Abstract
Isolated tissue bath assays are a classical pharmacological tool for evaluating concentration-response relationships in a myriad of contractile tissues. While this technique has been implemented for over 100 years, the versatility, simplicity and reproducibility of this assay helps it to remain an indispensable tool for pharmacologists and physiologists alike. Tissue bath systems are available in a wide array of shapes and sizes, allowing a scientist to evaluate samples as small as murine mesenteric arteries and as large as porcine ileum – if not larger. Central to the isolated tissue bath assay is the ability to measure concentration-dependent changes to isometric contraction, and how the efficacy and potency of contractile agonists can be manipulated by increasing concentrations of antagonists or inhibitors. Even though the general principles remain relatively similar, recent technological advances allow even more versatility to the tissue bath assay by incorporating computer-based data recording and analysis software. This video will demonstrate the function of the isolated tissue bath to measure the isometric contraction of an isolated smooth muscle (in this case rat thoracic aorta rings), and share the types of knowledge that can be created with this technique. Included are detailed descriptions of aortic tissue dissection and preparation, placement of aortic rings in the tissue bath and proper tissue equilibration prior to experimentation, tests of tissue viability, experimental design and implementation, and data quantitation. Aorta will be connected to isometric force transducers, the data from which will be captured using a commercially available analog-to-digital converter and bridge amplifier specifically designed for use in these experiments. The accompanying software to this system will be used to visualize the experiment and analyze captured data.
Keywords: Biochemistry, Issue 95, smooth muscle function, receptor pharmacology, signal transduction, tissue bath, rat, aorta, aortic rings, isometric contraction, concentration response curve
Introduction
The discipline of pharmacology has used the isolated tissue bath system for over 150 years. The versatility of this system has allowed scientists across the world to characterize receptors and receptor signal transduction, with this knowledge forming the basis of therapies that have treated millions of individuals with diseases or disorders such as hypertension, heart failure, diabetes, gastrointestinal disease, bladder dysfunction, asthma, and swallowing disorders, to name just a few. To this day, the isolated tissue bath remains an important facet of drug development and basic research, as it allows the tissue to function as a tissue. In this JoVE lesson, a formal protocol is shared to demonstrate a visual and virtual experiment utilizing the data from an isolated tissue bath experiment that measures isometric contraction, which permits receptor characterization.
The primary advantage of this technique is that the tissue is living and functions as a whole tissue, with a physiological outcome (contraction or relaxation) that is relevant to the body. It is a synthesis of steps (drug-receptor interaction, signal transduction, second messenger generation, change in smooth muscle excitability, and change in tissue function). While other techniques allow study of each of these steps (e.g. radioligand binding for drug affinity, measurement of second messengers), the isolated tissue bath technique allows for integration of all these steps1. Another advantage is that retaining tissue function permits calculation of important pharmacological variables that are more meaningful in a tissue vs a cellular setting; it comes closer to how the drugs examined would work in the body as a whole.
Protocol
NOTE: All procedures described in this paper are performed according to guidelines established by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University.
1. System Preparation and Setup
- Make 5 L of a physiological salt solution (PSS), which is the amount needed for a tissue bath contraction experiment that uses up to 50 ml tissue baths; see Table 2 for PSS recipe. Calculate total required volume by multiplying number of tissue baths times the bath volume and then multiplying by the number of required tissue washes.
- Use Table 2 as a guide to making PSS. Dissolve the salts in approximately 4 L of water. NOTE: HPLC – Type I water is recommended
- Add 8 ml of 1 M CaCl2 solution (147 g/L if using the dihydrate salt) to the solution, so the end solution is 1.6 mM Calcium.
- Quantum sufficit PSS solution to 5 L.
Preheat the tissue bath system to 37 °C by turning on the recirculating heated water bath. Critical Step: Each component of the system is water-jacketed, ensure that they are connected in serial to one another. The direction of flow is critical – ensure that water flows into each component at the lowest barbed connection and out at the highest barbed connection.
Turn on data acquisition system. Power on the force transducers at least 15 min prior the experiment to equilibrate temperature. NOTE: Most force transducers employ strain gauges that are sensitive to variations in temperature and exhibit thermal drift initially after power is applied.
Launch data acquisition software and ensure connection with data acquisition system. Please follow manufacture instructions for enabling data recording.
Make sure the force transducers are calibrated before tissue is placed in the tissue bath and before data recording has started; follow manufacturer instructions for calibration.
Connect the tissue bath system to a 95% O2 / 5% CO2 medical grade gas cylinder and check for gas leaks and then pressurize the system.
Fill the tissue bath reservoirs with PSS and allow the solution time to reach optimal temperature. Prime the system and remove any air bubbles within the system and tubing.
Check tissue bath aerators to ensure consistent solution aeration, which oxygenates the PSS buffer and provides Brownian motion to distribute drugs that will be introduced in the tissue bath during the experiment. Make sure aeration/bubbles does not cause tissue movement, which will disrupt data recordings; decrease gas flow as necessary. NOTE: The tissue bath and data acquisition system are now ready to start the experiment.
2. Tissue Preparation
Ideally, dissect tissues from the animal immediately prior to use and place directly into PSS. NOTE: Some tissues can be saved in PSS overnight at 4 °C, but appropriate controls must be done to validate tissue function after prolonged storage; see Section 6.
Use the thoracic aorta as the tissue in this exercise.
Anesthetize the rat in accordance with institutional guidelines and place the rat dorsal side down. Confirm anesthetization via a toe-pinch and the loss of reflex response to this painful stimulus. NOTE: This protocol uses 70 mg/kg of pentobarbital delivered via an intraperitoneal injection. Institutional guidelines for rodent anesthesia may differ.
Create a pneumothorax by creating an incision with scissors along the diaphragm at the bottom of the rib cage. Then, continue the incision from the bottom of the ribcage to the sternum and bisect.
Dissect away or place aside those organs that are on top of the spinal column and locate the aorta, which lies directly along the spinal column. NOTE: This step provides a clear working space to dissect out the aorta.
Sever all connections to the aorta; from the esophagus, lung, etc. and cut the aorta perpendicular to the spine at the level of the diaphragm.
Gently hold the aorta with forceps and use scissors to dissect the aorta from the spine. Start the dissection in the lower diaphragm area adjacent to the spine and work towards the heart. Make sure to take extra precaution to not pull or tug on the aortic tissue, which may damage the tissue.
Immediately place the aorta in the prepared dissection dish containing PSS. NOTE: Dissection of the aorta into rings is best done in a dissecting dish that has a black silastic foundation. This allows for the use of wires that can be used to cannulate the aorta and be fastened to the dish, providing stability while in the dissection dish. The black background provides contrast that aids dissection. Care should be taken in use of cannulating wires as the endothelial cell layer can be removed with excess rubbing of the wire against the lumen of the vessel.
Take a wire and make a small 90° angle at one end and push the angled end into the silastic foundation to anchor the guide wire. Place the whole aorta in the dissection dish.
Hold the end of the guide wire with forceps, and then gently thread the wire into the lumen of the aorta.
Use forceps and slide the aorta onto the wire. When the free end of the guide wire is visible, curve the wire gently and place the free end into the silastic foundation of the dish to stabilize the tissue.
Using small vannas scissors and forceps, remove the perivascular adipose tissue and all other extraneous tissue, blood clots, etc. until the thoracic aorta appears white and somewhat fibrous.
Remove the cleaned aorta from wire and use scissors to cut the aorta in rings that are approximately 3-5 mm in width.
Place an aortic ring on one of a pair of tissue hooks by holding the hook and using forceps to gently slide the ring onto hook. Repeat this process with the second hook. Take care to ensure the hooks do not tangle; see Figure 3.
Check that each hook has a different silk suture attached. Check that one hook has a small knotted loop of silk that allows for attachment to a glass or stainless steel rod, while the other is a 10-4 cm long piece suture that will be hand-tied to the force transducer; see Figure 3. NOTE: Once the aorta is secured to the hooks, the tissue is ready to be mounted in the tissue bath; see Figure 3.
Repeat steps 2.14-2.15, to mount a second aortic ring in the second tissue bath chamber.
3. Tissue Placement in Bath
Note that in this system, the tissue baths themselves are stationary. At this time, fill the tissue baths with warmed, aerated PSS and allow the solution to come to temperature.
- With the silk suture, tie one of the hooks on the tissue preparation to the peg on the stainless steel rod. Place this end into the tissue bath chamber. Connect the rod to a ring stand and place the other end in the staining dish filled with PSS to keep tissue immersed in buffer; see Figure 3.
- Place the rod and tissue in the tissue bath chamber and make sure the tissue is fully immersed in PSS and the rod is secure; see Figure 3.
- Tie the other suture to the force transducer and make sure to leave slack in the suture between the tissue and the force transducer. NOTE: This slack will be removed by adjusting the micrometer; see Figure 3.
Repeat these steps for the second aortic ring and tissue bath chamber.
4. Setting Passive Tension
NOTE: Each tissue has a length (Lo) at which smooth muscle cells respond optimally. Preliminary experiments to determine the optimal stretching tension which achieves this length must be performed for each individual tissue type to be examined. The rat thoracic aorta has an optimal passive stretching tension of 4 g.
Use the micrometer/rack and pinion to increase tension to 2 g and wait for the tissue to reach plateau. Once plateau is reached, increase tension another 2 g and wait for the tissue to plateau. NOTE: In the aortic rings, after the initial tension of 2 g has set and plateau reached, the tissue should then relax to ~1.6 g. A second application of 2 g would bring the tissue to ~3.6 g, from which the tissue will again relax and tension will decrease. Thus, after having added 4 g of total tension, the software should indicate roughly 3.2 g of tension.
Repeat these steps for the second aortic ring and tissue bath chamber.
5. Equilibration and Drug Making
Equilibrate the tissue for 60 min after applying passive tension to the tissue.
During the equilibrium phase, wash the tissue every 15-20 min. Drain the tissues bath and replace the PSS with PSS from a warmed reservoir. NOTE: During this time, the tissue will relax, which is indicated by the loss of passive tension.
During this time, prepare drugs for the experiment, which need to be at least 1,000 times more concentrated than the actual concentration in the bath, so only a small volume of the drug stock is needed to achieve the desired concentration.
6. Initial Challenge
At the end of the equilibration period, wash the tissue one final time, save data, and re-zero all the inputs.
- Pick an agonist (compound that will cause active contraction) to which the tissue responds. NOTE: In the rat thoracic aorta, the arterial smooth muscle will actively contract to an alpha adrenergic receptor agonist due to the innervation of the tissue by the sympathetic nervous system. An adrenergic agonist would not be useful in intestinal tissues given that adrenergic agonists cause relaxation. In this case a receptor agonist such as acetylcholine (cholinergic receptor) could be used. An alternative to both phenylephrine and acetylcholine is to use a non-receptor agonist such as high potassium (K) as the first challenge. In smooth muscle, high K operates to indirectly open calcium channels and thus is viewed as a general index of smooth muscle integrity.
- Perform the first challenge or the wake-up challenge by adding 10-5 M phenylephrine to the tissue bath. To achieve this concentration in 50 ml tissue bath, add 50 μl of a 10-2 M phenylephrine solution. NOTE: 50 μl into 50 ml of PSS is a 1/1,000 dilution, so the final concentration is 1,000x less than stock, or 10-5 M. Knowledge of volume and consistent maintenance of the volume within the tissue bath chamber is critical to determining the final drug concentration.
Add 10-5 M phenylephrine to the tissue bath and allow the contraction to peak and then plateau, when the slope of the data becomes zero. Make sure to record each experimental event to aid post-experimental analysis.
After plateau, wash the agonist out thoroughly by emptying and refilling the tissue bath chamber with new PSS. Make sure to record these events as well. NOTE: A rule of thumb is that the concentration of agonist in the bath is reduced 10 fold with each wash. Although, more than 3-4 washes may be necessary to bring the tissue back down to its equilibrium baseline tone (tension).
Let the tissue rest at baseline tone for ~10 min before proceeding.
7. Experiment
NOTE: Prazosin, an alpha adrenergic receptor antagonist, will be introduced to the tissue bath chamber to shift the concentration response curve to phenylephrine (PE); this is a demonstrable antagonism.
To one bath, add the vehicle (H2O) in which prazosin was dissolved. To another, add the appropriate concentration of prazosin. In this instance, add 25 µl of vehicle to one bath and 25 µl of 10-5 M concentration of prazosin to the other to achieve a 5 x 10-9 M or a 5 nM concentration in the bath. NOTE: While the addition of vehicle seems unnecessary in this example, it is imperative to do so when using other vehicles that have a potential to be vasoactive (e.g., DMSO, ethanol, acetate). Add the corresponding vehicle even if little evidence exists for it to be vasoactive.
- Leave vehicle/prazosin in the baths and do not wash the tissue for 1 hr.
- While tissues are equilibrating, prepare multiple concentrations of agonist (PE). Include a wide range of concentrations, from concentrations eliciting no response (e.g., 10-9 M PE) to concentrations that surpass the maximal response (e.g., 10-4 M PE). Since concentration-response curves are logarithmic in nature, make six separate stock solutions of PE (10-6-10-1 M) through serial dilutions from the 10-1 M stock solution. Make sure the volume needed for each concentration is slightly greater than triple the total volume needed for all baths (i.e. >300 µl).
- Proceed to adding agonist, as described in Table 1. NOTE: This experiment will generate a cumulative concentration response curve to PE; each iteration takes into account the amount of substance already added to the bath. Additions occur until they no longer increase contraction, or the whole curve has plateaued.
- Add each drug concentration individually until the tissue threshold is reached.
- If no change occurs, add the next concentration in the series; see Table 2. NOTE: The timing of these additions depends on the agonist used. For example, norepinephrine and phenylephrine contraction develops rapidly, and should plateau within minutes. By contrast, endothelin-1 contraction develops slowly and may not plateau for close to 45 min. Other experimentation can be done on the tissue after construction of a curve such as this, as long as (1) the substance washes out; and (2) it can be demonstrated that the tissue returns to normal contractile behavior and is not affected by the previous stimulations.
8. Data Analysis
Make sure to save data immediately prior to and after an intervention (e.g., wake up or full curve).
Find and set the tissue baseline for each tissue bath chamber/input channel, which will aid in data analysis.
Once the baseline is set, find the maximal response for each concentration, and record the change from baseline. Sequentially move through each of the concentrations and record the baseline shift.
Graph the resulting data. Do this in any graphing program. NOTE: Graph Pad Prism is designed for pharmacologists, and this is ideal for the type of experiment presented here.
Representative Results
In terms of agonism, the relative efficacy (EMAX) and potency (EC50) of an agonist in a given tissue can be calculated and compared to responses of other agonists in the same tissue1. In our experiment, the rat thoracic aorta was incubated with either vehicle or the α1 adrenergic receptor antagonist prazosin (5 nM) for one hour prior to adding the α1 adrenergic agonist phenylephrine to the tissue bath and generating contraction (Figure 1).
Figure 2 presents modified results of Figure 1. The green responses have marked the maximum contraction achieved by PE marked as max, the ½ maximum contraction marked as such and then associated with the PE concentration that caused that response. Identifying these values is identifying the effective concentration of an agonist that achieves a half max (50%) response or EC50 value. This was donegraphically in this example.Computer software that uses logistic functions for sigmoidal curves can be used for curve fitting and calculation of EC50 values.
In generating and interpreting these results, it is important to use both low and high concentrations of agonist to create the concentration-response curve. Without enough points to show a stable baseline and maximum plateau, EC50 and EMAX can only be estimated. This was done graphically in this example. Computer software that uses logistic functions for sigmoidal curves can be used for curve fitting and calculation of EC50 values.
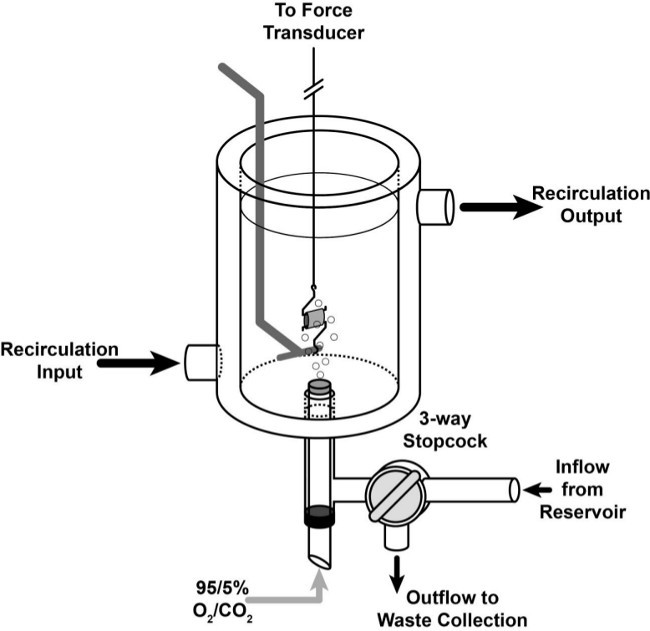 Figure 1: Tissue Bath Schematic. Cartoon representing a ring of tissue placed in the double-wall, water jacketed tissue bath. Buffer inflow and outflow are regulated by a 3-way glass stopcock integrated into the base of the chamber. O2/CO2 is bubbled in through an aerator that is sealed with a gasket to prevent leak of PSS or gas. Recirculation input is below the output to maintain proper recirculation and prevent formation of air pockets that would cause temperature disregulation.
Figure 1: Tissue Bath Schematic. Cartoon representing a ring of tissue placed in the double-wall, water jacketed tissue bath. Buffer inflow and outflow are regulated by a 3-way glass stopcock integrated into the base of the chamber. O2/CO2 is bubbled in through an aerator that is sealed with a gasket to prevent leak of PSS or gas. Recirculation input is below the output to maintain proper recirculation and prevent formation of air pockets that would cause temperature disregulation.
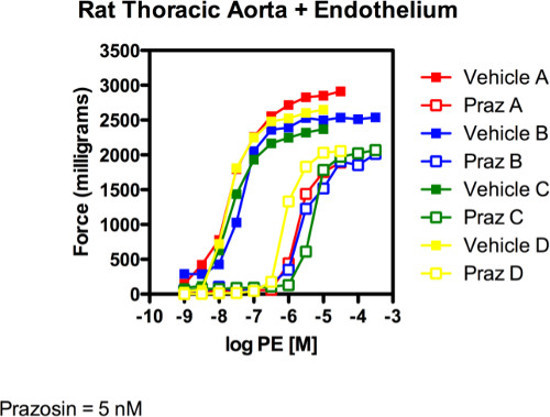 Figure 2: Rat Thoracic Aorta + Endothelium. The figure above depicts four separate experiments (different animals and done by different investigators on different set ups as represented by different colors) to test the ability of prazosin to shift a PE-induced contraction. Prazosin (5 nM) clearly shifted PE-induced contraction curve rightward in a parallel fashion.
Figure 2: Rat Thoracic Aorta + Endothelium. The figure above depicts four separate experiments (different animals and done by different investigators on different set ups as represented by different colors) to test the ability of prazosin to shift a PE-induced contraction. Prazosin (5 nM) clearly shifted PE-induced contraction curve rightward in a parallel fashion.
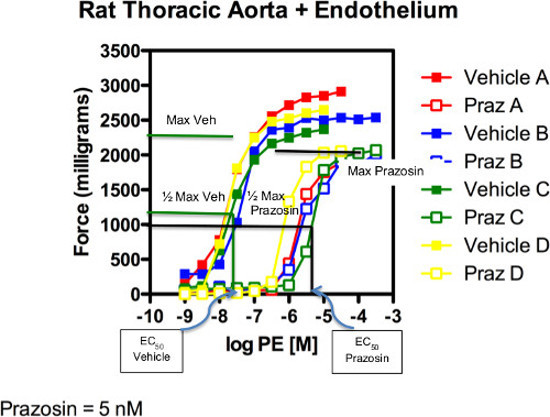 Figure 3: Rat Thoracic Aorta + Endothelium. Graphical estimation of EC50 values for PE in the absence (vehicle) and presence (prazosin) of antagonist. Green line (group C) only is marked.
Figure 3: Rat Thoracic Aorta + Endothelium. Graphical estimation of EC50 values for PE in the absence (vehicle) and presence (prazosin) of antagonist. Green line (group C) only is marked.
| Salt | MW (g/mole) | 5 Liters | FINAL mM |
| (grams) | |||
| NaCl | 58.45 | 37.99 | 130 |
| KCl | 74.56 | 1.75 | 4.7 |
| KH2PO4 | 136.1 | 0.8 | 1.18 |
| MgSO4•7H20 | 246.5 | 1.45 | 1.17 |
| NaHCO3 | 84.21 | 6.25 | 14.9 |
| Dextrose | 180.16 | 5 | 5.5 |
| EDTA | 380 | 0.05 | 0.03 |
Table 1: Normal Physiological Salt Solution (PSS) Recipe. Contents of the bicarbonate-buffered PSS used in this experiment. Included are the molecular weights of all salts and the amount added to 5 L of dH2O to achieve the desired concentration.
| Concentration in bath | Addition of agonist made to bath |
| 1 x 10-9 M | 50 ml 1 x 10-6 M |
| 3 x 10-9 M | 100 ml 1 x 10-6 M |
| 1 x 10-8 M | 35 ml 1 x 10-5 M |
| 3 x 10-8 M | 100 ml 1 x 10-5 |
| 1 x 10-7 M | 35 ml 1 x 10-4 M |
| 3 x 10-7 M | 100 ml 1 x 10-4 M |
| 1 x 10-6 M | 35 ml 1 x 10-3 M |
| 3 x 10-6 M | 100 ml 1 x 10-3 M |
| 1 x 10-5 M | 35 ml 1 x 10-2 M |
| 3 x 10-5 M | 100 ml 1 x 10-2 M |
| 1 x 10-4 M | 35 ml 1 x 10-1 M |
Table 2: Tissue Bath Additive Agonist Concentrations. The amount of agonist to be added to each bath to properly construct cumulative concentration-response curves. The desired bath concentration is achieved through addition of sequential amounts of increasing concentrations of agonist. Each addition takes into account the concentration of agonist already present in the bath. This is done to minimize the increase in volume in the tissue bath that would result from using increasing volumes of a single concentration of agonist.
Discussion
Measurement of isometric force as a research tool is over 150 years old, but it continues to be the prototypical technique for receptor characterization in contractile tissues2. The power of this technique is in its simplicity and versatility: by recording responses elicited by increasing concentrations of an agonist in the presence or absence of an antagonist, a myriad of information can be derived about the pharmacological characteristics of each drug and the receptor to which it binds3-5. Experiments of this type also garner information about the competitive versus non-competitive nature of the antagonists used, as well as receptor heterogeneity and non-specific drug effects6-8. Thus, with simple permutations to this isolated tissue bath experiment, a relatively complete pharmacological profile of the receptors that mediate agonist-induced muscle contraction can be generated.
In terms of agonism, the relative efficacy (EMAX) and potency (EC50) of an agonist in a given tissue can be calculated and compared to responses of other agonists in the same tissue1. In our experiment, the rat thoracic aorta was incubated with either vehicle or the α1 adrenergic receptor antagonist prazosin (5 nM) for one hr prior to adding the α1 adrenergic agonist phenylephrine to the tissue bath and generating contraction. Figure 2 presents modified results of Figure 1. The green responses have been marked with the maximum contraction achieved by PE marked as max, the ½ maximum contraction marked as such and then associated with the PE concentration that caused that response. Identifying these values is identifying the effective concentration of an agonist that achieves a half max (50%) response or EC50 value.
Unfortunately, using agonist-dependent parameters alone to determine receptor binding characteristics can be complicated9. Ideally, additional experiments using receptor antagonists allow the calculation of two important parameters that are key to defining the interaction between a drug and a receptor: the -log10 of the antagonist dissociation constant (pKB) and the -log10 of the molar antagonist concentration necessary to elicit two-fold rightward shift in the concentration-response curve (pA2)10. Both KB and pA2 gain their usefulness from the fact that they are agonist-independent values, and remain constant even between different tissues11. From the data in Figure 2, pKB can be calculated using the following equation and based on EC50 values calculated elsewhere:
EC50 in the absence of prazosin = 2 x 10-8 M EC50 in the presence of prazosin = 7 x 10-6 M
Solve for KB in the following equation: log (dr-1) = log [B] - log KB Where: [B] = antagonist concentration, or 5 x 10-9 M. log (5 x 10-9 M) = -8.3 dr = dose ratio of EC50 value in presence of antagonist/EC50 in absence. If there is a rightward shift, this value will be greater than one. Thus: dr = 7 x 10-6 M/2 x 10-8 M or 700 x 10-8 M/2 x 10-8 M = 700/2 = 350 Substituting for [B] and dr: log (350-1) = -8 - log KB 2.54 = -8 - log KB pKB = 10.54
This value for prazosin is consistent with the values obtained when prazosin interacts with an α1adrenergic receptor12,13, suggesting that the adrenergic receptor mediating contraction to phenylephrine is the α1 adrenergic receptor.
Several steps are critical to the success of these experiments. Tissues must remain in PSS after dissection to prevent loss of tissue viability. Any isometric muscle preparation has an optimal length-tension relationship that generates maximal force against passive tension14,15. For the experiment described in this protocol, optimal passive tension was previously determined by briefly adding increments of 0.5 g of passive tension to the tissue. The tissue should begin without tone and then after 30 min of equilibration, the tissue was challenged with a maximal concentration of KCl (80 mM), which generated active tone. Then, the tissue was washed and allowed to return to baseline tone. After 15 min at baseline, another 0.5 g of passive tension was placed and this process repeated until a plateau of active tension was achieved with additional passive tension. In preliminary experiments, 4 g total of passive tension was determined to achieve maximal active tension generation in aortic rings, and thus this total amount of tension is placed on the rings prior to equilibration. Procedurally, it’s best to zero prior passive tension application, but can be done at any time prior the experiment. Over-stretching tissues, at any point in the experiment or dissection, will negatively impact tissue viability and experimental outcomes. This is most imperative when placing tissues in the bath, as this step has the highest likelihood of excessive stretch. Adequate washing, in number and duration is required for reproducible effects. A second challenge too soon after an initial challenge, or before tissues have returned to baseline tension, will result in aberrant responses. If studying reversible antagonists/inhibitors, tissues should not be washed prior to agonist addition, as the inhibitor concentration will be decreased.
There are notable advantages and disadvantages to the isolated tissue bath assays.
Disadvantages: Tissues may experience different degrees of damage during surgical removal or placement of rings on hooks. Since the endothelial cell is the inner lining of the aortic ring, care must be taken on the placement of the ring on hooks so as not to damage this cell layer. Tissues may also have distinctly different lengths of time in which they are viable in the tissue bath, and this has to be determined; not all tissues are the same. Along these lines, tissues may change in their responsiveness throughout the day such that time controls become a necessary control for every experiment. A good example of this is the guinea pig trachea that improves in contractility maximum by 100% during a typical 8 hr experiment. Drugs that are poorly soluble in water may precipitate out in the PSS. Finally, cumulative and non-cumulative additions of a drug that is an agonist could result in different outcomes if receptor desensitization to the agonist occurs; angiotensin II is one such drug which shows rapid tachyphylaxis.
Advantages: One of the primary advantages of tissue bath experiments is that it is real time; one can see the experiment as it unfolds and can rapidly make conclusions and plan next steps, as well as troubleshoot during an experiment. An experiment takes a day to do. Multiple tissues can typically be prepared from one animal such that an animal can serve as its own control, and that adds strength to an experiment. One also can isolate the tissue from other factors so as to test a relatively pure response of the tissue to the drug. In vitro experiments such as the tissue bath system also allow use of a small amount of drug compared to an in vivo experiment.
The basic experimental design described herein can be extensively modified to allow for the recording of additional parameters or the introduction of other external stimuli. For example, addition of electrodes allow for electric field stimulation of innervating nerves16,17. With the addition of thermal or pH probes, the effects of temperature and pH on contractile responses can also be measured18,19. Similarly, oxygen can be substituted partially or in full with N2 to evaluate hypoxia induced effects. Furthermore, the same basic principles of isometric contractility measurement used in this video can be used to develop systems that allow concomitant measurement of isometric tension development and changes in intracellular calcium20. Signal transduction is also readily studied, since systems exist that can rapidly freeze a tissue sample during a response, such that the activity of a signal transduction pathway system can be verified biochemically.
The variation of equipment that can be used to do this is enormous. This whole system, either hand constructed or automated, can be purchased from multiple different companies. The tissue baths and tissue holders used in this protocol were hand-blown (tissue baths) and hand constructed (holders) by an in-house Michigan State University machine shops.
Disclosures
Authors have nothing to disclose.
Acknowledgments
The Watts Laboratory through the years, and Dr. Marlene Cohen and Kathy Schenck for teaching us this assay over two decades ago. NIGMS R25GM074119 supported development of this teaching module for a Short Course in Integrative and Systems Pharmacology held on the campus of MSU from 2005 to 2013.
References
- Kenakin TP. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- Scheindlin S. A brief history of modern pharmacology. Modern Drug Discovery. 2001;4:87–88. [Google Scholar]
- Christopoulos A, El-Fakahany EE. Qualitative and quantitative assessment of relative agonist efficacy. Biochem. Pharmacol. 1999;58:735–748. doi: 10.1016/s0006-2952(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin TG. protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Braverman AS, Kohn IJ, Luthin GR, Ruggieri MR. Prejunctional M1 facilitory and M2 inhibitory muscarinic receptors mediate rat bladder contractility. Am. J. Physiol. 1998;274:R517–523. doi: 10.1152/ajpregu.1998.274.2.r517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, et al. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G1206–1213. doi: 10.1152/ajpgi.00286.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Adner M, Edvinsson L. Characterization of endothelin receptors in the cerebral vasculature and their lack of effect on spreading depression. J. Cereb. Blood Flow Metab. 1996;16:698–704. doi: 10.1097/00004647-199607000-00021. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Agonist-activated ion channels. Br. J. Pharmacol. 2006;147(1):S17–26. doi: 10.1038/sj.bjp.0706502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Chen PE. Taking the time to study competitive antagonism. Br. J. Pharmacol. 2007;150:541–551. doi: 10.1038/sj.bjp.0706997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild HO. pA, a new scale for the measurement of drug antagonism. 1947. Br. J. Pharmacol. 1997;120(4):29–46. doi: 10.1111/j.1476-5381.1997.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshita M, Kigoshi S, Muramatsu I. Pharmacological characterization of two distinct alpha 1-adrenoceptor subtypes in rabbit thoracic aorta. Br. J. Pharmacol. 1993;108:1071–1076. doi: 10.1111/j.1476-5381.1993.tb13507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, et al. Binding and functional characterization of alpha1-adrenoceptor subtypes in the rat prostate. Eur. J. Pharmacol. 1999;366:119–126. doi: 10.1016/s0014-2999(98)00895-4. [DOI] [PubMed] [Google Scholar]
- Cooke PH, Fay FS. Correlation between fiber length, ultrastructure, and the length-tension relationship of mammalian smooth muscle. J. Cell Biol. 1972;52:105–116. doi: 10.1083/jcb.52.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Gore RW. Length-tension relationship of vascular smooth muscle in single arterioles. Am. J. Physiol. 1989;256:H630–H640. doi: 10.1152/ajpheart.1989.256.3.H630. [DOI] [PubMed] [Google Scholar]
- Davis RP, et al. One-month serotonin infusion results in a prolonged fall in blood pressure in the deoxycorticosterone acetate (DOCA) salt hypertensive rat. ACS Chem. Neurosci. 2013;4:141–148. doi: 10.1021/cn300114a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, et al. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J. Physiol. 2009;587:5275–5288. doi: 10.1113/jphysiol.2009.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga TV, Wray S. On the mechanisms whereby temperature affects excitation-contraction coupling in smooth muscle. J. Gen. Physiol. 2002;119:93–104. doi: 10.1085/jgp.119.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvelin JM, O'Connor C, McLoughlin P. Effect of changes in pH on wall tension in isolated rat pulmonary artery: role of the RhoA/Rho-kinase pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L673–L684. doi: 10.1152/ajplung.00331.2003. [DOI] [PubMed] [Google Scholar]
- Tykocki NR, Thompson JM, Jackson WF, Watts SW. Ryanodine receptors are uncoupled from contraction in rat vena cava. Cell Calcium. 2013;53:112–119. doi: 10.1016/j.ceca.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


