Abstract
This video demonstrates in detail an in vitro single-fiber electrophysiological recording protocol using a mouse colorectum-nerve preparation. The approach allows unbiased identification and functional characterization of individual colorectal afferents. Extracellular recordings of propagated action potentials (APs) that originate from one or a few afferent (i.e., single-fiber) receptive fields (RFs) in the colorectum are made from teased nerve fiber fascicles. The colorectum is removed with either the pelvic (PN) or lumbar splanchnic (LSN) nerve attached and opened longitudinally. The tissue is placed in a recording chamber, pinned flat and perfused with oxygenated Krebs solution. Focal electrical stimulation is used to locate the colorectal afferent endings, which are further tested by three distinct mechanical stimuli (blunt probing, mucosal stroking and circumferential stretch) to functionally categorize the afferents into five mechanosensitive classes. Endings responding to none of these mechanical stimuli are categorized as mechanically-insensitive afferents (MIAs). Both mechanosensitive and MIAs can be assessed for sensitization (i.e., enhanced response, reduced threshold, and/or acquisition of mechanosensitivity) by localized exposure of RFs to chemicals (e.g., inflammatory soup (IS), capsaicin, adenosine triphosphate (ATP)). We describe the equipment and colorectum–nerve recording preparation, harvest of colorectum with attached PN or LSN, identification of RFs in the colorectum, single-fiber recording from nerve fascicles, and localized application of chemicals to the RF. In addition, challenges of the preparation and application of standardized mechanical stimulation are also discussed.
Keywords: Medicine, Issue 95, visceral afferent, colorectum, single-fiber, extracellular recording, teased fiber, pelvic, lumbar splanchnic, mechanically-insensitive afferent, MIA, silent afferent
Introduction
Pain and hypersensitivity are the predominant complaints of patients suffering from functional gastrointestinal disorders, including irritable bowel syndrome (IBS), which exist in the absence of apparent pathobiological cause or tissue damage. For example, IBS patients exhibit hypersensitivity, including heightened responses to rectal balloon distension and increased sensitivity during normal bowel function, as well as hypersensitivity of somatic referral (i.e., tenderness to palpation of the abdominal area)1. Because targeting colorectal afferents has proven to be effective in alleviating pain and hypersensitivity in IBS patients (e.g., intra-rectal instillation of local anesthetics2,3; oral ingestion of the guanylate cyclase-C agonist linaclotide4-6), improved understanding of the afferent innervation of the colorectum is important.
Visceral afferents, including colorectal afferents, are capable of responding to chemical/nutrient- and thermal modalities (e.g., 7-9). However, visceral afferents responding to mechanical stimuli (i.e., mechanosensitive afferents) have been the most thoroughly studied because mechanical stimuli (e.g., luminal distension, stretch) are those that generally give rise to conscious sensations, including discomfort and pain10-16. In addition, the viscera are also innervated by mechanically insensitive afferents (MIAs), commonly termed silent or sleeping nociceptors17. Under normal physiological conditions, MIAs do not respond to mechanical stimulation or have very high response thresholds18, but can become active and acquire mechanosensitivity in pathophysiological conditions and contribute to hypersensitivity.
Using the in vitro preparation and protocol described here, we developed and employed an electrical stimulus strategy to search for receptive endings, permitting unbiased identification of both mechanosensitive and MIA endings in the colorectum19. The colorectal innervation is derived from lumbar splanchnic (LSN) and pelvic nerve (PN) pathways, and includes colorectal afferents that can be categorized into five mechanosensitive classes (serosal, mucosal, muscular, muscular-mucosal, mesenteric) and one MIA class20. Using this in vitro preparation, we found that colorectal MIAs acquired mechanosensitivity (sensitize) following brief exposure of their receptive fields to an inflammatory soup (IS), which sensitized 71% of MIAs in the PN pathway and 23% of MIAs in the LSN pathway19. We also documented long-term sensitization (up to 28 days) of MIAs in the context of long-lasting behavioral visceral hypersensitivity (i.e., in mice receiving intracolonic treatments with zymosan21 or 2,4,6-trinitrobenzenesulfonic acid (TNBS)22).
Among mechanosensitive afferents, muscular and muscular-mucosal afferents are the only classes that tonically encode circumferential stretch of the colorectum (i.e., are stretch-sensitive) and subserve the encoding of noxious colorectal distension23,24. Using a computer-controlled force actuator, we applied a standard, homogeneous, and reproducible ramped stretch in the circumferential direction of the flattened colorectal tissue and further categorized stretch-sensitive afferents as low-threshold and high-threshold23. In addition, the time course of sensitization of stretch-sensitive afferents after intracolonic zymosan21 or TNBS22 treatment corresponds to the onset, persistence, and/or recovery of behavioral visceral hypersensitivity, suggesting a role of stretch-sensitive colorectal afferents in visceral pain and hypersensitivity.
Protocol
NOTE: This protocol was reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
1. Preparation of Modified Krebs Solution and Test Drug Aliquots
Make 6 L of modified Krebs solution that contains (in mM): 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, 11.1 D-glucose, 2 sodium butyrate, 20 sodium acetate, 0.004 nifedipine (to block spontaneous muscle contractions), and 0.003 indomethacin (to block synthesis of endogenous prostaglandins). Use ice-cold and warm Krebs solutions for tissue dissection and single-fiber recording, respectively.
Prepare any chemical solutions (e.g., IS, capsaicin, ATP) in aliquots at desired concentrations.
2. Dissection of the Colorectum-nerve Tissue
Anesthetize and euthanize male mice (6 - 8 weeks old, 20 - 30 g) in a CO2 chamber at a flow rate that displaces 10 - 30% of the chamber volume per minute until mice stop breathing completely as indicated by the absence of chest movement.
Immediately following euthanasia, exsanguinate by cutting open the thoracic chamber, perforating the right atrium, and immersing the mouse carcass in an ample volume (~500 ml) of ice-cold Krebs (4 °C) solution bubbled with carbogen (95% O2, 5% CO2).
Carefully remove all viscera but the colon and pelvic organs. Transect the mouse in half across the T12 spinal segment slightly above the thoracic diaphragm and transfer the caudal half to a dissection chamber containing ice cold, bubbled Krebs solution.
Under a stereomicroscope, remove the bladder and the reproductive organs by transecting at their junctions to the urethra, and remove the descending/abdominal aorta until it bifurcates into common iliac arteries. Free the PN or the LSN from their surrounding tissues by blunt dissection and follow the nerve from outside the iliac crest till its ventral entry point into the L6 and S1 vertebral column (for PN) or T13 and L1 vertebral column (for LSN).
Cut the pubic symphysis and right and left acetabular joints, and remove the iliac bone. Carefully free either the PN or LSN from the attached muscle and connective tissue from close to the colorectum until where the nerve enters the vertebral column.
Carefully resect the iliac bone to expose the distal colorectum. Dissect out the distal colorectum with the attached PN or LSN in continuum.
Transfer the colorectum with the nerve attached to the bath compartment of the tissue chamber. Remove excessive connective tissue by further dissection, and open the colorectum longitudinally along the anti-mesenteric border.
With mucosal side facing up, pin the mesenteric edge of the colorectum adjacent to the recording compartment into the silicone base of the chamber and attach the antimesenteric length of the colorectum to a rake of hooks connected to a force actuator (illustrated in Figure 1 and photographed in Figure 2A).
Extend the PN or LSN into the recording compartment, which is connected to the bath compartment by a mouse hole and gate. Gently lay the nerve trunk onto a small glass mirror in the recording compartment, which provides a hydrophilic surface for the nerve to adhere to. Superfuse the bath compartment with warm (30 - 32 °C), oxygenated Krebs solution and fill the recording compartment with mineral oil.
3. Single-fiber Recording and Localization of the Receptive Field
Carefully peel back the epineurium (nerve sheath) from the PN or LSN under the stereomicroscope at high magnification (50 - 60X). Using fine forceps, tease the nerve trunk into 5 - 8 nerve bundles of ~100 µm thickness.
Place the platinum-iridium reference electrode in contact with the Krebs solution in the tissue chamber. Sequentially place the individual nerve bundles onto the recording electrode made of the same material.
Use a soft paint brush to evoke APs from the colorectal afferents by gently stroking up and down the colorectal surface. Locate the nerve bundle(s) that innervate the colon through detectable AP (action potential) recordings. NOTE: The PN and LSN also innervate the urinary bladder and other pelvic organs.
Use a pair of 30 G needle tips to further split the nerve bundle into fine fascicle filaments of ~10 µm thickness and place an individual filament onto the recording electrode.
Place the round-tipped concentric electrode perpendicular to the mucosal surface to electrically excite afferent endings at suprathreshold stimulus intensity (10 mA magnitude, 0.5 msec duration @ 0.3 Hz), which produces a ~2 mm radius of current spread. Move the electrode systematically (~1.5 mm steps) along the length and width of the flattened colorectum to localize receptive endings.
When an afferent ending is excited, adjust the electrode position to pinpoint the site of activation (receptive field, RF) that requires minimum stimulus intensity (stimulus threshold). Discard endings with a stimulus threshold >3 mA19.
Calculate the conduction velocity (CV) from 1) the distance between the stimulating electrode at the receptive field (RF) and the recording site and 2) the conduction delay (e.g., Figure 2B) between the stimulus artifact and the onset of the action potential. CV (m/sec) = distance (mm) / conduction delay (msec).
4. Functional Classification of Mechanosensitive Colorectal Afferents
- After locating a RF by electrical stimulation, apply the following three mechanical stimuli to the RF:
- Conduct the probing stimulus by pressing the tip of a calibrated von Frey-like nylon monofilament (0.4 and 1 g force) perpendicularly towards the RF on the flattened colorectum.
- Conduct the stroking stimulus by gently stroking the colorectal mucosa with a fine nylon filament strand (10 mg force) to generate a small surface shear stress at the RF.
- Conduct the circumferential stretch using a computer-controlled force actuator, which delivers a ramped stretch force (0 - 170 mN at 5 mN/sec) in circumferential direction along the anti-mesenteric edge of the colorectum via the rake of hooks described in step 2.8.
Classify afferents as serosal (respond only to blunt probing), mucosal (respond to mucosal stroking and blunt probing), muscular (respond to circumferential stretch and blunt probing) muscular/mucosal (respond to circumferential stretch, mucosal stroking and blunt probing), or MIA (not responsive to any of the three mechanical stimuli).
For mesenteric afferents (only in the LSN innervation) that are difficult to activate selectively by electrical stimulation, locate their receptive endings by mechanical stroking/probing of the mesentery.
For stretch-sensitive afferents (muscular and muscular-mucosal), determine the response threshold, which is defined as the force that evokes the first AP during ramped stretch.
For serosal afferents, record their responses to ascending levels of punctate probing of the receptive field driven by the computer-controlled force actuator.
5. Chemical Application/Modulation of Receptive Endings
Record a baseline response to a mechanical stimulus (i.e., response to ramped stretch, punctate probing, or mucosal stroking).
Coat the bottom edge of a piece of tubing (brass or stainless steel, 10 mm high and 4 x 4 mm2 square or 4 - 5 mm diameter) with petrolatum and place it over the receptive field on the colorectum.
Remove the Krebs solution inside the tubing, and expose the receptive ending for 3 - 5 min to 170 μl of the solution containing the chemical(s) to be tested.
Monitor the response of the afferent during chemical application (some afferents are chemosensitive).
Remove the chemical solution and tubing to terminate the action of the chemical. Within 4 - 6 min, test the afferent response to the same mechanical stimulus as in the baseline response.
Reapply the mechanical stimulus again after sufficient period of wash-out (>15 min).
6. Recording and Discriminating AP spikes
Digitize the electrical signals recorded from axons at 20 kHz and save the data to a computer. Monitor the signal on-line by an audio monitor.
Analyze the AP spikes off-line and discriminate single units based upon principal component analysis of individual spike waveforms25. NOTE: One record should contain no more than two easily discriminable active units.
Representative Results
The setup is illustrated in Figure 1. It includes a custom-made tissue chamber that houses the colorectum in a silicone-lined bath compartment and the attached nerve in a contiguous mineral oil-filled compartment. The two-compartment chamber was machined from a solid block of acrylic plastic by a CNC machine; the bottom of both compartments was subsequently lined with firm silicone to allow easy pin down of the colorectal tissue. Extracellular APs from teased nerve fascicles are recorded using a low-noise, battery-powered differential amplifier with a high common-mode rejection ratio (CMRR ~ 60 dB). The gain of the amplifier is set to x10,000 and the band filter range at 0.3 to 10 kHz. Electrical stimulation of the colorectum is delivered by an optically coupled stimulator in constant current mode via a concentric electrode in contact with the colorectal mucosa. Mechanical stimulation (colorectal stretch and punctate probing) is delivered by a computer-controlled force actuator. An A-D converter and appropriate software oversee both stimulation and recording processes by sending voltage command outputs to initiate mechanical and electrical stimuli as well as recording and digitizing the extracellular AP signals from the differential amplifier. To isolate from mechanical and electrical noise sources, the tissue chamber, microscope and differential amplifier are placed inside a Faraday cage mounted on a pneumatic air table.
As shown in Figure 2A, the colorectum with the attached nerve is dissected out from a mouse, cut open along the anti-mesenteric edge, and pinned flat in the silicone-lined tissue chamber; the nerve is placed on a glass mirror in the adjacent recording chamber. Displayed in Figure 2B is a representative record of an action potential (AP) in response to electrical stimulation of the RF at threshold. The AP in this record lags behind the stimulus artifact (•) by 49 msec due to the conduction delay from the RF to the recording electrode, resulting in a calculated conduction velocity of 0.43 m/sec, well in the range of an unmyelinated C-fiber. Displayed in Figure 2C are typical responses of an afferent to stimuli delivered by hand (probing of the RF with von Frey-like monofilaments, 1 g, and fine mucosal stroking of the RF, 10 mg). This record contains two easily discriminable afferents; only the large amplitude afferent responds to stroking. As shown in Figure 2D, afferent responses to probing were also assessed by a computer-controlled force actuator that delivers to the same site on the colorectum a series of precisely timed and reproducible mechanical forces (5, 10, 20, 40 and 80 mN, 5 sec duration). Similarly, circumferential stretch of the colorectum (0 - 170 mN at 5 mN/sec) is delivered by the same actuator with a representative response displayed in Figure 2E.
As shown in Figure 3, colorectal afferents can be functionally categorized into six classes based upon their response profiles to three distinct mechanical stimuli (see step 4.2 above). All afferent endings except for mesenteric afferents were located by electrical stimulation (e-stim; left-most column, arrows indicate stimulus artifact). Mechanically insensitive afferents (MIAs) do not respond to any of the three mechanical stimuli. In contrast, all mechanosensitive endings respond to probing (0.4 - 1.4 g). Among them, muscular and muscular-mucosal endings are also activated by circumferential stretch (0 - 170 mN), and thus are termed stretch-sensitive afferents; muscular-mucosal endings are also activated by stroking (10 mg). Mucosal endings are also activated by stroking (10 mg) but not stretch and serosal endings are not activated by either stretch or stroking. Mesenteric endings are identified by mechanically brushing the mesentery.
Displayed in Figure 4A are representative responses from a stretch-sensitive afferent evoked by three consecutive circumferential stretches separated by 5 min. The spike numbers are evenly binned into three bins and displayed as stimulus-response functions in Figure 4B, revealing high reproducibility in both response magnitude (spike number) and response threshold.
This in vitro colorectum-nerve preparation also permits local application of chemicals to afferent receptive endings. The exposure to chemicals is restricted to regions around the afferent RF by placing brass or stainless steel tubing atop the colorectal mucosa to physically isolate the RF from the rest of the colorectum. Typical results following chemical application include: direct activation of afferents upon application of an acid hypertonic solution (AHS26; Figure 5A), no activation, but acquisition of mechanosensitivity by an MIA after application of an inflammatory soup (IS19; Figure 5B), increased response (i.e., sensitization) to mechanical stretch after application of IS (Figure 5C), and reduced response to mechanical stretch after application of cGMP (Figure 5D).
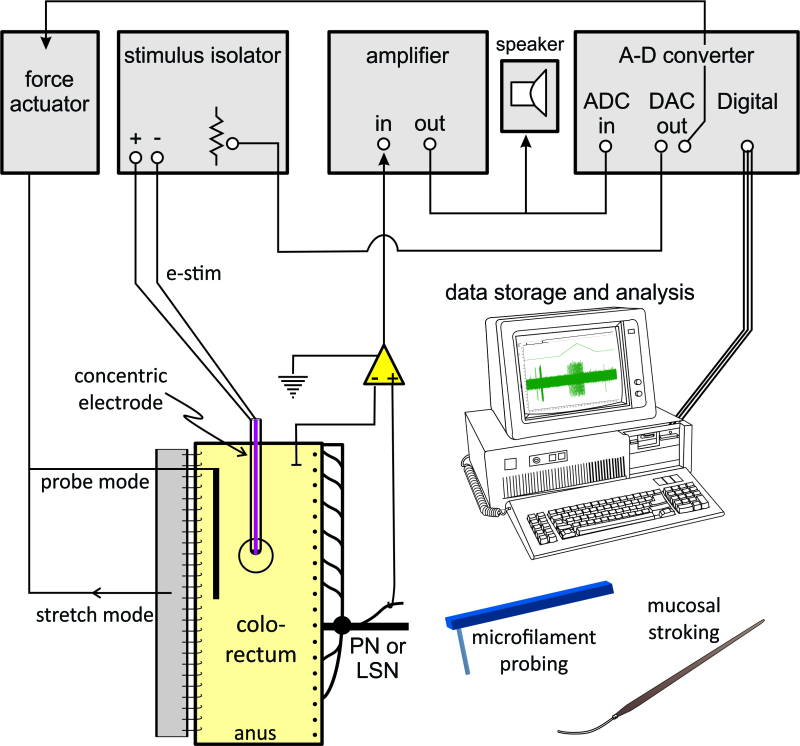 Figure 1. Schematic representation of the experimental setup. The colorectum-nerve is placed in a two-compartment tissue chamber and isolated along with the differential amplifier head stage from other electronic equipment by a Faraday cage. Each afferent RF is identified by electrical stimulation (e-stim) of the colorectum and tested by three mechanical stimuli: nylon monofilament probing, mucosal stroking, and circumferential stretch. Please click here to view a larger version of this figure.
Figure 1. Schematic representation of the experimental setup. The colorectum-nerve is placed in a two-compartment tissue chamber and isolated along with the differential amplifier head stage from other electronic equipment by a Faraday cage. Each afferent RF is identified by electrical stimulation (e-stim) of the colorectum and tested by three mechanical stimuli: nylon monofilament probing, mucosal stroking, and circumferential stretch. Please click here to view a larger version of this figure.
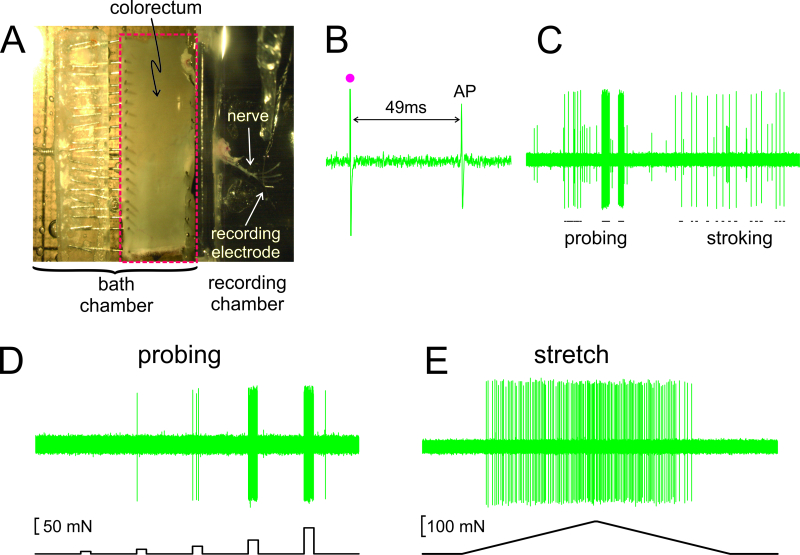 Figure 2. An image through the stereomicroscope of the dissected colorectum with attached pelvic nerve (A).B – E show representative records. (B) An action potential (AP) evoked by electrical stimulation (stimulus artifact, •). (C) Typical responses to hand-held monofilament probing and mucosal stroking. (D,E) Responses to probing and to circumferential stretch delivered by the computer-controlled force actuator, respectively. Please click here to view a larger version of this figure.
Figure 2. An image through the stereomicroscope of the dissected colorectum with attached pelvic nerve (A).B – E show representative records. (B) An action potential (AP) evoked by electrical stimulation (stimulus artifact, •). (C) Typical responses to hand-held monofilament probing and mucosal stroking. (D,E) Responses to probing and to circumferential stretch delivered by the computer-controlled force actuator, respectively. Please click here to view a larger version of this figure.
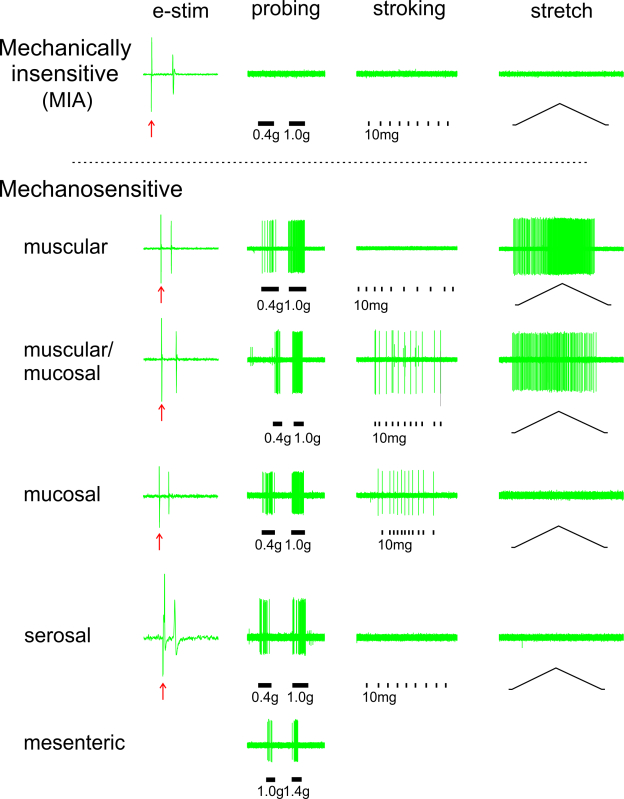 Figure 3. Functional characterization of colorectal afferent classes. Afferents are located by electrical stimulation (e-stim, ↑) of the colorectum and categorized into five mechanosensitive classes and one mechanically-insensitive afferent (MIA) class based upon their respective response profiles to three mechanical stimuli: probing, stroking, and stretch. Please click here to view a larger version of this figure.
Figure 3. Functional characterization of colorectal afferent classes. Afferents are located by electrical stimulation (e-stim, ↑) of the colorectum and categorized into five mechanosensitive classes and one mechanically-insensitive afferent (MIA) class based upon their respective response profiles to three mechanical stimuli: probing, stroking, and stretch. Please click here to view a larger version of this figure.
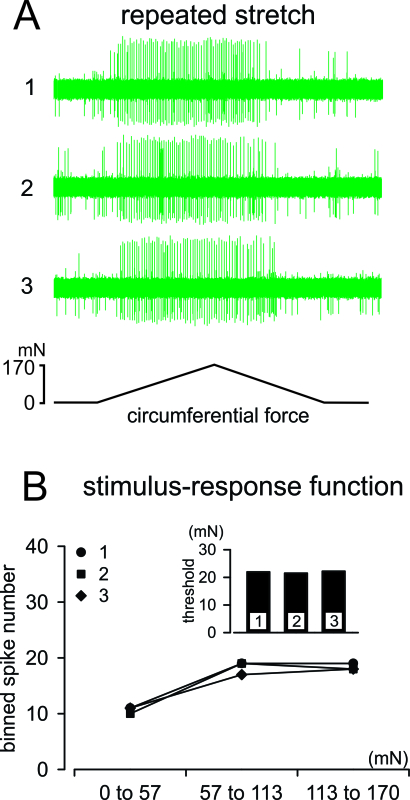 Figure 4. Afferent responses to reproducible, computer-controlled mechanical stimulation. (A) Responses of a muscular-mucosal afferent to three consecutive ramped stretches (0 - 170 mN at 5 mN/sec; 5 min inter-stimulus interval). (B) Responses (action potential spikes) were evenly binned into three bins (0 - 57, 57 - 113, and 113 - 170 mN) and displayed as stimulus-response functions; response threshold is displayed in the inset. Please click here to view a larger version of this figure.
Figure 4. Afferent responses to reproducible, computer-controlled mechanical stimulation. (A) Responses of a muscular-mucosal afferent to three consecutive ramped stretches (0 - 170 mN at 5 mN/sec; 5 min inter-stimulus interval). (B) Responses (action potential spikes) were evenly binned into three bins (0 - 57, 57 - 113, and 113 - 170 mN) and displayed as stimulus-response functions; response threshold is displayed in the inset. Please click here to view a larger version of this figure.
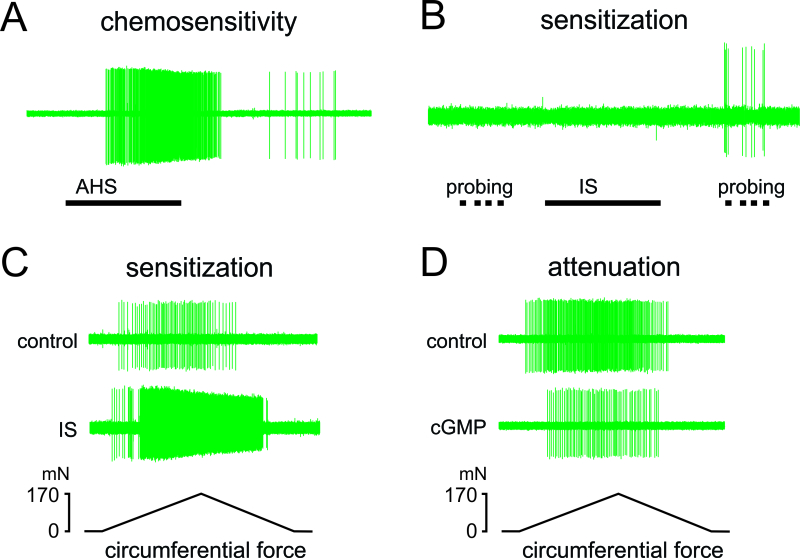 Figure 5. Afferent responses to localized chemical application to receptive endings. (A) An example of chemosensitivity of a serosal afferent to application of acidic hypertonic solution (AHS). (B) An example of acquisition of mechanosensitivity (sensitization) by a MIA ending. This MIA did not respond directly to an inflammatory soup (IS), but responded to 1.4 g monofilament probing afterwards. (C) Sensitization (an increase in response magnitude and reduction in response threshold) to stretch of a muscular afferent after exposure of its ending to IS. (D) Attenuation of the response to stretch by a muscular-mucosal afferent after exposure of its ending to cyclic guanosine monophosphate (cGMP). Please click here to view a larger version of this figure.
Figure 5. Afferent responses to localized chemical application to receptive endings. (A) An example of chemosensitivity of a serosal afferent to application of acidic hypertonic solution (AHS). (B) An example of acquisition of mechanosensitivity (sensitization) by a MIA ending. This MIA did not respond directly to an inflammatory soup (IS), but responded to 1.4 g monofilament probing afterwards. (C) Sensitization (an increase in response magnitude and reduction in response threshold) to stretch of a muscular afferent after exposure of its ending to IS. (D) Attenuation of the response to stretch by a muscular-mucosal afferent after exposure of its ending to cyclic guanosine monophosphate (cGMP). Please click here to view a larger version of this figure.
Discussion
The in vitro colorectum-nerve preparation described here has proven to be a powerful approach to study neural encoding functions of individual colorectal afferents, which nicely complements other non-functional approaches (e.g., cellular, molecular, and histological studies) on visceral sensory neurons (see review 27 for details). Neuronal mechanisms contributing to nociception and long-term colorectal hypersensitivity have been revealed and pharmacological manipulations have been performed that have revealed targets that could alleviate visceral pain. The following key points associated with successful implementation of this preparation are discussed below: 1) reduction of electrical noise, 2) increase in signal detection, and 3) selection of a standardized stimulus to evaluate changes of afferent encoding. In addition, several limitations of this technique are discussed.
Action potentials (APs) propagating intracellularly along nerve axons generally have a transmembrane potential of 100 - 130 mV. However, due to the small specific capacitance of the axon membrane, this relatively large depolarization only results in a small electrical charge displacement across the cell membrane, which can easily dissipate into the surrounding extracellular tissue/interstitial fluid (which has an electrical impedance considerably lower than the lipid membrane). For extracellular recording from nerve filaments/axons, the electrical signal is usually in the range of microvolts, close to the magnitude of the thermal/white noise associated with a typical bioelectrical recording setup, rendering noise reduction the first priority for successful recording. To most effectively insulate from ambient electrical noise, it can be helpful to place the tissue chamber, recording and grounding electrodes, the differential amplifier (DC battery-powered) and the stereomicroscope in a Faraday cage. If movement artifacts occur, placing the Faraday cage on a pneumatic air table to dampen mechanical vibration is helpful. Ideally, the recording and reference electrodes that enter the “+” and “–” ports of the differential amplifier, respectively, should have comparable impedance relative to their common ground and be located close to each other. Thus, any external noise will be recorded about equally by both electrodes and subjected to the stringent common mode rejection by the differential amplifier.
In our setup (Figure 2A), the reference electrode is dipped into the Krebs perfusion solution in the tissue chamber whereas the recording electrode is in contact with a fine nerve filament of considerable impedance. This single-electrode configuration with a nontrivial impedance mismatch is not usually ideal for noise reduction. However, this configuration offers the convenience of placing the fine nerve filament onto only one electrode, which is particularly appealing when recording from mouse colorectal nerve filaments of limited length (10 - 15 mm). Based upon our experience, the single-electrode configuration is acceptable when the peak-to-peak background noise is below 20 µV in the record. Otherwise, further noise reduction would demand a two-electrode recording configuration in which the fine nerve filament has to be in contact with both recording and reference electrodes placed parallel to each other. All the large metal parts inside the Faraday cage need to be grounded in a star-like manner to one common ground, a copper block in our setup. Care has to be taken to avoid formation of ground loops.
To ensure extracellular detection of APs, the first step is successful dissection of the colorectum-nerve tissue. Pinching or pulling the nerve during dissection must be avoided, which can irreversibly damage the nerve and affect AP conduction. The dissected nerve stem also needs to be free of any connected muscle tissue, which leaks potassium when damaged and can block nerve conduction by depolarization. This dissection skill is generally acquired through diligent practice over weeks to months and demands a high level of eye-hand coordination and dexterity in handling and using surgical instruments. In addition, to avoid damage to the colorectal tissue, the electrical search strategy utilizes a concentric electrode that has a blunt, rounded tip and relatively large diameter (external Φ0.55 mm, internal Φ0.125 mm) and is connected to a micromanipulator by a compliant bridge, resulting in a modest mechanical force applied to the mucosal surface (~100 mg). In order to acquire a larger signal in the recording trace, the AP-induced transmembrane current needs to be channeled and “trapped” on the electrode by creating a small impedance bridge between the nerve axon(s) and the electrode surface. Thus, the epineurium and perineurium that insulate the nerve need to be dissected free during the process of splitting the nerve into fine filaments of ~10 µm thick. Because the AP-induced transmembrane current dissipates considerably within a short distance from the axon membrane, a thinner nerve filament usually results in a better signal-to-noise ratio due to the axons’ closer proximity to the electrode surface. In the mineral oil chamber, the mirror that the nerve is placed upon often attracts a thin layer of Krebs solution (the glass surface is hydrophilic). It is thus necessary that the recording electrode and nerve filament are not in contact with the mirror surface during recording. Any residual droplets of Krebs solution, which provide a low-impedance bridge between the electrode and the mirror surface (i.e., shunting) will significantly reduce the signal amplitude in the record.
This colorectum-nerve preparation permits the study of functional changes of afferents after exposing RFs to a variety of chemical mediators and insults in vitro as well as in the context of long-term pathophysiological conditions (e.g., colorectums taken from previously treated mice). An objective measure of functional changes of afferents depends upon the following: 1) a standardized stimulus with high precision and reproducibility and 2) afferent responses that are robust and reproducible. Of the three mechanical stimuli applied to the colorectum, probing and stroking stimuli of the RF are often delivered by hand-held von Frey-like monofilaments. For probing, the monofilament is usually calibrated to deliver a reproducible perpendicular force when bending. However, von Frey-like monofilaments (0.4 and 1 g) have small and different cross-sectional diameters (0.2 and 0.3 mm, respectively), resulting in a high nominal stress when applied perpendicular to the colorectal surface (124.8 kPa for 0.4 g and 138.7 kPa for 1 g), an intense, punctate mechanical stimulus beyond the normal physiological range. In addition, the sharp edge of the filament likely causes uneven distribution of stresses with focal peak stress considerably higher than the nominal stress (stress concentration). Given that the typical RF size (1 mm2) is significantly larger than the cross-section of a monofilament, and the inability to reproducibly stimulate the identical site with a hand-held monofilament, it is common to observe responses to repeated stimuli that differ significantly in AP frequency and duration. As an example, the responses to probing shown in Figure 2C by the same hand-held monofilament (1 g) varied considerably which likely are contributed to by an inability to reproducibly probe the identical site and variable duration and interval between consecutive stimuli. Mucosal stroking delivered by a hand-held filament is similarly challenging and also tends to evoke variable responses from the same afferent. Stimulus reproducibility can be improved by using a computer-controlled force actuator to deliver precise probing (and stretch) forces. For probing, we use a monofilament with a larger diameter (e.g., #6.45, 1 mm) that more fully covers a typical afferent RF24,28. Computer-controlled circumferential stretch, as opposed to other tissue stretching approaches directed to the RF, allows homogeneous deformation throughout the length of the colorectum, making possible the correlation with colorectal distension in its original cylindrical configuration based upon comparable circumferential mechanical stress (i.e., 0 - 170 mN stretch is equivalent to 0 - 45 mm Hg intraluminal pressure23). Since the stretch force is applied uniformly at the anti-mesenteric edge, not directly to the RF, the evoked local mechanical stress at the afferent RF is reproducible between consecutive applications of stretch. In addition, the L-type Ca2+ channel blocker nifedipine added to the bath to inhibit spontaneous smooth muscle contraction, contributes to maintenance of colorectal compliance between ramped stretch tests23. Finally, the afferent responses to the ramped stretch protocol have proven to be reproducible with small variability in both the stimulus-response function and response threshold (e.g., Figure 4). Thus, afferent responses to ramped stretch have been widely used as an objective assessment of changes of afferent function in studying neuronal mechanisms of visceral pain and hypersensitivity (e.g., 19-22,24,26,28-31).
The colorectum-nerve preparation is a powerful tool for the study of colorectal visceral afferents. However, it also has some limitations. First, the axons of the cell bodies of the sensory neurons in the dorsal root ganglion are transected in the preparation, precluding the study of molecular identities of those cell bodies (e.g., single cell RT-PCR or transcriptome analysis of the different classes of colorectal afferents). Second, the low signal-to-noise ratio of the single-fiber recording demands optimal surgical dissection/nerve splitting skills and low-noise recording, significantly limiting wider application of this protocol in other laboratories. Third, this in vitro preparation may not be applicable to investigations of systemic factors that modulate visceral sensation, such as the autonomic nervous system, circulating hormones and cytokines, intestinal microbiota, and descending modulation from the central nervous system.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Supported by NIH award R01 DK093525 (GFG). We greatly appreciate the scientific review and grammatical editing of the manuscript by Dr. Amber Shaffer (University of Pittsburgh) and thank Michael Burcham for assistance in preparation of figures.
References
- Naliboff BD, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Verne GN, Sen A, Price DD. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. Journal of Pain. 2005;6:493–496. doi: 10.1016/j.jpain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Chey WD, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–1712. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- Rao S, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714–1724. doi: 10.1038/ajg.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby RW, et al. Pharmacologic properties, metabolism, and disposition of linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J Pharmacol Exp Ther. 2013;344:196–206. doi: 10.1124/jpet.112.199430. [DOI] [PubMed] [Google Scholar]
- Mei N. Intestinal chemosensitivity. Physiol Rev. 1985;65:211–237. doi: 10.1152/physrev.1985.65.2.211. [DOI] [PubMed] [Google Scholar]
- Mei N, Lucchini S. Current data and ideas on digestive sensitivity. J Auton Nerv Syst. 1992;41:15–18. doi: 10.1016/0165-1838(92)90122-w. [DOI] [PubMed] [Google Scholar]
- Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol. 1998;80:2632–2644. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF. Spinal neurones with long projections activated from the abdominal viscera of the cat. J Physiol. 1982;322:1–20. doi: 10.1113/jphysiol.1982.sp014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Sann H. Mechanically evoked responses of afferent fibres innervating the guinea-pig's ureter: an in vitro study. J Physiol. 1989;412:245–266. doi: 10.1113/jphysiol.1989.sp017613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. A novel type of unmyelinated chemosensitive nociceptor in the acutely inflamed urinary bladder. Agents Actions. 1988;25:219–221. doi: 10.1007/BF01965016. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibres in the sural nerve of the rat. J Physiol. 1991;435:229–242. doi: 10.1113/jphysiol.1991.sp018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol. 2011;300:G170–G180. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1085–G1098. doi: 10.1152/ajpgi.00542.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, et al. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2012;302:G676–G683. doi: 10.1152/ajpgi.00490.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, et al. Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G817–G824. doi: 10.1152/ajpgi.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol. 2010;298:G402–G409. doi: 10.1152/ajpgi.00487.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, et al. Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci. 2013;33:9831–9839. doi: 10.1523/JNEUROSCI.5114-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe IT. Principal component analysis. 2nd. New York, NY: Springer; 2002. [Google Scholar]
- La JH, Feng B, Schwartz ES, Brumovsky PR, Gebhart GF. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2012;303:G802–G809. doi: 10.1152/ajpgi.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, et al. plasticity and modulation of visceral afferents. Brain Research Reviews. 2009;60:171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin ME, Feng B, Schwartz ES, Gebhart GF. Combined genetic and pharmacological inhibition of TRPV1 and P2X3 attenuates colorectal hypersensitivity and afferent sensitization. Am J Physiol Gastrointest Liver Physiol. 2013;305:G638–G648. doi: 10.1152/ajpgi.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1250–G1258. doi: 10.1152/ajpgi.00329.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF. Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor family receptor α-3 in colorectal afferents. Am J Physiol Gastrointest Liver Physiol. 2011;300:G418–G424. doi: 10.1152/ajpgi.00456.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


