Abstract
Discs large (Dlg) is a conserved member of the membrane-associated guanylate kinase family, and serves as a major scaffolding protein at the larval neuromuscular junction (NMJ) in Drosophila. Previous studies have shown that the postsynaptic distribution of Dlg at the larval NMJ overlaps with that of Hu-li tai shao (Hts), a homologue to the mammalian adducins. In addition, Dlg and Hts are observed to form a complex with each other based on co-immunoprecipitation experiments involving whole adult fly lysates. Due to the nature of these experiments, however, it was unknown whether this complex exists specifically at the NMJ during larval development.
Proximity Ligation Assay (PLA) is a recently developed technique used mostly in cell and tissue culture that can detect protein-protein interactions in situ. In this assay, samples are incubated with primary antibodies against the two proteins of interest using standard immunohistochemical procedures. The primary antibodies are then detected with a specially designed pair of oligonucleotide-conjugated secondary antibodies, termed PLA probes, which can be used to generate a signal only when the two probes have bound in close proximity to each other. Thus, proteins that are in a complex can be visualized. Here, it is demonstrated how PLA can be used to detect in situ protein-protein interactions at the Drosophila larval NMJ. The technique is performed on larval body wall muscle preparations to show that a complex between Dlg and Hts does indeed exist at the postsynaptic region of NMJs.
Keywords: Neuroscience, Issue 95, adducin, body wall dissection, developmental biology, Discs large, Drosophila, Hu-li tai shao, immunohistochemistry, neuromuscular junction, neuroscience, protein-protein interaction, Proximity Ligation Assay, third instar larvae
Introduction
Drosophila Discs large (Dlg) is a conserved member of the membrane-associated guanylate kinase family of scaffolding proteins that help orchestrate the assembly of large protein complexes at specific sites of the plasma membrane. Originally identified as a tumor suppressor protein, Dlg serves as an important determinant of epithelial apicobasal polarity 1,2,3. Dlg also serves as a major scaffolding module at the neuromuscular junction (NMJ) of glutamatergic motor neurons during larval development 4. Dlg plays diverse roles at the larval NMJ, and its pleiotropism relies on its ability to associate with multiple proteins 5,6. One such protein is Hu-li tai shao (Hts), a homologue to the mammalian adducins that have mainly been described in regards to their roles in regulating the actin-spectrin cytoskeleton 7. It has previously been shown that Dlg and Hts can form a complex with each other based on in vitro co-immunoprecipitation experiments involving whole adult fly lysates 8. One shortcoming of these results, however, is that they do not indicate where this complex forms. With the use of immunohistochemistry, the distributions of Dlg and Hts are observed to overlap at the postsynaptic membrane of larval NMJs, but are they in a complex in this region 8? As recently shown and detailed further here, Proximity Ligation Assay (PLA) is used to look for an in situ association between Dlg and Hts specifically at the larval NMJ 27.
PLA is a relatively new technique used mostly in cell and tissue culture that can detect protein-protein interactions in situ 9. In this assay, primary antibodies against the two proteins of interest are detected with a pair of species-specific secondary antibodies, termed PLA probes, which are conjugated to oligonucleotides (Figure 1A, B). If the two proteins are in close proximity to each other (i.e. within a few tens of nanometers), the distance between the attached PLA probes can be bridged through hybridization of two additional connector oligonucleotides (Figure 1C). In this conformation, the free ends of the connector oligonucleotides are close enough to make contact with each other, and a closed circular DNA molecule can be formed upon in situ ligation (Figure 1D). The circular DNA molecule serves as a template for in situ rolling circle amplification, which is primed by one of the oligonucleotides conjugated to the PLA probes (Figure 1E). Sequences within the resulting amplified, concatemeric DNA product can then be visualized with fluorescently-labeled, complementary oligonucleotide probes (Figure 1F). Since the amplified DNA remains attached to one of the PLA probes, the subcellular localization of the protein-protein interaction within a tissue can be readily ascertained.
Several methods are commonly used to detect protein-protein interactions including in vitro techniques such as co-immunoprecipitation, pull-down assays and yeast two-hybrid screening, and in vivo techniques such as Förster Resonance Energy Transfer (FRET) and Bimolecular Fluorescence Complementation (BiFC). A pitfall of the in vitro techniques is that they do not identify where the interaction is endogenously occurring, while the aforementioned in vivo techniques involve the artificial expression of fusion proteins that may not reflect the native behavior of their endogenous counterparts. One major advantage of PLA is that it is capable of determining within a tissue the subcellular localization of endogenous protein interactors that are in close proximity to each other and likely forming a complex, with the degree of closeness required to generate a signal being comparable to FRET and BiFC. PLA can detect interactions with high specificity and sensitivity due to the coupling of antibody recognition and DNA amplification. Thus, the assay can generate discrete, bright signals in the form of puncta that reveal the exact position of the interaction. In addition, scarcely visible antigens can be detected. Finally, PLA is a relatively simple technique to perform, and it takes no longer than a standard immunohistochemical procedure to complete. Therefore, PLA provides a technical advantage over other protein-protein interaction assays that are often plagued with long preparation times and extensive troubleshooting.
This protocol demonstrates how PLA can be applied to the Drosophila larval NMJ for the purpose of detecting endogenous protein-protein interactions in situ. Here, PLA is performed on larval body wall muscle preparations where Dlg and Hts are shown to indeed exist in a complex at the postsynaptic region of NMJs. PLA has not been previously used to study the larval NMJ, and there are at present only a handful of published papers that have used this assay in Drosophila tissue. It is hoped that further exposure of PLA to the Drosophila community will result in its increased use as an additional tool to complement other, more commonly used protein-protein interaction assays.
Protocol
1. Body Wall Preparation
NOTE: Preparation of third instar larval body walls (for study of the NMJs which innervate the body wall muscles) was performed as previously described in Brent et al. 10, or Ramachandran and Budnik 11,12, but with some modifications.
- Dissection
- Raise fly stocks and crosses at 25 °C for five to six days using standard procedures 13.
- Pick crawling third instar larvae from vials or bottles using fine forceps.
- Wash the larvae in a small Petri dish containing Phosphate Buffer Saline (PBS) to remove any food particles.
- Place a single larva onto a sylgard disc and immerse it in a few drops of ice-cold PBS. Using ice-cold PBS will help stun the larva making it easier to manipulate. Throughout the dissection, ensure that the preparation is always submerged in PBS to prevent it from drying.
- Position the larva with its dorsal side facing up so that the two tracheal tracts are visible under a dissecting microscope (Figure 2A). Using the forceps to grasp a minutien pin, pin the larva down at the posterior end near the spiracles (Figure 2B). With another pin, pierce through the cuticle at the anterior end near the mouth hooks. Gently stretch the larva out lengthwise, then pin it down (Figure 2B).
- Using microdissection scissors, pinch the posterior end near the pin to create a small opening. The incision should be superficial enough to just pass through the cuticle.
- Placing the tip of the bottom blade of the scissors into the incision, cut along the entire length of the dorsal midline between the two tracheal tracts (Figure 2C). Point the scissor blades slightly upwards when cutting to avoid damaging the ventral body wall muscles.
- Make a small horizontal incision slightly anterior to the posteriorly-placed pin (Figure 2C). Make another similar incision slightly posterior to the anteriorly-placed pin (Figure 2C). The incisions should just pass through the cuticle. NOTE: The three incisions from steps 1.1.7 and 1.1.8 combined should resemble an "
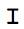 " when completed, i.e., a left- and right-hand flap on the dorsal side of the larval body should be produced.
" when completed, i.e., a left- and right-hand flap on the dorsal side of the larval body should be produced. - Carefully clean out the internal organs with the forceps. Adding a few forceful drops of PBS will help displace the organs out of the larval body, thus making it easier to remove them. Avoid poking the larval body as it will cause damage to the body wall muscles.
- Unfurl the larval body open and pin the corners down (Figure 2D). When pinning, stretch the body wall both horizontally and vertically to form an evenly-tensioned rectangle (see Figure 2G for the shape), taking care not to tear the body wall muscles in the process.
- Finish removing any remaining internal organs (Figure 2E).
- Fixation and Permeabilization
- Immerse the pinned body walls in several drops of Bouin's Solution. Incubate for 15 min on ice. Alternatively, use 4% paraformaldehyde (PFA) as an alternative fixative; incubate for 30 min.
- Rinse three times with Phosphate Buffer Saline with Triton (PBT).
- Using fine forceps, carefully remove the pins and transfer the body walls by their corners into a siliconized 0.65 ml microcentrifuge tube.
- Store the body walls in PBT at 4 °C until ready for PLA. For optimal results, start immunostaining the body walls within a day or two of dissection. NOTE: To save on reagents and to ensure that all body walls are treated equally during the assay, different genotypes can be placed into a single tube. Genotypes can be distinguished by cutting the corners of the body walls differently (see Figure 2F for examples).
2. Immunohistochemistry
NOTE: Immunostaining of third instar larval body walls was performed as previously described in Brent et al. 14 and Ramachandran and Budnik 11, but with some modifications 11,14. NOTE: Perform all steps at room temperature and with gentle agitation unless otherwise stated.
- Blocking
- Wash the body walls with PBT three times for 10 min each.
- Block with 1% Bovine Serum Albumin (BSA) for 1 hr.
- Immunostaining
- Incubate the body walls with mouse and rabbit primary antibodies against the two proteins of interest (diluted in 1% BSA) for 2 hr at room temperature, or overnight at 4 °C. In this case, use 1:10 mouse anti-Dlg and 1:250 rabbit anti-HtsM 15,16. Antibodies against markers that are not made in mouse or rabbit can also be included — e.g., use 1:200 goat anti-Hrp to delineate the neuronal membranes.
- Wash with PBT three times for 10 min each.
- Incubate with fluorophore-conjugated secondary antibodies to detect the markers (diluted in 1% BSA) for 2 hr at room temperature, or overnight at 4 °C. As the PLA signal is later visualized with a red fluorophore, another fluorophore must be used to detect the marker — e.g., use 1:200 FITC-conjugated anti-goat to detect the goat anti-Hrp antibody. Due to the use of light-sensitive reagents, keep the tubes in the dark from this point onwards. NOTE: The kit that is used allows for PLA to be performed between primary antibodies raised in mouse and rabbit, with the signal visualized on the red channel under confocal microscopy. If desired, other kits are available that allow the assay to be done with primary antibodies raised in other species, and the signal visualized on other channels.
3. Proximity Ligation Assay
NOTE: Perform all steps at room temperature and with gentle agitation unless otherwise stated.
- PLA Probes
- Wash the body walls with PBT three times for 10 min each.
- Incubate with PLA probes (1:5 dilution each in 1% BSA) for 2 hr at 37 °C. In this case, use 40 µl of PLA probe anti-mouse MINUS, 40 µl of PLA probe anti-rabbit PLUS and 120 µl of 1% BSA to ensure the proper immersion and mixing of 5-10 body walls. Up to a 1:25 dilution of the PLA probes can still result in an adequate signal-to-noise ratio (i.e., for this experiment).
- Ligation
- Wash the body walls with Wash Buffer A twice for 5 min each.
- Incubate with Ligation solution (1:40 dilution of Ligase in Ligation buffer) for 1 hr at 37 °C. In this case, use 5 µl of Ligase, 40 µl of 5 Ligation buffer and 155 µl of high purity water to ensure the proper immersion and mixing of 5-10 body walls.
- Amplification
- Wash the body walls with Wash Buffer A twice for 2 min each.
- Incubate with Amplification solution (1:80 dilution of Polymerase in Amplification buffer) for 2 hr at 37 °C. In this case, use 2.5 µl of Polymerase, 40 µl of 5 Amplification buffer and 157.5 µl of high purity water to ensure the proper immersion and mixing of 5-10 body walls.
- Preparation for Imaging
- Wash the body walls with Wash Buffer B twice for 10 min each.
- Wash with 0.01x Wash Buffer B once for 1 min.
- Equilibrate in a few drops of mounting solution for at least 30 min before mounting, or store overnight at 4 °C.
- Using fine forceps, carefully transfer the body walls onto a platform slide with their cuticles facing down. Position the body walls in rows and in the same orientation within a drop or two of mountant. Place a 22 mm x 40 mm coverslip over the preparation taking care not to generate air bubbles, then seal the slide with clear nail polish.
- Store the slides in the dark at -20 °C until ready for confocal imaging.
Representative Results
In wild-type third instar larval NMJs, Dlg is predominately found at the postsynaptic membrane of type I glutamatergic boutons, with Dlg immunoreactivity levels being more pronounced in type Ib boutons than type Is boutons (Figure 3A) 4. Hts is present throughout the muscle but concentrates at the postsynaptic region with Hts immunoreactivity levels appearing equal in both type I boutons, and is also found presynaptically (Figure 3A') 8,17. Note that the distributions of Dlg and Hts largely overlap at the postsynaptic region (Figure 3A'') 8.
To determine if Dlg and Hts exist in a complex at the NMJ, PLA between the two proteins was performed on larval body wall muscle preparations. For this assay, a mouse anti-Dlg antibody that detects the second PDZ domain at the N-terminus and a rabbit anti-HtsM antibody that detects the MARCKS-homology domain at the C-terminus were used 15,16. In wild-type, PLA signal between Dlg and Hts was observed specifically at the NMJ (Figure 3C-C''). The signal mostly localized circumferentially to the presynaptic membrane of type I boutons, which was demarked by Hrp, thus indicating that Dlg and Hts are in close proximity to each other and likely form a complex at the postsynaptic region. The result was determined to be specific as our negative control, involving hts01103 mutant NMJs that lack Hts immunoreactivity (Figure 3B-B''), showed no observable PLA signal (Figure 3D) 8,17,18. In addition, PLA performed without adding the rabbit anti-HtsM antibody produced no signal (data not shown). High magnification views of the boutons revealed that Dlg and Hts immunoreactivity grossly overlapped at the postsynaptic region (Figure 3E), whereas PLA between the two proteins resulted in discreet puncta indicating that only a subset of the total Dlg and Hts proteins are in complex (Figure 3F).
To further test the reliability of PLA as a tool to study the larval NMJ, we also evaluated interactions between the serine/threonine p21-activated kinase, Pak, and other synaptic proteins 19. Previous co-immunoprecipitation experiments have shown that Pak is a member of the Scribble complex, of which Dlg is also a member 20. In wild-type NMJs, Pak localizes to the postsynaptic density (Figure 4A,C), where it is required for Dlg localization 15,21. The immunoreactive distributions of Pak and Dlg overlap at the postsynaptic region (Figure 4A''), and PLA between the two proteins resulted in a positive signal specifically at the NMJ (Figure 4B). These results indicate that the two proteins are in close proximity to each other and are likely forming a complex in this region. Another synaptic protein we tested for Pak complex binding was Wingless (Wg), the Drosophila Wnt ligand, which plays numerous roles at the NMJ 22. In wild-type, Wg is enriched at the presynaptic and postsynaptic sides of type I boutons, but is also present as puncta throughout the muscle cytoplasm (Figure 4C') 23. Interestingly, though the immunoreactive distributions of Pak and Wg partially overlapped at the postsynaptic region (Figure 4C''), PLA between the two proteins produced no observable signal (Figure 4D). Therefore, Pak and Wg are not in close proximity to each other at the NMJ. This result demonstrates that not all proteins that show overlapping immunoreactivities through traditional co-localization experiments will generate a PLA signal, and serves as an additional negative control.
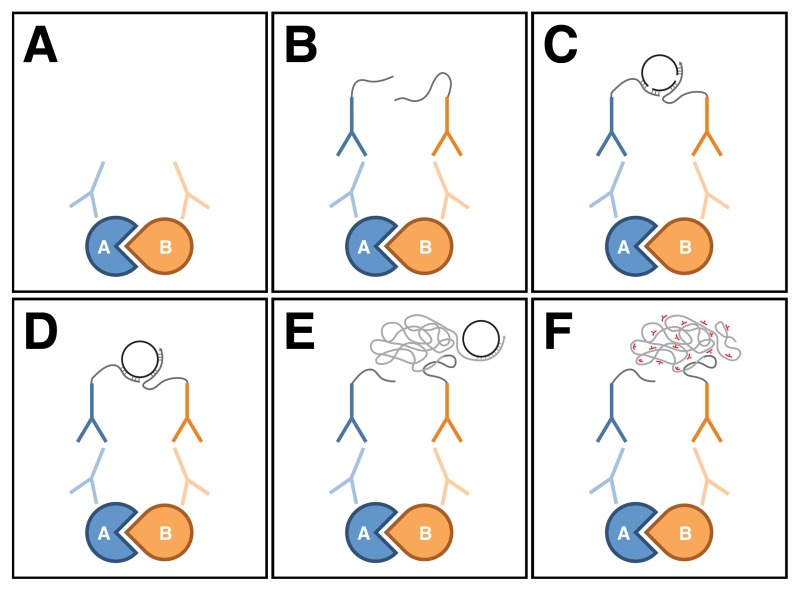 Figure 1: Schematic diagram of Proximity Ligation Assay. (A) Primary antibodies bind to the two proteins of interest using standard immunohistochemical procedures. (B) The primary antibodies are then detected with a pair of species-specific secondary antibodies, termed PLA probes, which are conjugated to oligonucleotides. (C) If the two proteins are in close proximity to each other, the distance between the attached PLA probes can be bridged through hybridization of two additional connector oligonucleotides. (D) In this conformation, the connector oligonucleotides can form a closed circular DNA molecule upon in situ ligation. (E) The circular DNA molecule serves as a template for in situ rolling circle amplification, which is primed by one of the oligonucleotides conjugated to the PLA probes. (F) Sequences within the amplified DNA product are then detected with fluorescently-labeled, complementary oligonucleotide probes. Note that the figure was based on Weibrecht et al.
24. Please click here to view a larger version of the figure.
Figure 1: Schematic diagram of Proximity Ligation Assay. (A) Primary antibodies bind to the two proteins of interest using standard immunohistochemical procedures. (B) The primary antibodies are then detected with a pair of species-specific secondary antibodies, termed PLA probes, which are conjugated to oligonucleotides. (C) If the two proteins are in close proximity to each other, the distance between the attached PLA probes can be bridged through hybridization of two additional connector oligonucleotides. (D) In this conformation, the connector oligonucleotides can form a closed circular DNA molecule upon in situ ligation. (E) The circular DNA molecule serves as a template for in situ rolling circle amplification, which is primed by one of the oligonucleotides conjugated to the PLA probes. (F) Sequences within the amplified DNA product are then detected with fluorescently-labeled, complementary oligonucleotide probes. Note that the figure was based on Weibrecht et al.
24. Please click here to view a larger version of the figure.
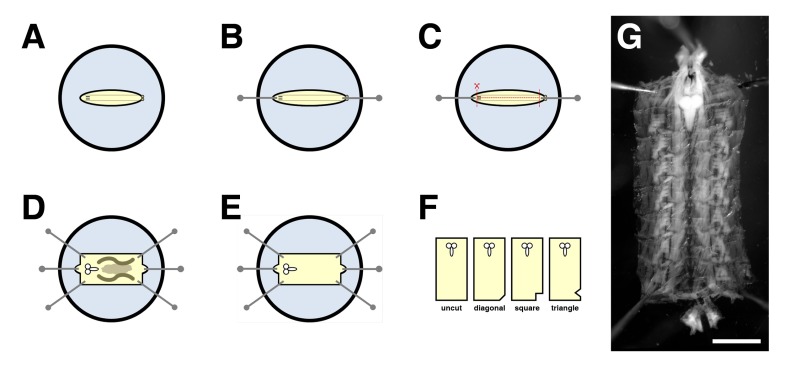 Figure 2: Preparation of third instar larval body walls. (A-F) Schematic drawings representing a body wall dissection. (A) Place a single larva onto a sylgard disc with its dorsal side facing up so that the two tracheal tracts are visible. (B) Pin the larva down at the anterior and posterior ends with minutien pins, stretching the larva out lengthwise in the process. (C) Make three incisions in the dorsal side of the larval body with microdissection scissors. The incisions should resemble an ''I'' when completed. (D) Unfurl the larval body open and pin the corners down, stretching the body wall both horizontally and vertically in the process to form an evenly-tensioned rectangle. (E) Clean out any remaining internal organs. (F) Shown are examples of the different ways to cut the corner of the body walls in order to distinguish different genotypes. (G) View of a stretched body wall preparation when completed. The picture was taken with a digital camera mounted onto a dissecting microscope. Scale bar in Panel F represents 1 mm. Note that the figure was based on Ramachandran and Budnik, 2010 12. Please click here to view a larger version of the figure.
Figure 2: Preparation of third instar larval body walls. (A-F) Schematic drawings representing a body wall dissection. (A) Place a single larva onto a sylgard disc with its dorsal side facing up so that the two tracheal tracts are visible. (B) Pin the larva down at the anterior and posterior ends with minutien pins, stretching the larva out lengthwise in the process. (C) Make three incisions in the dorsal side of the larval body with microdissection scissors. The incisions should resemble an ''I'' when completed. (D) Unfurl the larval body open and pin the corners down, stretching the body wall both horizontally and vertically in the process to form an evenly-tensioned rectangle. (E) Clean out any remaining internal organs. (F) Shown are examples of the different ways to cut the corner of the body walls in order to distinguish different genotypes. (G) View of a stretched body wall preparation when completed. The picture was taken with a digital camera mounted onto a dissecting microscope. Scale bar in Panel F represents 1 mm. Note that the figure was based on Ramachandran and Budnik, 2010 12. Please click here to view a larger version of the figure.
 Figure 3: Dlg and Hts exist in a complex at the postsynaptic region of larval NMJs.(A-B'') Distributions of Dlg (red) and Hts (green) in wild-type and hts mutant NMJs. (A-A'') In wild-type NMJs, Dlg is predominantly found at the postsynaptic membrane of type I boutons, with Dlg levels being more pronounced in type Ib than Is boutons. Hts is present throughout the muscle but also concentrates around the postsynaptic region, with Hts levels being similar in both type I boutons. The distributions of Dlg and Hts overlap at the postsynaptic region. (B-B'') NMJs mutant for hts lack Hts immunoreactivity. Note that the hts01103 mutation effects NMJ branching 8. (C-D'') PLA between Dlg and Hts (red) performed on wild-type and hts mutant NMJs. Hrp is used to mark the neuronal membrane (green). Shown are high magnification views of a few boutons. (C-C'') In wild-type, PLA signal is observed specifically at the NMJ. The signal is mostly localized circumferentially to the presynaptic membrane of type I boutons, indicating that Dlg and Hts are in close proximity to each other and exist in a complex at the postsynaptic region. (D) NMJs mutant for hts showed no observable PLA signal. (E-F) Shown are high magnification views of single boutons. (E) Hts and Dlg immunoreactivity grossly overlap at the postsynaptic region. (F) PLA between Dlg and Hts results in distinct puncta indicating that only a subset of the proteins are in a complex. Scale bar in Panel B'' represents 40 µm (A-B''); scale bar in Panel D'' represents 10 µm (C-D''); scale bar in Panel F represents 5 µm (E-F). All NMJs shown are from muscles 6/7 in abdominal segment 4. Images were taken as merged stacks using a Nikon A1R laser scanning confocal microscope with NIS-Elements software, and processed with Adobe Photoshop. Please click here to view a larger version of the figure.
Figure 3: Dlg and Hts exist in a complex at the postsynaptic region of larval NMJs.(A-B'') Distributions of Dlg (red) and Hts (green) in wild-type and hts mutant NMJs. (A-A'') In wild-type NMJs, Dlg is predominantly found at the postsynaptic membrane of type I boutons, with Dlg levels being more pronounced in type Ib than Is boutons. Hts is present throughout the muscle but also concentrates around the postsynaptic region, with Hts levels being similar in both type I boutons. The distributions of Dlg and Hts overlap at the postsynaptic region. (B-B'') NMJs mutant for hts lack Hts immunoreactivity. Note that the hts01103 mutation effects NMJ branching 8. (C-D'') PLA between Dlg and Hts (red) performed on wild-type and hts mutant NMJs. Hrp is used to mark the neuronal membrane (green). Shown are high magnification views of a few boutons. (C-C'') In wild-type, PLA signal is observed specifically at the NMJ. The signal is mostly localized circumferentially to the presynaptic membrane of type I boutons, indicating that Dlg and Hts are in close proximity to each other and exist in a complex at the postsynaptic region. (D) NMJs mutant for hts showed no observable PLA signal. (E-F) Shown are high magnification views of single boutons. (E) Hts and Dlg immunoreactivity grossly overlap at the postsynaptic region. (F) PLA between Dlg and Hts results in distinct puncta indicating that only a subset of the proteins are in a complex. Scale bar in Panel B'' represents 40 µm (A-B''); scale bar in Panel D'' represents 10 µm (C-D''); scale bar in Panel F represents 5 µm (E-F). All NMJs shown are from muscles 6/7 in abdominal segment 4. Images were taken as merged stacks using a Nikon A1R laser scanning confocal microscope with NIS-Elements software, and processed with Adobe Photoshop. Please click here to view a larger version of the figure.
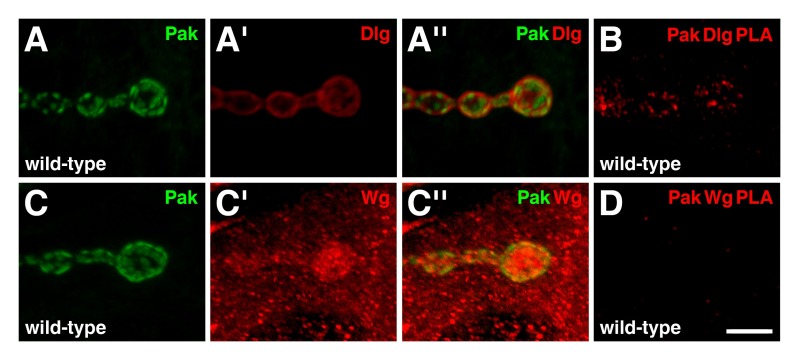 Figure 4: Pak exists in a complex with Dlg, but not Wg, at the postsynaptic region of larval NMJs.(A-D) Shown are high magnification views of a few boutons. (A-A'') Distributions of Pak (green) and Dlg (red) in wild-type NMJs. Pak localizes to the postsynaptic density. Note that the immunoreactive distributions of Pak and Dlg overlap at the postsynaptic region. (B) PLA between Pak and Dlg (red) performed on wild-type NMJs. PLA signal is observed specifically at the NMJ, indicating that the two proteins are in close proximity to each other in this region. (C-C'') Distributions of Pak (green) and Wg (red) in wild-type NMJs. Wg is enriched at both the presynaptic and postsynaptic sides of the NMJ, but is also present as puncta throughout the muscle. Note that the immunoreactive distribution of Pak is also observed to partially overlap with Wg at the postsynaptic region. (D) PLA between Pak and Wg (red) performed on wild-type NMJs. No specific PLA signal is observed, indicating that the two proteins are not in close proximity to each other at the NMJ despite their overlapping distributions. Scale bar in Panel D represents 5 µm (A-D). All NMJs innervate muscles 6/7 in abdominal segment 4. Images were taken as merged stacks using a Nikon A1R laser scanning confocal microscope with NIS-Elements software, and processed with Adobe Photoshop. Please click here to view a larger version of the figure.
Figure 4: Pak exists in a complex with Dlg, but not Wg, at the postsynaptic region of larval NMJs.(A-D) Shown are high magnification views of a few boutons. (A-A'') Distributions of Pak (green) and Dlg (red) in wild-type NMJs. Pak localizes to the postsynaptic density. Note that the immunoreactive distributions of Pak and Dlg overlap at the postsynaptic region. (B) PLA between Pak and Dlg (red) performed on wild-type NMJs. PLA signal is observed specifically at the NMJ, indicating that the two proteins are in close proximity to each other in this region. (C-C'') Distributions of Pak (green) and Wg (red) in wild-type NMJs. Wg is enriched at both the presynaptic and postsynaptic sides of the NMJ, but is also present as puncta throughout the muscle. Note that the immunoreactive distribution of Pak is also observed to partially overlap with Wg at the postsynaptic region. (D) PLA between Pak and Wg (red) performed on wild-type NMJs. No specific PLA signal is observed, indicating that the two proteins are not in close proximity to each other at the NMJ despite their overlapping distributions. Scale bar in Panel D represents 5 µm (A-D). All NMJs innervate muscles 6/7 in abdominal segment 4. Images were taken as merged stacks using a Nikon A1R laser scanning confocal microscope with NIS-Elements software, and processed with Adobe Photoshop. Please click here to view a larger version of the figure.
Discussion
This report demonstrates how PLA can be applied to the Drosophila larval NMJ. The assay is performed on larval body wall muscle preparations for the purpose of detecting endogenous protein-protein interactions present at the NMJ. With this technique, Dlg and Hts are shown to be in close proximity to each other, and thus exist in a complex, specifically at the postsynaptic region 27. In support of this result, a previous study has provided evidence of their association with the following data: 1) the immunoreactive distributions of Dlg and Hts overlap at the postsynaptic region of larval NMJs, 2) Dlg and Hts form a complex based on co-immunoprecipitation experiments involving whole adult fly lysates, and 3) Dlg and Hts interact in both epithelial and synaptic junctions 8. PLA signal at the postsynaptic region was also observed between Dlg and Pak, an interaction previously identified in other studies, thereby providing further credence of using this assay at the NMJ 15,20,21. Interestingly, PLA between Pak and Wg resulted in no observable signal, even though their immunoreactive distributions overlapped at the NMJ. This result demonstrates that not all proteins that show overlapping immunoreactive distributions will generate a PLA signal, therefore indicating that PLA provides a higher resolution in detecting protein-protein interactions than traditional co-localization studies.
Multiple changes were made to the original PLA protocol in order to optimize the assay for the larval NMJ (data not shown) 9,25. First, it was determined that 1% BSA is a better blocking agent for the body walls instead of the provided Blocking Solution. Second, to produce a strong signal-to-noise ratio, proper immersion and mixing of the body walls in the reaction solutions is critical. A minimum of 200 µl for five to ten body walls in a 0.65 ml microcentrifuge tube was deemed suitable when incubating with the PLA probes, ligase or polymerase, though the volumes can be increased accordingly when processing more body walls. Signal strength was also enhanced by increasing the reaction times by 30 minutes over the recommended times. Third, a serial dilution test of the PLA probes was performed to optimize the balance between reagent conservation and signal strength. For the experiments performed in this report, up to a 1:25 dilution — from the recommended 1:5 dilution — can still produce a reasonable signal-to-noise ratio. Note that optimization of the ligation and amplification reactions were not performed. Finally, as PLA can be sensitive to minute changes, it is strongly recommended that the different genotypes of a single experiment are placed in a single tube so that the controls and experiments are treated equally during the assay. Genotypes can be distinguished by cutting the corners of the body walls differently.
Several methods are used to detect Drosophila protein-protein interactions including yeast two-hybrid screening, co-immunoprecipitation and FRET. But how do these methods compare against PLA and its ability to visualize protein-protein interactions in situ? Yeast two-hybrid can detect direct binding between Drosophila proteins expressed in yeast, and it has been used in high-throughput genome-wide screens. Though many of the identified interactions are biologically relevant, the assay often produces false-positives. In addition, false negatives can occur for multiple reasons: the proteins are fused to transcription factor domains which may interfere with binding, many interactions require post-translational modifications which do not occur in yeast, and the nucleus (where the assay takes place) may not be a suitable environment for some interactions to properly form. Such issues are not encountered with PLA as it deals with endogenous proteins in their native environment. One method that can be used to detect interactions between endogenous proteins is co-immunoprecipitation. Experiments involving Drosophila lysates can reveal the tissue and stage in the life cycle in which an interaction occurs. However, as lysate formation involves disrupting the cells to extract the proteins, the cells within the tissue in which the interaction occurs in, as well as its subcellular localization, cannot be assessed — unlike in PLA. Furthermore, co-immunoprecipitation detects protein complex binding and cannot distinguish which proteins within the complex are in close proximity to each other. There are methods such as FRET available to Drosophilists that allow the visualization of protein-protein interactions within the cell. However, FRET involves the overexpression of transgenic proteins fused to fluorescent tags and may not reflect endogenous protein behavior as in PLA.
Despite its advantages, there are some limitations when using PLA. One such limitation is the availability of primary antibodies against the proteins of interest which are made in different species. This problem can easily be circumvented with the use of tagged transgenic proteins, though they may not be truly representative of the endogenous interaction. Another issue is that PLA may produce false negatives, e.g. when the two primary antibodies sterically hinder each other or when the epitope of one of the primary antibodies involves the protein-protein interaction site. In addition, although PLA detects proteins which are in close proximity to each other, it does not distinguish between direct and indirect protein-protein interactions, unlike in pull-down assays and yeast two-hybrid screening. Furthermore, PLA between endogenous proteins will not identify which domains are responsible for the interaction. Thus, other protein-protein interaction assays will still be needed to fully characterize the interaction molecularly.
Researchers have begun using super-resolution microscopy to map the spatial architecture of the Drosophila larval NMJ, with resolutions of tens of nanometers being achieved 26. PLA, with its comparable molecular resolution, should provide a low cost, technically simple complement to these studies that will aid in building up a detailed view of the organization of the numerous proteins at the NMJ.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank the Bloomington Drosophila Stock Center for providing fly stocks. We also thank the Developmental Studies Hybridoma Bank and Dr. Lynn Cooley (Yale University) for providing antibodies. A special thanks goes to AhHyun Yoo for her help on the manuscript. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (Krieger), the William and Ada Isabelle Steel Fund (Krieger), and the Canadian Institutes of Health Research (Harden).
References
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- Humbert PO, et al. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Budnik V, Thomas U. Scaffolding proteins at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:181–216. doi: 10.1016/S0074-7742(06)75009-7. [DOI] [PubMed] [Google Scholar]
- Thomas U, Kobler O, Gundelfinger ED. The Drosophila larval neuromuscular junction as a model for scaffold complexes at glutamatergic synapses: benefits and limitations. J Neurogenet. 2010;24:109–119. doi: 10.3109/01677063.2010.493589. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. Drosophila adducin regulates Dlg phosphorylation and targeting of Dlg to the synapse and epithelial membrane. Dev Biol. 2011;357:392–403. doi: 10.1016/j.ydbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Soderberg O, et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45:227–232. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Brent JR, Werner KM, McCabe BD. Drosophila larval NMJ dissection. J Vis Exp. 2009. [DOI] [PMC free article] [PubMed]
- Ramachandran P, Budnik V. Immunocytochemical staining of Drosophila larval body-wall muscles. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5470. [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Budnik V. Dissection of Drosophila larval body-wall muscles. Cold Spring Harb Protoc. 2010. [DOI] [PubMed]
- Ashburner M. Drosophila: A Laboratory Manual. Cold Spring, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Brent J, Werner K, McCabe BD. Drosophila larval NMJ immunohistochemistry. J Vis Exp. 2009. [DOI] [PMC free article] [PubMed]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Petrella LN, Smith-Leiker T, Cooley L. The Ovhts polyprotein is cleaved to produce fusome and ring canal proteins required for Drosophila oogenesis. Development. 2007;134:703–712. doi: 10.1242/dev.02766. [DOI] [PubMed] [Google Scholar]
- Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW. Hts/Adducin controls synaptic elaboration and elimination. Neuron. 2011;69:1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, et al. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-Activated Kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bahri S, et al. The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development. 2010;137:2023–2032. doi: 10.1242/dev.045088. [DOI] [PubMed] [Google Scholar]
- Albin SD, Davis GW. Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci. 2004;24:6871–6879. doi: 10.1523/JNEUROSCI.1538-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles K, Budnik V. Wnt signaling in neuromuscular junction development. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibrecht I, et al. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev Proteomics. 2010;7:401–409. doi: 10.1586/epr.10.10. [DOI] [PubMed] [Google Scholar]
- Thymiakou E, Episkopou V. Detection of signaling effector-complexes downstream of bmp4 using PLA, a proximity ligation assay. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Maglione M, Sigrist SJ. Seeing the forest tree by tree: super-resolution light microscopy meets the neurosciences. Nat Neurosci. 2013;16:790–797. doi: 10.1038/nn.3403. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. Phospho-regulated Drosophila adducin is a determinant of synaptic plasticity in a complex with Dlg and PIP2 at the larval neuromuscular junction. Biol. Open. 2014;3(12):1196–1206. doi: 10.1242/bio.20148342. [DOI] [PMC free article] [PubMed] [Google Scholar]


