Abstract
The success of a small animal model to study critical illness is, in part, dependent on the ability of the model to simulate the human condition. Intra-tracheal inoculation of a known amount of bacteria has been successfully used to reproduce the pathogenesis of pneumonia which then develops into sepsis. Monitoring hemodynamic parameters and providing standard clinical treatment including infusion of antibiotics, fluids and drugs to maintain blood pressure is critical to simulate routine supportive care in this model but to do so requires both arterial and venous vascular access. The video details the surgical technique for implanting carotid artery and common jugular vein catheters in an anesthetized rat. Following a 72 hr recovery period, the animals will be re-anesthetized and connected to a tether and swivel setup attached to the rodent housing which connects the implanted catheters to the hemodynamic monitoring system. This setup allows free movement of the rat during the study while continuously monitoring pressures, infusing fluids and drugs (antibiotics, vasopressors) and performing blood sampling.
Keywords: Medicine, Issue 95, catheter, rat, carotid, artery, jugular, vein, vascular access, blood sampling, hemodynamic measures
Introduction
Arterial and venous catheterization of rats has long been used in laboratory research.1, 2 Catheterization can be used for monitoring of hemodynamic parameters including systolic, diastolic and mean arterial pressure (MAP), heart rate (HR) and central venous pressure (CVP).3,4 In addition, these catheters allow the infusion of standard treatments or potential therapies as well as blood sampling to further analyze the underlying mechanism of an illness or treatment. Therefore, having vascular access in a small animal model is critical for studying clinical performance measures and treatment effects.
To study the underlying causes of critical illness, it is important to first develop a model to simulate the human condition. Intra-tracheal inoculation of a known amount of bacteria has been successfully used to reproduce the pathogenesis of pneumonia, a severe pulmonary infection which then develops into sepsis.3, 5 Monitoring hemodynamic measures and providing standard clinical treatment is critical to simulate routine supportive care. Standard clinical treatment includes infusion of antibiotics which aids in clearing the underlying infection and the administration of fluids and vasopressors, two therapies employed in septic patients to maintain blood pressure.5-7 Vascular access, specifically implantation of patent catheters is especially important when we study investigational therapies for sepsis.
For many years, the ability to administer standard clinical treatment to rodents was limited by the availability of the materials with the needed properties for constructing implantable catheters and the ability of the infusion technology to deliver small volumes accurately over time. In addition, the ability to trigger an infusion in response to the continuous monitoring of a specific hemodynamic measure allows for a consistent and reproducible standard treatment model when using larger sample sizes typical of rodent studies.8, 9 These technological limitations in materials and precision hardware have been overcome but to simultaneously provide routine therapies while monitoring hemodynamic parameters, both arterial and venous vascular access must be available.
The video details the surgical technique for implanting carotid artery and common jugular vein catheters in an anesthetized rat. Following a 72 hr recovery period, the animals will be re-anesthetized and connected to a tether and swivel setup attached to the rodent housing used to connect the implanted catheters to the hemodynamic monitoring system. During the study, the system allows for a fluid infusion based on either delivering a given volume at a certain infusion rate or an automated system that delivers an infusion rate depending on the mean arterial pressure detected to maintain a given pressure range. The fluid infusion system uses programmable syringe pumps that interface with the data acquisition system’s digital outputs and are controlled by the software monitoring the MAP. The tether and swivel setup allows free movement of the rat during the study while monitoring pressures, infusing fluids and vasopressors, and performing blood sampling without having to manipulate the animals. Simultaneous monitoring of up to 12 instrumented animals with 2 catheters each (in our setup) with the hardware expandable to monitoring 24 instrumented animals allows for a great deal of flexibility for studying multiple stratified groups in each experiment.
This catheterization procedure can be beneficial to research facilities that use similar rodent models requiring vascular access for monitoring, sampling and treatment for up to 11 days. If the research facility considering this procedure has experience with rodents and a proper support structure for maintaining these animals then developing these skills can provide a significant cost saving, reducing total cost per catheterized rat from more than $180 to less than $50 (including naïve rat, catheter components and technical costs) and increasing scheduling flexibility (naïve rats are readily available vs. company delivery schedule of implanted rats). The success of this procedure is dependent on the skills of the individual performing the catheterizations. The training animals required to become proficient in this procedure has ranged from 20 to 40 rats with a resulting catheter patency rate of >90%.
Protocol
The procedures described below were performed as part of a protocol approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health.
1. Preparation for Surgery
Gas sterilize catheters and instruments.
2. Prepare the Catheters
Flush the catheters with heparinized glycerol (250 IU heparin/1 ml glycerol) to ensure patency and avoid leakage. Plug end of the catheter with a metal stopper (20 ga, 15 mm) to retain heparinized glycerol.
3. Prepare Aseptic Workstation
Use 70% alcohol to spray the workstation and instrument trays. Use a sterile drape to cover the operating table and instrument trays and place instruments on the tray.
4. Prepare Rats for Surgery
Place rat individually in a Lucite chamber and expose to an anesthetic; isoflurane (3-5%) mixed with oxygen until unconscious. Remove rat from the chamber and shave fur from anterior and posterior areas of the neck. Apply lubricant ointment to eyes.
5. Catheter Implantation
Place anesthetized rat (confirmed by toe pinch) onto a heated surgical table in the ventral position; place its nose into the nose cone to maintain anesthesia with spontaneous respirations (isoflurane; 3.5%). Gently scrub the surgical area 3 times alternating use of Betadine and 70% alcohol. Make a 0.5 cm midline skin incision between the scapulae using a surgical scissor.
Reposition rat in the dorsal position; gently restrain the legs to each side of the table using rubber bands; maintain anesthesia. Place two rolled sterile 4x4 gauze under neck to slightly hyperextend for better exposure. Make a 2 cm ventral cervical skin incision right of the midline of the neck at the level of the clavicle using a scalpel.
6. Right Jugular Vein Catheterization
Using a hemostat, bluntly dissect the right jugular vein, separate out the salivary and lymphatic tissues to visualize and isolate a 5 mm section of the vessel. Using 4-0 silk suture, place a loose tie on both cranial and caudal ends of the vessel to maximize the exposure of the vessel. Using a micro surgical scissor make an incision large enough to pass the catheter, in line with the vessel between the two ligatures and tie the cranial ligature around the vessel.
Insert the venous catheter into the vessel towards the heart with the assistance of the micro dissecting hook and forceps and advance the catheter until all of the PU 3F segment is in the vessel. Use the ligatures at the cranial and caudal ends to secure the catheter to the vessel.
7. Left Carotid Artery Catheterization
Using a hemostat, bluntly dissect the omohyoid muscle longitudinally to expose the left carotid artery and isolate a 5mm section of the vessel. Ensure the vagus nerve (white in color) is completely separated from the artery. Take care not to shred or break the nerve.
Using 4-0 silk suture, place a loose tie on the caudal end of the vessel, tie off the cranial end of the vessel and place a bulldog clamp caudally above the suture to stop the blood flow following the incision. Using a micro surgical scissor, make an incision, large enough to pass the catheter, in line with the vessel between the two ligatures. Insert the arterial catheter towards the heart with the assistance of the micro dissecting hook and forceps.
Use a smooth needle holder without lock to hold the portion of the catheter inside the vessel tight before removing the bulldog clamp. Advance the catheter with a pair of forceps while loosening needle holder slowly until the entire PU 2F segment of catheter is in the vessel. Tie the loose caudal ligature around the catheter and vessel to secure, but not so tight as to occlude, the catheter.
8. Using a Straight Hemostat, Tunnel a 5 cm Tube back Subcutaneously behind the Ear and through the Incision between the Scapulae. Exteriorize the Catheters through the Tube and Remove the Tube.
9. Close the Ventral Incision with Three Stainless Steel Wound Clips, and the Dorsal Incision with 4-0 Silk Sutures to Secure the Exteriorized Catheters in Place.
10. Post-surgical Monitor and Care
Following catheterization, terminate the anesthesia and recover the rat in lateral decubitus position in a cage with cellulose bedding. Observe the rats at 2 hr intervals for at least 4 hr or until showing no signs of pain and then once daily. Give Ketoprofen [5 mg/kg, subcutaneous injection (SQ)] immediately post procedure and every 12 hr if the rat shows signs of pain or distress. In our experience, no rats have required additional analgesia for pain following the initial injection. Characteristics observed that would elicit consideration for additional doses of analgesia include but not limited to; abnormal posture; increase/decrease in respiratory rate, unthrifty/ungroomed, infection/inflammation of the incision site, eye discharge, piloerection (“spiked haircoat”), reduction in activity in response to audible or tactile stimuli. NOTE: Rats recovered for 72 hr that gain >10 g from pre-catheterization weight will be enrolled in the study.
11. Connection to Hemodynamic System
Following recovery, enroll healthy rats (weight gain > 10g) in the study. Place rats in Lucite chamber and anesthetize as previously described.
Place sterile drape on surgical table with sterile hemostat, micro forceps, 1 ml syringes with blunt needle attached. NOTE: The tips of the hemostat should be covered with rubber to avoid damage to the catheter. Place anesthetized rat on a surgical table in the ventral position and place nose into the nose cone to maintain anesthesia (3-5%) with spontaneous respirations.
Clamp arterial and venous catheters using a pair of rubber capped hemostats just below the metal plug pins. Use a pair of forceps to remove the metal pin at the end of the catheter and attach a 1 ml syringe filled with heparin saline with a blunt needle attached. In turn, remove the hemostats and withdraw 0.1 ml to ensure the catheters are patent.
Re-clamp the catheters, remove the needle and attach this end to a longer catheter (pre-flushed with heparin saline) that’s connected to a swivel and tethered on top of the shoebox cage. Connect these catheters to the transducers for data acquisition and recording, sampling, or infusion. NOTE: All catheters are protected with coiled spring conduits long enough to allow an animal’s full range of motion in their cage.
Following catheter connection, terminate anesthesia and recover the animals in the sternal position in a cage with cellulose bedding. To maintain arterial catheter patency, manually flush the lines with 0.05 ml heparin saline (50 IU/ml) every 1 hr for 24 hr which coincide with the regular assessments of the animals.
12. Blood Sampling, Pressure Monitoring and Drug Administration
Attach empty syringe to stopcock connected to arterial catheter and withdraw 0.6 ml inline flush. Attach sample syringe, take sample, and return the 0.6 ml inline flush followed by 0.7 ml heparinized saline flush.
Use data acquisition hardware and software to measure and record MAP and heart rate continuously.
Connect the automated drug delivery system (ADDS) to the venous catheter. NOTE: ADDS adjusts the rate of vasopressor infusion (high, low, or 0 dose) based on the MAP detected to maintained pressures within the normal physiologic range.
Representative Results
Changes in vascular pressure are transmitted through the fluid filled catheters (Figure 1) and converted into electrical signals represented by the hemodynamic waveforms (Figures 2, 3). Without vascular access, these measures could not be made. Real-time streaming of the waveforms allows for detection and analysis of changes on a beat to beat basis (Figure 2). Compressing the time scale of the waveforms allows for quantifying changes that occur over a longer duration (minutes to days) (Figure 3, upper 4 waveforms) which can be correlated with changes in vasopressor infusion rates (Figure 3, lower 4 waveforms).
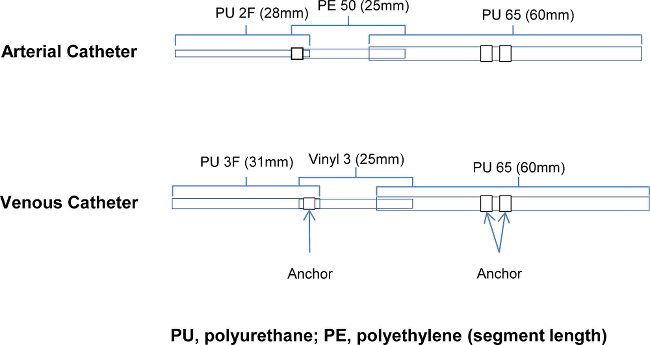 Figure 1. Catheter Design: Details of the components of the arterial and venous catheters. PU, polyurethane; PE, polyethylene (segment length) Please click here to view a larger version of this figure.
Figure 1. Catheter Design: Details of the components of the arterial and venous catheters. PU, polyurethane; PE, polyethylene (segment length) Please click here to view a larger version of this figure.
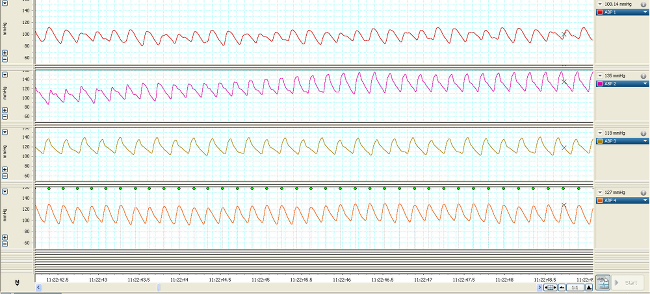 Figure 2. Real-time MAP waveforms of 4 typical rodents 7 days post-catheterization over 7 sec.
Please click here to view a larger version of this figure.
Figure 2. Real-time MAP waveforms of 4 typical rodents 7 days post-catheterization over 7 sec.
Please click here to view a larger version of this figure.
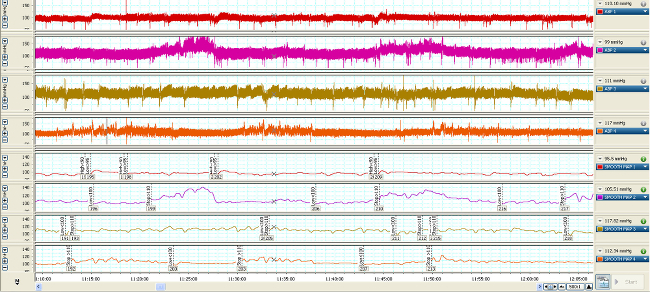 Figure 3. Time-compressed MAP waveforms of 4 typical rodents (upper 4 waveforms) 7 days post-catheterization over 1 hr. When the MAP is averaged (lower 4 waveforms), that value is used to trigger ADDS high (< 90 mmHg), low (<100 mmHg) or stop (>110) flow response from the infusion pump. Please click here to view a larger version of this figure.
Figure 3. Time-compressed MAP waveforms of 4 typical rodents (upper 4 waveforms) 7 days post-catheterization over 1 hr. When the MAP is averaged (lower 4 waveforms), that value is used to trigger ADDS high (< 90 mmHg), low (<100 mmHg) or stop (>110) flow response from the infusion pump. Please click here to view a larger version of this figure.
Discussion
Investigating therapeutic approaches to a clinical model of illness necessitates the ability to accurately perform hemodynamic monitoring, thus requiring vascular access. In our model of sepsis, arterial catheterization provides systemic pressure monitoring and venous catheterization allows for the application of standard clinical therapy. Standard therapy for sepsis includes the infusion of antibiotics to treat bacterial infection and the infusion of fluids and vasopressors to maintain arterial pressure within the desired range. Both catheters are used for blood sampling. In this design, the application of programmable infusion pumps with automatic triggering of the vasopressor infusion rate based on real-time mean arterial pressures is an advance over the clinical setting which requires manual titration by the medical staff and in the pre-clinical setting makes the use of this treatment modality possible for studying large numbers of animals.
Some complications were experienced early in the development of the model. These included partial occlusion of both arterial and venous catheters from over tightening, ties on PU2 and PU3 components, (1-2%); misplacement of jugular venous catheter into a small branch, (<1%); unexplained sudden death after recovery (no pulmonary thrombus or other organ damage evident), (1-2%) resolved by keeping isoflurane concentration 2-3.5% with O2 flow rate of 2 L/min. Other common surgical complications of hemorrhage, local infection and self-inflicted damage were not observed. Once surgical proficiency is attained, each procedure should be able to be completed in no more than 15 min. The rate of success measured as a healthy recovery and patent catheters, can be expected to exceed 90% once fully trained. An additional animal is included in each study to minimize the loss of data and the total number of animals needed and account for a failed catheter that may be “infusion only” or mortality due to complications.
The most critical step in this procedure is the design and placement of the catheters. The materials need to be soft at the tip so as not to pierce the vessel wall without crimping too easily. The body of the catheter has to be stiffer (than the tip) to be able to advance into the vessel sufficiently and be secured in place. Figure 1 shows the 3 diameters of tubing that are connected for the arterial catheter and 2 different diameter tubing connected for the venous catheters. These components are slid within each other to reduce catheter diameter at the tip and are glued together with anchors added to ensure placement of the catheter in the vessel or heart is maintained.
Once recovered and connected to the swivel and tether, the MAP and CVP waveforms should appear similar to the data represented in Figure 2. To maintain arterial catheter patency during monitoring, an hourly heparin saline flush (0.05 ml) should be performed. Continuous infusion maintains the venous catheter patency. During line connection and flush, close attention must be paid to insure there are no air bubbles in the line. An air bubble of ≥0.1ml can result in an embolism in the lung, brain or other organs. Hemodynamic waveforms are recorded and analyzed over the course of the study. When compressing time in the x axis, Figure 3 shows the changes in MAP and CVP over the course of 1 hr as well as the changes in rate and effect of vasopressor infusion.
The procedure has several potential limitations. In our studies, the catheters remain patent for up to 11 days. It is unknown how long the catheters would remain patent beyond this period. To fit the catheters as described, there is a minimum vascular size, which roughly correlates with rodent weight, requiring animals enrolled in this study to be greater than 200 g. The use of a smaller catheter diameter would result in increasing resistances to flow reducing patency. Attaining central venous pressure measures requires precise catheter placement in the atrium sufficient to account for size and growth during the study period and can be a challenge.
Once mastered, arterial and vascular catheterization can provide the basis for a wide variety of rodent models requiring hemodynamic monitoring, blood sampling, and infusion of fluids or therapies while minimizing any pain and distress during instrumentation or handling once recovered. In fact, in conjunction with a previous JOVE publication10 describing catheterization of the femoral vein, we have successfully performed this model with 3 implanted catheters.
Disclosures
The authors have no potential conflicts of interest.
Acknowledgments
Intramural NIH program supported the development of this model. Publication support was provided by Harvard Apparatus Inc. and ADInstruments Inc. The work by the authors was done as part of US government–funded research; however, the opinions expressed are not necessarily those of the National Institutes of Health.
References
- Buckingham RE. Indwelling catheters for direct recording of arterial blood pressure and intravenous injection of drugs in the conscious rat. J Pharm. Pharmacol. 1976;28(5):459–461. doi: 10.1111/j.2042-7158.1976.tb04660.x. [DOI] [PubMed] [Google Scholar]
- Buckle JW, Nathaniels PW. Proceedings: A dual catheter system for the simultaneous infusion and sampling of the vascular system of the unrestrained rat. J. Physiol. 1974;242(2):55P–56P. [PubMed] [Google Scholar]
- Solomon SB, et al. Effective dosing of lipid A analogue E5564 in rats depends on the timing of treatment and the route of Escherichia coli infection. J. Infect. Dis. 2006;193(5):634–644. doi: 10.1086/500147. [DOI] [PubMed] [Google Scholar]
- Cui X, et al. Bacillus anthracis cell wall produces injurious inflammation but paradoxically decreases the lethality of anthrax lethal toxin in a rat model. Intensive Care Med. 2010;36(1):148–156. doi: 10.1007/s00134-009-1643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezado ZM, Natanson C. Systemic hemodynamic abnormalities and vasopressor therapy in sepsis and septic shock. Am. J. Kidney Dis. 1992;20(3):214–222. doi: 10.1016/s0272-6386(12)80693-7. [DOI] [PubMed] [Google Scholar]
- Perdue PW, Kazarian KK, Nevola J, Law WR, Williams T. The use of local and systemic antibiotics in rat fecal peritonitis. J. Surg. Res. 1994;57(3):360–365. doi: 10.1006/jsre.1994.1155. [DOI] [PubMed] [Google Scholar]
- Qiu P, et al. The individual survival benefits of tumor necrosis factor soluble receptor and fluid administration are not additive in a rat sepsis model. Intensive Care Med. 2011;37(10):1688–1695. doi: 10.1007/s00134-011-2324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, et al. Severity of sepsis alters the effects of superoxide anion inhibition in a rat sepsis model. J. Appl. Physiol. 2004;97(4):1349–1357. doi: 10.1152/japplphysiol.01161.2003. [DOI] [PubMed] [Google Scholar]
- Karzai W, et al. Protection with antibody to tumor necrosis factor differs with similarly lethal Escherichia coli versus Staphylococcus aureus pneumonia in rats. Anesthesiology. 2003;99(1):81–89. doi: 10.1097/00000542-200307000-00016. [DOI] [PubMed] [Google Scholar]
- Jespersen B, Knupp L, Northcott CA. Femoral Arterial and Venous Catheterization for Blood Sampling, Drug Administration and Conscious Blood Pressure and Heart Rate Measurements. J. Vis. Exp. 2012. p. e3496. [DOI] [PMC free article] [PubMed]


