateaus (Figure 4B)
Type 4: diffuse, confluent, and homogeneous scales with persistence of the first-order lines
Type 5: thick, uneven scales covering the entire SC surface, obliterating the shallow lines
Figure 4.
Xerosis appearance on CSSS.
Notes: (A) Type 2. (B) Type 3.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
Quantitative assessment of xerosis relies on analytical evaluations of SACD.3,14 The attenuation of optical light transmission has been used to measure the amount of scales collected on the discs. That method was improved by measuring reflectance colorimetry of the unstained sample deposited onto a colored reference plate. Thick scales abate the L* and a* values of reflectance colorimetry. Image analysis of SACD is possible for the quantification of desquamation disorders. The parameters of importance are the area (A) covered by scales, and their thickness rated on a 5-level gray scale. The percentage of scales (Tn) is calculated in relation to each thickness level (n). A desquamation index (DI) is yielded according to:
| (1) |
The squamometry index (SQMI) is yet another objective evaluation of xerosis and some other SC disturbances.17,18 SACD are stained for 1 minute by dropping the TBBF solution over the SC surface, followed by gentle tap-water rinsing. The stained SACD is placed over a hole cut out of a slide, which is placed onto a white color reference plate. The Chroma C* value measured by reflectance colorimetry correlates with the amount of removed scales and with the clinical rating of xerosis. A seemingly regular SC of the volar forearm is commonly characterized by SQMI values lower than 20. By contrast, a normal scalp without dandruff shows a weaker SQMI below 5. This technique is conveniently applied for assessing some inflammatory conditions such as sunburn (Figure 5), and to quantify the efficacy of antidandruff shampoos.
Figure 5.
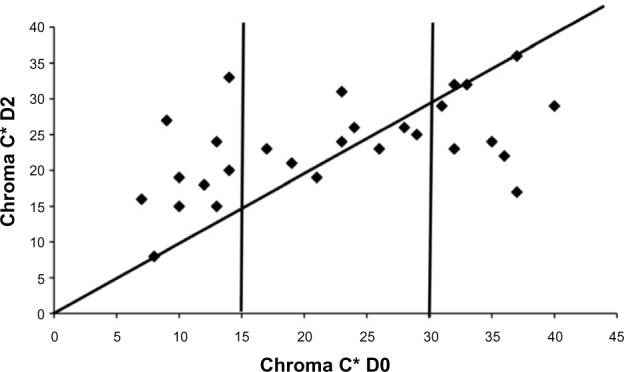
SQMI as assessed by Chroma C* at D0 and D2.
Note: Normal skin (Chroma C* D0<15) shows an increase in SQMI while xerotic skin (Chroma C* D0>30) responds with a decrease in SQMI values.
Abbreviations: D0, before acute photodamage; D2, 48 hours after acute photodamage; SQMI, squamometry index.
Xerosis of old age as well as wrinkling are possibly evaluated on CSSS. In particular, the wrinkling process and its correction by some cosmetics is conveniently substantiated by combining image analysis and optical profilometry.
Comedometry
Follicular casts and microcomedones are conveniently studied on CSSS collected from the face or the back. The horny material sampled from the upper portion of the follicular ducts corresponds to a follicular stripping,11,19 and it reflects the balance between comedo formation and lysis (Figure 6A). Comedometry allows for computerized quantification of the number and size of follicular casts collected on CSSS.11,19,20 The numerical density of follicles is influenced by the body site. For each site with absence of obvious comedogenesis, the interindividual variation remains small. This method finds application in comedogenesis- and comedolysis-related disorders and their treatments.20–23 Both the number and the size of follicular casts are influenced by some treatments. Comedometry on human skin showing comedogenesis reveals large interindividual differences in the number of horny follicular casts. CSSS are used to predict the comedogenic risk linked to products, and, by contrast, to quantify any comedolytic activity of cosmetics. The concentration in active compounds governs the propensity of modifying the microcomedo density and size. When an exogenous comedogenic factor is involved, the vast majority of the hair follicles are similarly involved. By contrast, endogenous comedogenic factors (androgens, acne, etc) typically affect to a variable extent a minority of hair follicles.10 The sensitivity of the method is such that it is possible to disclose signs of microcomedolysis after a short time (a couple of weeks) of adequate treatment.23
Figure 6.
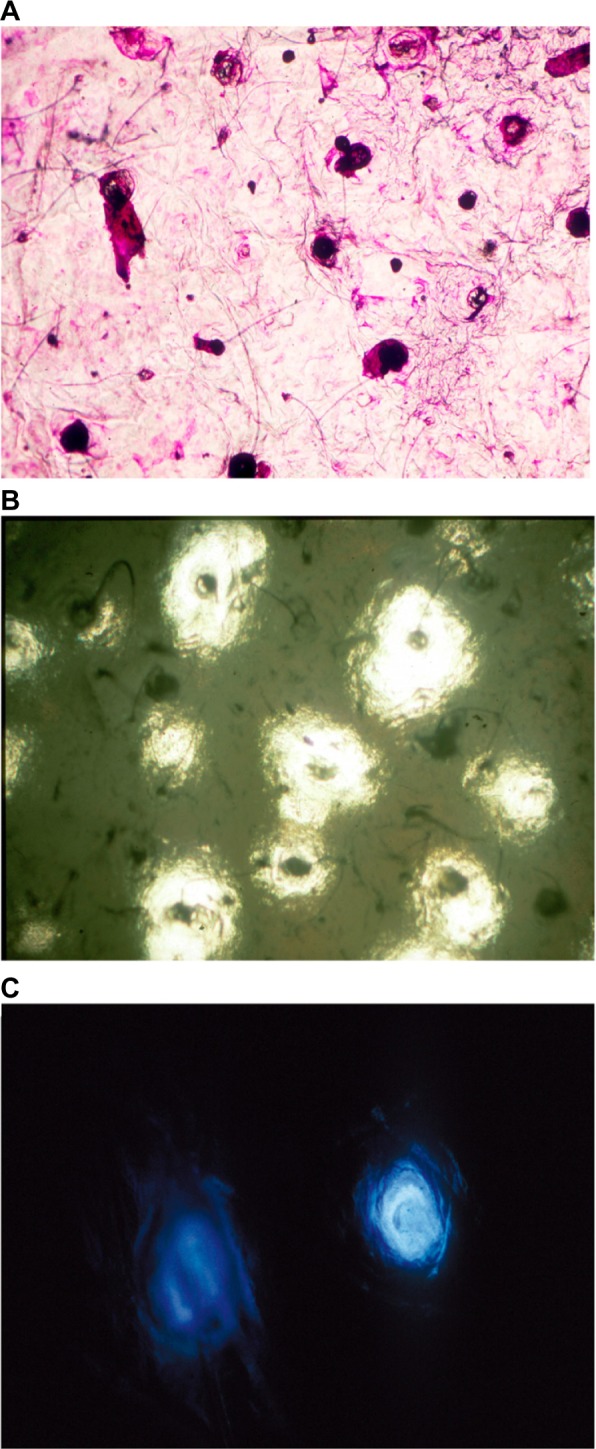
Microcomedones collected on CSSS from the face.
Notes: (A) Microcomedones and discrete follicular casts. (B) CSSS and a sebum-sensitive foil superposed on one another, revealing microcomedones (dark spots) pouring out (white area) or not (grey background) sebum at the skin surface. (C) Follicular fluorescence on a CSSS.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
Sebum-sensitive foils (SSF), available as Sebutape® (Cuderm corp; Dallas, TX, USA) and Sebufix® (CK Electronic), are conveniently used for assessing the sebum output at the skin surface. It is possible to combine such method with CSSS.24 In a first step, a non-sticky SSF is applied to the skin for a few seconds. The outlines of the foil are ink marked on the SC. In a second step, following removal of the SSF, CSSS is collected from the very same skin site. The ink mark is visible on such sampling. The CSSS and the foil are then exactly superposed using the ink mark as an adjusting mark. The dual SSF–CSSS samplings are examined under the microscope (Figure 6B) and submitted to image analysis, considering specifically the darker horny follicular casts and the clear transparent sebum spots. Some correlations are possibly established between the pore sizes, the follicular casts, the microcomedones, and the clear transparent spots of sebum.24
Analytical methods for evaluating some features of follicular casts rely on image analysis when illuminating the specimen under the microscope with either white light, polarized light, or fluorescent light. The assessment of the follicular fluorescence for evaluating the presence of porphyrins produced by Propionibacterium acnes in follicles is occasionally important for assessing any impact of cosmetics.25 In fact, some products possibly emit fluorescence by themselves (Figure 6C), while others such as sunscreens display a quenching effect by absorption of porphyrin fluorescence.
It is possible to assess the density of hair follicles over a defined surface area, as well as to observe the skin pores and the presence of follicular hyperkeratosis (kerosis), comedones, trichostasis spinulosa, intrafollicular bacteria, and mites (Demodex folliculorum).7,26–28 In some instances, other hair follicle structures including hair bulbs and follicular sheaths are visualized on CSSS. Skin pores distinctly corresponding to follicular or sudoral openings at the skin surface are conveniently explored using CSSS.27
Corneomelametry
Melanin production in melanocytes is under complex neuroendocrine controls.29 It is identified in regular corneocytes of phototype V and VI individuals. It is also present in the SC covering various pigmented lesion in clear-skinned people (Figure 7). It is mandatory to distinguish melanin-laden anucleated corneocytes from neoplastic dendritic nucleated melanocytes as seen after their migration inside the SC covering a malignant melanoma.10 For increasing the sensitivity of the procedure, the aspect of the dusty melanin load is typically increased using an argentaffin stain procedure. The relative darkness of these CSSS is conveniently assessed using corneomelametry. Such a method consists of measuring the reduction in light transmission through the CSSS using a photodensitometer device designed for photomicroscopy.30–33 Bleaching agent effects are possibly tested and compared using corneomelametry. Melasma and solar lentigines (aging spots) are typical lesions explored in that way.
Figure 7.
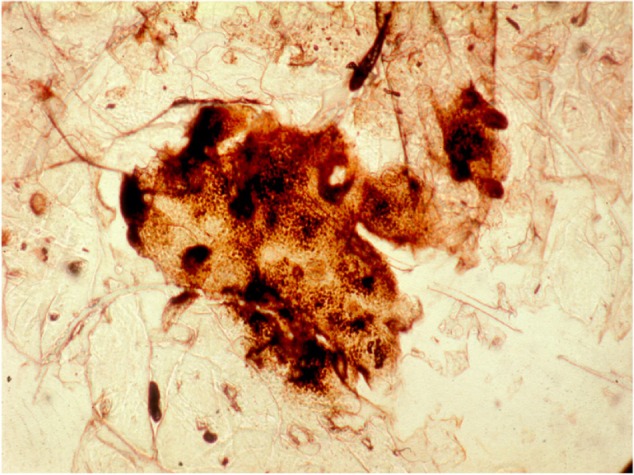
Abundant melanin deposits in clustered corneocytes as revealed by a CSSS.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
Corneodynamics
The dansyl chloride (DC) test was generally accepted as a noninvasive clinical attempt at evaluating the SC turnover. The overall time for fluorescence extinction depends on both the rate of transit of corneocytes through the SC and the thickness of that layer. This test proves to be difficult to interpret on clinical grounds due to the uneven fade-out of fluorescence at the skin surface. An improved method was introduced by the examination of CSSS harvested from a DC test area.33 It was further refined by replacing DC with the browning dihydroxyacetone (DHA) agent.34
Corneodynamics is assessed using CSSS from DC (Figure 8A) and DHA (Figure 8B) test sites at a predetermined time of a trial. Accordingly, at about day 10, CSSS are examined under a fluorescent light microscope because both DC and DHA are fluorescent. Image analysis applied to such pictures allows quantification of the nonfluorescent versus fluorescent areas of the SC. This ratio is an indicator of the rate of SC turnover. However, both the DC and DHA tests are dramatically influenced by cleansing agents and skincare products.35 The extraction of the dyes from the SC by these products is tentatively used to predict irritancy potential.35
Figure 8.
Corneodynamics on CSSS at day 10.
Notes: (A) Dansyl chloride test. (B) Dihydroxyacetone test.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
An inverse relationship has been suggested between the size of corneocytes and the speed of epidermal turnover. This aspect is possibly studied on skin strippings, particularly CSSS during corneodynamics.
Another facet of the SC dynamics is gained by squamometry, in particular UVL squamometry.3 For instance, the SQMI kinetics after acute photodamage show a complex response of the epidermis with early (±48 hours) and late (±10 days) changes.36 Indeed, a single regular SACD sampling corresponds at the most to the regular corneocyte daily shedding. Normal and moderately dry skin respond to UVL irradiation by an early increase in the SQMI value.
In vivo evaluation of the skin surface biocene
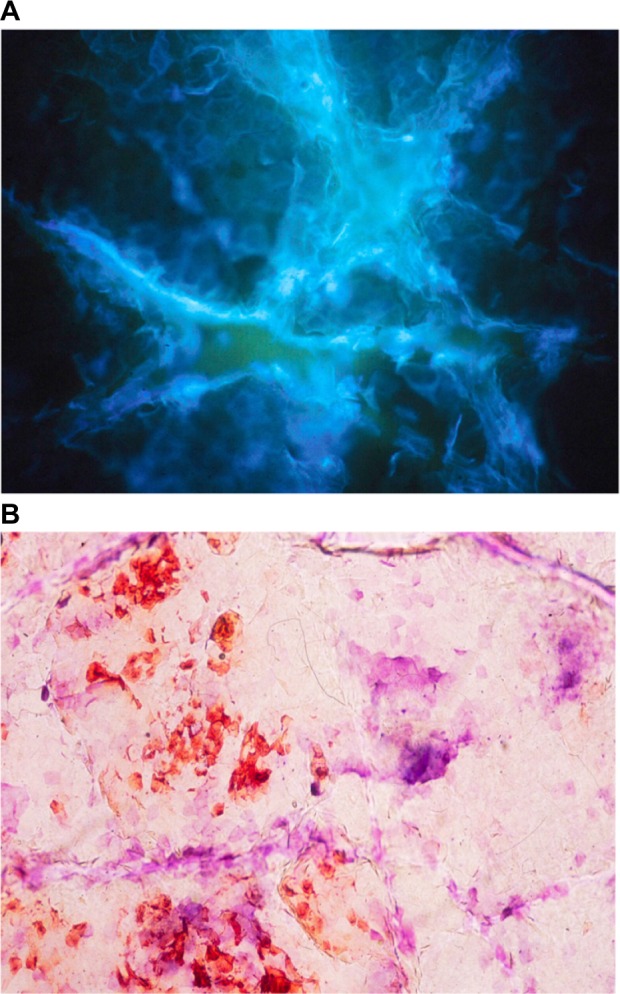
The biocene of resident bacteria and other saprophytic or pathogenic microorganisms is typically confined to the skin surface and the appendages.6,37–39 At the surface of the SC, the flora remains largely encased inside the cyanoacrylate bond during CSSS sampling. Thus, it is not accessible to staining procedures, and it remains invisible at microscopic examination. Therefore, the surface microflora is not adequately disclosed on CSSS. By contrast, samples of microorganisms entrapped inside the SC and follicular casts are distinctly collected from CSSS. In particular, the microflora is accessible by scraping out the horny spiky structures appended to the CSSS. Viability of the intrafollicular bacteria is conveniently assessed using the combination of vital stains (Figure 9A), such as neutral red,40 and flow cytometry.39
Figure 9.
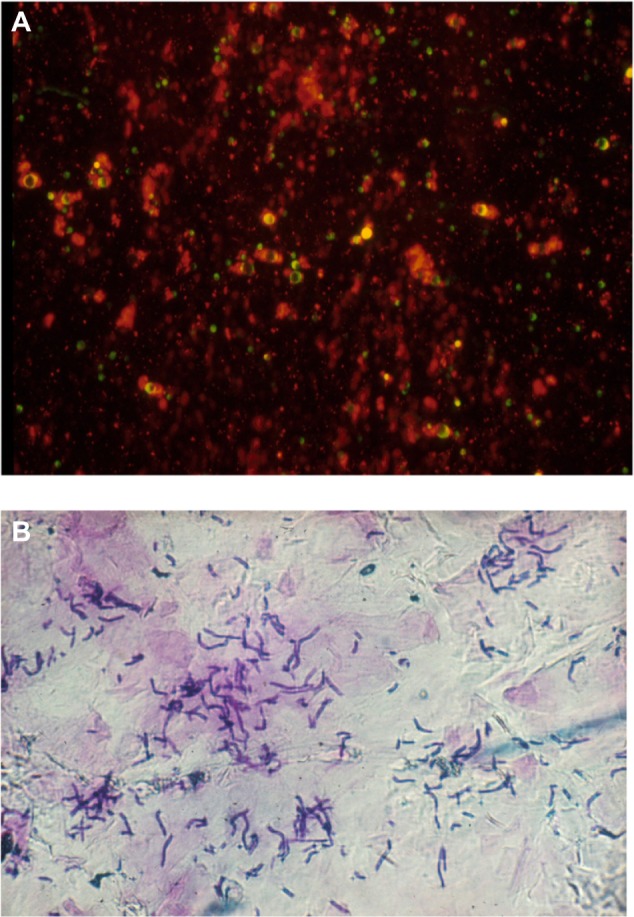
Superficial skin biocene.
Notes: (A) Bacteria revealed by the neutral red staining. (B) Malassezia sp. in the stratum corneum.
Abbreviation: CSSS, cyanoacrylate skin surface stripping.
The load of Malassezia spp. inside squames of dandruff and seborrheic dermatitis is assessed on SACD (Figure 9B). The efficacy of shampoos can be compared by combining squamometry and semiquantitative evaluations of the number of yeasts.41 The adhesion of Malassezia yeasts to corneocytes appears to be a prominent feature.42
Corneosurfametry
The presence of various tiny foreign bodies and microscopic parasites, and the impact of various chemical xenobiotics on the SC are conveniently assessed on CSSS.10,43 In particular, surfactants remove lipids, denature proteins, induce corneocyte swelling, alter the SC barrier function, and produce a feeling of skin roughness and dryness.44 The surest method for testing skin compatibility of surface-active agents was to use large panels of volunteers. However, such a procedure proved to be costly and time-consuming. Over the years, various alternative methods were proposed. Among them, corneosurfametry (CSM) refers specifically to the effects of surfactants and wash solutions on the SC.18,44,45 CSM is a noninvasive quantitative test rating the interaction between surfactants and human or animal SC (Figure 10). It is used as a predictive irritancy test that compares favorably with conventional in vitro tests.
Figure 10.
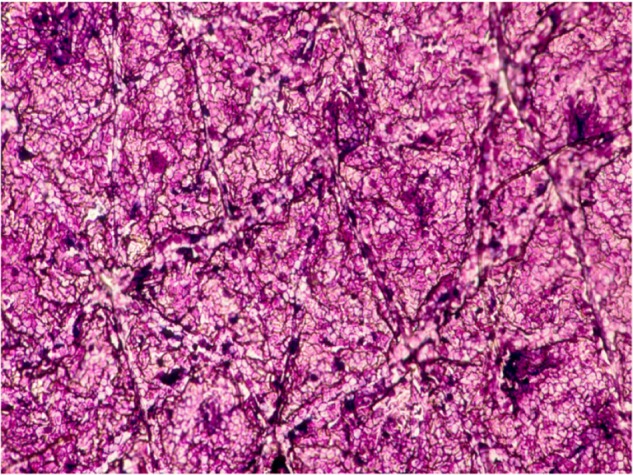
Corneosurfametry showing the dense staining of corneocytes after contact with a harsh soap.
CSM entails collection of CSSS from healthy skin of volunteers followed by a predetermined contact time of the SC samples with neat or diluted surfactants. A 2-hour experiment is usually convenient to disclose any differences in SC reactivity among products. The usual test concentrations for proprietary cleansing skincare products ranges from 3% to 10%, but higher dilutions are possibly required for harsh products. Each surfactant solution is sprayed uniformly on about 20 CSSS placed in a slide tray, which is then covered by a lid. At completion of the predetermined assessment time, CSSS are thoroughly rinsed with tap water, dried, and stained for 3 minutes with the TBBF dye solution. After removing any excess dye and copious water rinsing, samples are examined under the microscope. In addition, reflectance colorimetry is applied to the dry samples for recording both L* and Chroma C*. To be adequately interpreted, the L* value should be over 45, and superior to that of Chroma C*.18 Data out of range suggest an excess surfactant concentration for the test. For instance, the maximum concentration for testing sodium laurylsulfate (SLS) is approximately 2%.
CSM results are expressed by two parameters: namely, the CSM index (CSMI) and the colorimetric index of mildness (CIM). CSMI of any test product corresponds to the color difference between water-treated control samples and those exposed to the test product. Thus, CSMI is conveniently calculated as follows:
| (2) |
There is a linear negative correlation between values of L* and Chroma C*, and CIM of each sample is calculated as follows:
| (3) |
According to such analytical assessments, mild skincare products containing anionic surfactants are characterized by the combination of a low CSMI value and a high CIM value. The converse is true for harsh skincare products and most household cleaners. However, a low paradoxical CSMI value is experienced with some aggressive products yielding to a cascade of corneocyte swelling, lysis, and loss from the stratum disjunctum. Usually, interindividual variations are larger for SQMI than for CIM.18
Microwave CSM is a more rapid procedure than the regular CSM.46 In this procedure, CSSS are immersed in a flask containing the test surfactant solution, and they are placed in a microwave oven containing a 500 mL water load. Microwave CSM is typically run at 750 W for 30 seconds. The next steps are identical to those of the regular CSM procedure.
Responsive CSM is a variant of the method where skin to be sampled is preconditioned before harvesting CSSS.47 The method, based on repeated subclinical injuries by surfactants, is monitored in a controlled forearm immersion test. At completion of the in vivo procedure, CSSS are harvested for a regular or microwave CSM bioassay using the same surfactant as in the preliminary in vivo procedure. Preconditioning the skin in this way increases CSM sensitivity to discriminate between different potential irritations from mild surfactants.
Shielded CSM is used for testing skin-protective products (SPP), also called skin barrier formulations, claiming some protection against noxious external agents. In shielded CSM, CSSS are first covered by a controlled amount of the test SPP before performing regular CSM using a reference surfactant. Comparative screenings of various SPP are conveniently performed using shielded CSM, avoiding exposure of volunteers to any hazardous agents for in vivo testing.
Corneoxenometry
The corneoxenometry (CXM) bioassay is offered for testing any chemical xenobiotic other than surfactants.48,49 The compounds consist of any hazardous chemical including organic solvents, acids, bases, toxic agents, etc. The basic procedure is similar to CSM and its variants. It entails CSSS collection followed by its controlled immersion in the test solution for 1–120 minutes. The subsequent TBBS color darkening correlates with the test solution aggressiveness toward the SC. One main indication is found in the field of skin irritation while avoiding the risk of in vivo hazards. Skin barrier formulations are conveniently tested using CXM.50 Another indication deals with comparative assessments of penetration enhancers commonly used in some topical formulations.51
Regional SC reactivity to irritant xenobiotics
One of the most important functions of the epidermis is the formation of an efficient barrier between the body and a diversity of xenobiotics. Much research has been undertaken to understand the variability in the skin barrier efficacy, which resides in the SC. Measurements of the transepidermal water loss represent indirect noninvasive evaluations of such a function. Values are kept low when the barrier function is optimal, and they increase with the severity of the barrier defect. It is generally assumed that the cutaneous barrier is disrupted through intercellular lipid removal and protein denaturations. For instance, such a condition is reached following applications of various organic solvents to the skin. Since workers are exposed to solvents at many workplaces, methods are needed for the evaluation of the potential solvent toxicity. Such hazards call for ex vivo predictive bioassays on human skin or SC, such as CSM and CXM.
Large interindividual differences in CIM were reported regarding different surfactants and solvents.48 In addition, distinct regional differences are present regarding irritancy and percutaneous absorption. Both the dorsal hand and volar forearm appeared to be the least reactive skin locations on the CSM bioassay. By contrast, the same test revealed that the neck, forehead, back, and dorsal foot were more reactive sites.
In normal subjects, the extent of alterations induced in the human SC by solvents during CXM are more variable than those induced by diluted surfactants at the CSM bioassay. In addition, a heterogeneity in corneocyte alterations exists among solvents and between distinct body sites. For instance, hexane–methanol and chloroform–methanol strongly alter the SC structure. Indeed, the chloroform–methanol mixture is a potent lipid extractor from biological samples. However, it is not the top ranked aggressor on the CXM bioassay.48
The intensity of the skin response to irritant xenobiotics is subject to anatomic site variations. The initial mechanism governing such biological response presumably corresponds to the direct damage to the SC. Subtle regional variations in irritancy are possibly tested using CSM and CXM.44 Regional variations in skin reactivity presumably correspond in part to an uneven preconditioning by in vivo contacts with a previous irritant stress. A similar, but probably weaker process takes place in the condition of reactive skin.52 This concept illustrates the failure of drawing general conclusions from CSM and CXM performed from a given body site. Such a finding contrasts with the frequent variability in CIM data from distinct body sites, and with the inconsistency in severity of irritant reactions at different anatomical regions, as assessed by transepidermal water loss measurements at in vivo patch test sites.
Reactive (sensitive) skin
The term reactive (sensitive) skin covers a broad spectrum of different conditions, sharing in common the occurrence of unwanted changes in response to external environmental factors including personal-care products. Reactive skin is a heterogeneous problem characterized by a reduced cutaneous tolerance to specific environmental factors (cold, heat, wind, wool, topical products, etc).53 Clinical manifestations consist mainly of subjective symptoms linked to sensory irritation including discomfort, itching, stinging, and burning sensations. There are no specific signs discernable on CSSS except occasional discrete xerosis and parakeratosis. Other biometrological procedures are possibly useful.54
Some individuals complaining of sensitive skin are more readily deranged by some specific chemical and physical irritation. A series of these conditions are likely related to a defective SC barrier function. CSM performed with a reference surfactant (SLS 1%) shows that CSSS from patients with reactive skin react more severely than those harvested from healthy individuals without sensitive skin. SQMI is commonly higher on reactive skin than on otherwise normal SC. CIM is significantly lower (approximately 25% reduction) in patients with reactive skin than in normal nonsensitive individuals, indicating a higher susceptibility to irritation induced by surfactants and some other skincare products.52
Potency of squamolytic agents
CSSS, using a method similar to CSM, is a substrate for the study of the actual bioactivity of emollients and squamolytic (keratolytic) agents. The test product is applied to CSSS for various periods of time ranging from 15 minutes to 4 hours (Figure 11). After abundant rinsing and staining with TBBF, reflectance colorimetric values are recorded and CSMI and/or CIM parameters are calculated in a way similar to CSM.
Figure 11.
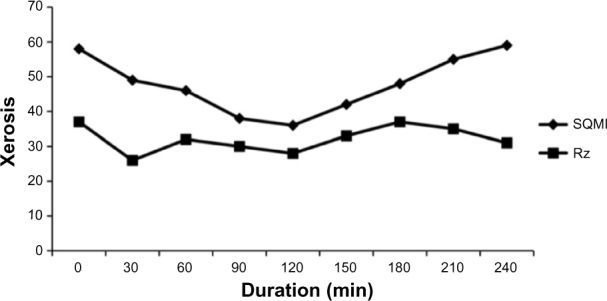
Xerosis evolution according to squamometry (SQMI parameter) and skin roughness (Rz) of CSSS in contact with a 50% glycolic acid solution for various durations of contact.
Abbreviations: SQMI, squamometry index; CSSS, cyanoacrylate skin surface stripping.
The effect of squamolytic agents and emollients is conveniently assessed on skin strippings collected in clinical trials (Figure 12). In general, it is appropriate to harvest samples at entry into the study, as well as 2 weeks and 4 weeks after treatment. After a 2-week period of treatment corresponding to the regression phase, late samples are collected. Xerosis is rated on CSSS. Both SQMI and DI show the kinetics of improvement followed by the posttreatment regression. The typical kinetics show first an aggravation in the test values followed by a drop in these values, this latter phase indicating a loss of corneocytes from the CSSS. Optical profilometry performed on the same samples shows the immediate smoothing effect of the products with reduction in the Rz value followed by desquamation and increased Rz.
Figure 12.
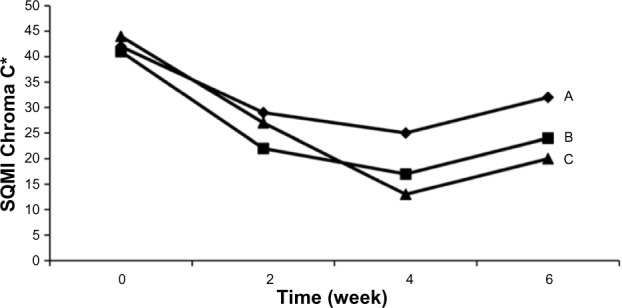
Evolution in the squamometry values (SQMI).
Note: Different kinetics of xerosis improvement during treatment (weeks 0–4) with three emollients (A, B, C) and the following regression phase (weeks 4–6).
Abbreviation: SQMI, squamometry index.
Conclusion
The possibilities of using skin strippings in dermocosmetic science seem endless. The emergence of new analytical methods increases the validity of the information yielded by SACD and CSSS. With regulations avoiding animal experiments and ethical considerations in human testing, there is a need and a real possibility to develop new predictive bioassays using controlled skin strippings as substrate.
Meaningful analytical morphologic aspects are open to SACD and CSSS samplings. Fortunately, sound mathematical relationships are perceived between the original structure and the images, and many of these relationships appear quite obvious. In general, multivariate statistical analysis is required to validate a meaningful conclusion. When testing xenobiotic effects on SC, the stratum disjunctum response as assessed by SQMI on SACD is different from CSMI on the thicker SC collected by CSSS. Nevertheless, both methods appear valuable and complementary.
Beyond SC scrapings, SACD and CSSS provide useful information in the field of cosmetology. These simple, low-cost, and minimally invasive methods allow the clinician, the cosmetologist, and the experimentalist to avoid invasive procedures within limits of well-defined indications. Less than 3 minutes are required between SC sampling and its microscopic examination. There are obvious features and subtle characteristics discernible in the CSSS structure for establishing key points in a variety of skin conditions. It is important to stress that no single criterion should usually be relied upon for a definitive feature on CSSS. Rather, a constellation of clues should be sought. A range of quantifications are made possible on CSSS using computer-assisted image analysis.
The SC holds a large amount of information about the skin itself, and the internal milieu as well. In many instances, CSSS appears to represent a convenient way that is currently available for exploring many facets of cosmetic science. There is no limitation to its use in cosmetic science. It represents a safe and time-saving method. The main advantages of CSSS are as follows:
Swift and safe method for SC samplings
Multiple diagnostic possibilities
Cheap procedure
Hygienic procedure
Minimally invasive
Easy handling
Stable samples over time
Defined skin stripping for scientific applications
Established method, known for more than 30 years
Supported by a number of scientific contributions
Acknowledgments
No sources of funding were used to assist in the preparation of this paper. The authors appreciate the excellent secretarial assistance of Mrs Ida Leclercq.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pierard GE, Goffin V, Hermanns-Le T, Pierard-Franchimont C. Corneocyte desquamation. Int J Mol Med. 2000;6:217–221. doi: 10.3892/ijmm.6.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Bashir SJ, Chew AL, Anigbogu A, Dreher F, Maibach HI. Physical and physiological effects of stratum corneum tape stripping. Skin Res Technol. 2001;7:40–48. doi: 10.1034/j.1600-0846.2001.007001040.x. [DOI] [PubMed] [Google Scholar]
- 3.Piérard-Franchimont C, Henry F, Piérard GE. The SACD method and the XLRS squamometry tests revisited. Int J Cosmet Sci. 2000;22:437–446. [PubMed] [Google Scholar]
- 4.Marks R, Dawber RP. Skin surface biopsy: an improved technique for the examination of the horny layer. Br J Dermatol. 1971;84:117–123. doi: 10.1111/j.1365-2133.1971.tb06853.x. [DOI] [PubMed] [Google Scholar]
- 5.Agache P, Mairey J, Boyer JP. Stripping of the stratum corneum with cyanoacrylate. Value in cutaneous physiology and pathology. J Med Lyon. 1972;53:1017–1022. French. [PubMed] [Google Scholar]
- 6.Lachapelle JM, Gouverneur JC, Boulet M, Tennstedt D. A modified technique (using polyester tape) of skin surface biopsy. Its interest for the investigation of athlete’s foot. Br J Dermatol. 1977;97:49–52. doi: 10.1111/j.1365-2133.1977.tb15426.x. [DOI] [PubMed] [Google Scholar]
- 7.Pierard-Franchimont C, Pierard GE. Skin surface stripping in diagnosing and monitoring inflammatory, xerotic, and neoplastic diseases. Pediatr Dermatol. 1985;2:180–184. doi: 10.1111/j.1525-1470.1985.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 8.Piérard-Franchimont C, Piérard GE. Assessment of aging and actinic damages by cyanoacrylate skin surface strippings. Am J Dermatopathol. 1987;9:500–509. doi: 10.1097/00000372-198712000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Katz HI. Skin surface touch print: review of indications and uses. Adv Dermatol. 1990;5:197–213. [PubMed] [Google Scholar]
- 10.Piérard GE, Piérard-Franchimont C, Paquet P, Hermanns-Lê T, Radermacher J, Delvenne P. Cyanoacrylate skin surface stripping and the 3S-Biokit advent in tropical dermatology: a look from Liège. Scientific World Journal. 2014;2014:462634. doi: 10.1155/2014/462634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groh DG, Mills OH, Jr, Kligman AM. Quantitative assessment of cyanoacrylate follicular biopsies by image analysis. J Soc Cosmet Chem. 1992;43:101–112. [Google Scholar]
- 12.Hirao T, Denda M, Takahashi M. Identification of immature cornified envelopes in the barrier-impaired epidermis by characterization of their hydrophobicity and antigenicities of the components. Exp Dermatol. 2001;10:35–44. doi: 10.1034/j.1600-0625.2001.100105.x. [DOI] [PubMed] [Google Scholar]
- 13.Harding CR, Long S, Richardson J, et al. The cornified cell envelope: an important marker of stratum corneum maturation in healthy and dry skin. Int J Cosmet Sci. 2003;25:157–167. doi: 10.1046/j.1467-2494.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 14.Piérard GE. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: evaluation by stratum corneum strippings. Skin Res Technol. 1996;2:3–11. doi: 10.1111/j.1600-0846.1996.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm KP, Kaspar K, Schumann F, Articus K. Development and validation of a semiautomatic image analysis system for measuring skin desquamation with D-Squames. Skin Res Technol. 2002;8:98–105. doi: 10.1034/j.1600-0846.2002.00322.x. [DOI] [PubMed] [Google Scholar]
- 16.Piérard GE. What do you mean by dry skin? Dermatologica. 1989;179:1–2. doi: 10.1159/000248089. [DOI] [PubMed] [Google Scholar]
- 17.Piérard GE, Piérard-Franchimont C, Saint Léger D, Kligman AM. Squamometry: the assessment of xerosis by colorimetry of D-Squame adhesive discs. J Soc Cosmet Chem. 1992;43:297–305. [Google Scholar]
- 18.Piérard GE, Goffin V, Piérard-Franchimont C. Squamometry and corneosurfametry for rating interactions of cleansing products with stratum corneum. J Soc Cosmet Chem. 1994;45:269–277. [Google Scholar]
- 19.Mills OH, Jr, Kligman AM. The follicular biopsy. Dermatologica. 1983;167:57–63. doi: 10.1159/000249749. [DOI] [PubMed] [Google Scholar]
- 20.Piérard GE, Piérard-Franchimont C, Goffin V. Digital image analysis of microcomedones. Dermatology. 1995;190:99–103. doi: 10.1159/000246655. [DOI] [PubMed] [Google Scholar]
- 21.Mills OH, Jr, Kligman AM. A human model for assaying comedolytic substances. Br J Dermatol. 1982;107:543–548. doi: 10.1111/j.1365-2133.1982.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 22.Mills OH, Jr, Kligman AM. A human model for assessing comedogenic substances. Arch Dermatol. 1982;118:903–905. [PubMed] [Google Scholar]
- 23.Uhoda E, Piérard-Franchimont C, Piérard GE. Comedolysis by a lipohydroxyacid formulation in acne-prone subjects. Eur J Dermatol. 2003;13:65–68. [PubMed] [Google Scholar]
- 24.Piérard GE. Rate and topography of follicular sebum excretion. Dermatologica. 1987;175:280–283. doi: 10.1159/000248834. [DOI] [PubMed] [Google Scholar]
- 25.Szepetiuk G, Piérard S, Pierard-Franchimont C, Caucanas M, Quatresooz P, Pierard GE. Recent trends in specular light reflectance beyond clinical fluorescence diagnosis. Eur J Dermatol. 2011;21:157–161. doi: 10.1684/ejd.2010.1243. [DOI] [PubMed] [Google Scholar]
- 26.Pagnoni A, Kligman AM, el Gammal S, Stoudemayer T. Determination of density of follicles on various regions of the face by cyanoacrylate biopsy: correlation with sebum output. Br J Dermatol. 1994;131:862–865. doi: 10.1111/j.1365-2133.1994.tb08590.x. [DOI] [PubMed] [Google Scholar]
- 27.Uhoda E, Piérard-Franchimont C, Petit L, Piérard GE. The conundrum of skin pores in dermocosmetology. Dermatology. 2005;210:3–7. doi: 10.1159/000081474. [DOI] [PubMed] [Google Scholar]
- 28.Gerber PA, Kukova G, Buhren BA, Homey B. Density of Demodex folliculorum in patients receiving epidermal growth factor receptor inhibitors. Dermatology. 2011;222:144–147. doi: 10.1159/000323001. [DOI] [PubMed] [Google Scholar]
- 29.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 30.Hermanns JF, Petit L, Piérard-Franchimont C, Paquet P, Piérard GE. Assessment of topical hypopigmenting agents on solar lentigines of Asian women. Dermatology. 2002;204:281–286. doi: 10.1159/000063359. [DOI] [PubMed] [Google Scholar]
- 31.Petit L, Piérard GE. Analytic quantification of solar lentigines lightening by a 2% hydroquinone-cyclodextrin formulation. J Eur Acad Dermatol Venereol. 2003;17:546–549. doi: 10.1046/j.1468-3083.2003.00808.x. [DOI] [PubMed] [Google Scholar]
- 32.Thirion L, Piérard-Franchimont C, Piérard GE. Whitening effect of a dermocosmetic formulation: a randomized double-blind controlled study on melasma. Int J Cosmet Sci. 2006;28:263–267. doi: 10.1111/j.1467-2494.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 33.Piérard GE. Microscopic evaluation of the dansyl chloride test. Dermatology. 1992;185:37–40. doi: 10.1159/000247400. [DOI] [PubMed] [Google Scholar]
- 34.Piérard GE, Piérard-Franchimont C. Dihydroxyacetone test as a substitute for the dansyl chloride test. Dermatology. 1993;186:133–137. doi: 10.1159/000247324. [DOI] [PubMed] [Google Scholar]
- 35.Paye M, Simion FA, Pierard GE. Dansyl chloride labelling of stratum corneum: its rapid extraction from skin can predict skin irritation due to surfactants and cleansing products. Contact Dermatitis. 1994;30:91–96. doi: 10.1111/j.1600-0536.1994.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 36.Piérard GE, Piérard-Franchimont C. Squamometry in acute photodamage. Skin Res Technol. 1995;1:137–139. doi: 10.1111/j.1600-0846.1995.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 37.Katz HI, Prawer SE, Hien NT, Mooney JJ. Skin-surface touch print for diagnosing fungal infections. Am Fam Physician. 1985;31:189–194. [PubMed] [Google Scholar]
- 38.Piérard GE, Piérard-Franchimont C, Dowlati A. Skin surface biopsy in clinical and experimental dermatology. Rev Europ Dermatol MST. 1992;4:445–466. [Google Scholar]
- 39.Piérard-Franchimont C, Gaspard U, Lacante P, Rhoa M, Slachmuylders P, Piérard GE. A quantitative biometrological assessment of acne and hormonal evaluation in young women using a triphasic low-dose oral contraceptive containing gestodene. Eur J Contracept Reprod Health Care. 2000;5:275–286. doi: 10.1080/13625180008500405. [DOI] [PubMed] [Google Scholar]
- 40.Naka W, Hanyaku H, Tajima S, Harada T, Nishikawa T. Application of neutral red staining for evaluation of the viability of dermatophytes and Candida in human skin scales. J Med Vet Mycol. 1994;32:31–35. doi: 10.1080/02681219480000051. [DOI] [PubMed] [Google Scholar]
- 41.Piérard-Franchimont C, Uhoda E, Loussouarn G, Saint-Léger D, Piérard GE. Effect of residence time on the efficacy of antidandruff shampoos. Int J Cosmet Sci. 2003;25:267–271. doi: 10.1111/j.1467-2494.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- 42.Piérard GE, Xhauflaire-Uhoda E, Piérard-Franchimont C. The key role of corneocytes in pityrosporoses. Dermatology. 2006;212:23–26. doi: 10.1159/000089017. [DOI] [PubMed] [Google Scholar]
- 43.Pierard GE, Renkin A, Pierard-Franchimont C. Trichome. Dermatologica. 1985;171:72–75. French. [PubMed] [Google Scholar]
- 44.Henry F, Goffin V, Maibach HI, Piérard GE. Regional differences in stratum corneum reactivity to surfactants. Quantitative assessment using the corneosurfametry bioassay. Contact Dermatitis. 1997;37:271–275. doi: 10.1111/j.1600-0536.1997.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 45.Xhauflaire-Uhoda E, Loussouarn G, Haubrechts C, Léger DS, Piérard GE. Skin capacitance imaging and corneosurfametry. A comparative assessment of the impact of surfactants on stratum corneum. Contact Dermatitis. 2006;54:249–253. doi: 10.1111/j.0105-1873.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 46.Goffin V, Piérard GE. Microwave corneosurfametry and the short-duration dansyl chloride extraction test for rating concentrated irritant surfactants. Dermatology. 2001;202:46–48. doi: 10.1159/000051585. [DOI] [PubMed] [Google Scholar]
- 47.Uhoda E, Goffin V, Pierard GE. Responsive corneosurfametry following in vivo skin preconditioning. Contact Dermatitis. 2003;49:292–296. doi: 10.1111/j.0105-1873.2003.0269.x. [DOI] [PubMed] [Google Scholar]
- 48.Goffin V, Letawe C, Piérard GE. Effect of organic solvents on normal human stratum corneum: evaluation by the corneoxenometry bioassay. Dermatology. 1997;195:321–324. doi: 10.1159/000245980. [DOI] [PubMed] [Google Scholar]
- 49.Xhauflaire-Uhoda E, Piérard-Franchimont C, Piérard GE. Effects of various concentrations of glycolic acid at the corneoxenometry and collaxenometry bioassays. J Cosmet Dermatol. 2008;7:194–198. doi: 10.1111/j.1473-2165.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 50.Xhauflaire-Uhoda E, Macarenko E, Denooz R, Charlier C, Piérard GE. Skin protection creams in medical settings: successful or evil? J Occup Med Toxicol. 2008;3:15. doi: 10.1186/1745-6673-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goffin V, Henry F, Piérard-Franchimont C, Piérard GE. Penetration enhancers assessed by corneoxenometry. Skin Pharmacol Appl Skin Physiol. 2000;13:280–284. doi: 10.1159/000029934. [DOI] [PubMed] [Google Scholar]
- 52.Goffin V, Piérard-Franchimont C, Piérard GE. Sensitive skin and stratum corneum reactivity to household cleaning products. Contact Dermatitis. 1996;34:81–85. doi: 10.1111/j.1600-0536.1996.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 53.Paquet F, Piérard-Franchimont C, Fumal I, Goffin V, Paye M, Piérard GE. Sensitive skin at menopause; dew point and electrometric properties of the stratum corneum. Maturitas. 1998;28:221–227. doi: 10.1016/s0378-5122(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 54.Piérard GE, Hermanns-Lê T, Piérard-Franchimont C, Courtois J, Piérard SL. Analytical search of sensory irritation to shampoos in reactive scalp. Labome Mat Meth. 2014;4:1097. [Google Scholar]


