Abstract
In recent decades the mouse has become the primary animal model of a variety of lung diseases. In models of emphysema or fibrosis, the essential phenotypic changes are best assessed by measurement of the changes in lung elasticity. To best understand specific mechanisms underlying such pathologies in mice, it is essential to make functional measurements that can reflect the developing pathology. Although there are many ways to measure elasticity, the classical method is that of the total lung pressure-volume (PV) curve done over the whole range of lung volumes. This measurement has been made on adult lungs from nearly all mammalian species dating back almost 100 years, and such PV curves also played a major role in the discovery and understanding of the function of pulmonary surfactant in fetal lung development. Unfortunately, such total PV curves have not been widely reported in the mouse, despite the fact that they can provide useful information on the macroscopic effects of structural changes in the lung. Although partial PV curves measuring just the changes in lung volume are sometimes reported, without a measure of absolute volume, the nonlinear nature of the total PV curve makes these partial ones very difficult to interpret. In the present study, we describe a standardized way to measure the total PV curve. We have then tested the ability of these curves to detect changes in mouse lung structure in two common lung pathologies, emphysema and fibrosis. Results showed significant changes in several variables consistent with expected structural changes with these pathologies. This measurement of the lung PV curve in mice thus provides a straightforward means to monitor the progression of the pathophysiologic changes over time and the potential effect of therapeutic procedures.
Keywords: Medicine, Issue 95, Lung compliance, Lung hysteresis, Pulmonary surfactant, Lung elasticity, Quasistatic compliance, Fibrosis, Emphysema
Introduction
The mouse is now the primary animal model of a variety of lung diseases. In models of emphysema or fibrosis, the essential phenotypic changes are best assessed by measuring the changes in lung elasticity. Although there are many ways to measure elasticity, the classical method is that of the total pressure-volume (PV) curve measured from residual volume (RV) to total lung capacity (TLC). This measurement has been made on adult lungs from nearly all mammalian species dating back almost 100 years1-3. Such PV curves also played a major role in the discovery and understanding of the function of pulmonary surfactant in fetal lung development4-7. Despite the PV curve’s importance as a measurement of the lung’s phenotype, there has been no standardized way to perform this measurement. It has been done simply by inflating and deflating the lung with discrete steps (waiting a variable time for equilibration after each) or with pumps that can continuously inflate and deflate the lung. The PV curve is often done over a volume range between zero and some user-define lung capacity, but the time duration of each pressure volume loop reported by different labs has been extremely variable, varying from a few sec8 to hr2. Some investigators refer to this total lung PV curve as static or quasistatic, but these are qualitative terms that offer little insight, and they are not used here. In addition, the PV curve has not been widely reported in the mouse, despite the fact that it can provide useful information on the macroscopic effects of structural changes in the lung.
Several issues have resulted in variability in PV curve acquisition including: 1) the rate of inflation and deflation; 2) the pressure excursions for inflation and deflation; and 3) the means to determine an absolute lung volume measurement. In the method present here, a rate of 3 ml/min was chosen as a compromise, being not too short as to reflect the dynamic elasticity associated with normal ventilation and not too slow as to make the measurement impractical, particularly when studying large cohorts. Since a nominal total lung capacity in a C57BL/6 healthy mouse is on the order of 1.2 ml9, this rate typically allows two complete closed PV loops to be done in about 1.5 min.
In the extended literature where PV curves have been reported, the peak inflation pressure used has been extremely variable, varying from as low as 20 to over 40 cm H2O. Part of this variability may be related to species, but a primary goal of setting the upper pressure limit for PV curves is to inflate the lung to total lung capacity (TLC), or maximal lung volume. The TLC in humans is defined by the maximal voluntary effort an individual can make, but unfortunately this can never be duplicated in any animal model. Thus, the maximal volume in experimental PV curves is determined by a maximal pressure arbitrarily set by the investigator. The goal is to set a pressure where the PV curve is flat, but unfortunately the inflation limb of a mammalian lung PV curve is never flat. So most investigators set a pressure where the inflation curve begins to flatten substantially, typically 30 cm H2O. In the mouse, however, the PV curve is even more complex with a double hump on the inflation limb, and where this inflation limb is often still rising steeply at 30 cm H2O10, so 30 is not a good end point for the PV curve. For this reason, we use 35 cm H2O as the pressure limit for the mouse PV curve, which is a pressure at which the inflation limbs of all strains we have examined begin to flatten.
Since the PV curve itself is very nonlinear, the appearance of a PV loop will depend on the volume from where the curve starts. Some commercial ventilators allow users to do large PV loops, starting from FRC, but if the FRC volume is unknown then it is impossible to interpret changes in such PV curve with any pathology, since these changes could simply result from a change in starting volume, and not structural alterations in the lung. Thus without an absolute volume measurement, PV curves are almost impossible to interpret and thus have little utility. Although, there are several ways to measure lung volumes, these are often cumbersome and require special equipment. In the simple approach described here, the PV curve starts at zero volume after an in vivo degassing procedure.
In summary, this paper demonstrates a straightforward method to standardize lung PV curve measurement in the mouse lung, and defines several metrics that can be calculated from this curve that are linked to lung structure. The PV curve thus provides a pulmonary function test that has direct application in being able to detect phenotypic structural changes in mice with common lung pathologies such as emphysema and fibrosis.
Protocol
The Johns Hopkins University Animal Care and Use Committee approved all animal protocols.
1. Equipment
The composite system set up, ready to measure the PV curve is shown in Figure 1.
- Volume measurement:
- Generate a constant rate of inflation and deflation by using a syringe pump with a switch that allows the user to quickly reverse the pump after reaching the pressure limits. For mouse PV curves, use a very lightly greased 5 ml glass syringe with the initial volume (prior to inflation) set at 3 ml of air. 3 ml is sufficiently large to measure volumes in nearly all mouse PV curves.
- Measure the volume delivered by the pump by attaching a linear differential transformer to the pump housing, with a small sensor rod connected to the moving syringe plunger. Note: An empirical means to correct for gas compression in the system is described under the PV curve recording section.
- Pressure measurement:
- Use a standard inexpensive pressure gauge with a range of 0-60 cm H2O (0-1 PSI).
- Recording measurement:
- To record the PV curve use any digital recorder with XY capabilities (e.g., PowerLab). Set one channel to record the corrected volume signal and another channel to record the transpulmonary pressure (Ptp), in order to graph the PV curve. Use a bridge preamplifier that connects to the main Powerlab to measure the pressure. Calibrate the pressure channel from 0-40 cm H2O, and calibrate the volume channel from 0-3 ml.
2. Correction for Gas Compression
Note: This is a critical initial step in the set up, since as the pressure increases, the gas volume decreases, and thus the volume of air delivered to the mouse will be increasingly less than the displacement of the syringe barrel.
- Close the stopcock that will connect the PV system to the lungs, so no gas can leave the system. Start the infusion and observe if the corrected Volume channel on the recorder shows any measurable changes as the pressure increases to about 40 cm H2O. If so, then correct as in the next steps.
- Correct for gas compression empirically by subtracting from the plunger displacement measurement (i.e., the uncorrected volume) a term proportional to the inflation pressure. Do this on a Powerlab channel (called Vc) to show the volume signal minus a coefficient times the pressure.
- Determine the coefficient in the equation. First, make an initial guess, turn the chart recording on, and start the pump. Since the inflation tubing is sealed, adjust the pressure coefficient multiplier to make the Vc channel read zero as the pressure rises from 0-40 cm H2O. If it goes up or down, simply adjust the correction factor until it stays flat over this pressure range. This correction factor will always be the same, if the same 3 ml starting volume in the syringe is not changed.
3. Experimental Tests in Mice
- Procedure for measurement of the PV curve in mice. All animal protocols were approved by the Johns Hopkins University Animal Care and Use Committee.
- Anesthetize mice (C57BL/6 mice at 6-12 weeks of age) with ketamine (90 mg/kg) and xylazine (15 mg/kg), and confirm anesthesia by the absence of reflex motion. Note: The PV curve can be completed in anesthetized mice in less than 10 min and is a terminal procedure.
- Tracheostomize the mice with an 18 G stub needle cannula. Do this by making a small incision in the skin overlying the trachea, locating the trachea, then making a small slit in the trachea, where the stub needle can be inserted. Secure the cannula by tying with thread.
- Allow the mice to breath 100% oxygen for at least 4 min. This can be via spontaneous breathing from a bag or with a ventilator nominally set with a tidal volume of 0.2 ml at 150 breaths/min.
- Close the tracheal cannula and allow 3-4 min for the mouse to absorb all the oxygen. This oxygen absorption procedure results the death of the animals and in a nearly complete degassing of the lung11. Confirm death of the mouse by measuring the cessation of the heartbeat with ECG electrodes or direct observation.
- Once the degassing of the lung is complete and the lung volume is zero, begin inflating the lung with room air in the syringe pump at a rate of 3 ml/min. Monitor the pressure trace on the digital recorder, and when it reaches 35 cm H2O, reverse the pump.
- Follow the deflation curve until pressure reaches negative 10 cm H2O, by which time the airways have collapsed, trapping air in alveoli preventing further volume reduction. Immediately reverse the pump again, allowing the lung to reinflate as the collapsed airways open. This heterogeneous opening is normally apparent by the noisy looking inflation limb at the initial part of this 2nd inflation.
- When the pressure again reaches 35 cm H2O, reverse the pump direction, and continue to deflate the lung until this 2nd deflation limb reaches 0 cm H2O. Then stop the pump.
- View the PowerLab chart record of pressure and flow and the PV curve. Then analyze the PV curve to detect phenotypic changes in lung parenchyma that occur with different lung pathologies.
Representative Results
Although the procedure for the PV curves is demonstrated in the video only for control healthy mice, we examined the ability of the PV curve to detect functional and pathologic changes in mice with two different common pathologies, emphysema and fibrosis. Details of these traditional models described elsewhere12,13. Very briefly, after anesthesia with 3% isoflurane the emphysema was caused by 3 or 6 U porcine pancreatic elastase instilled into the trachea and studied 3 weeks later, and the fibrosis was caused by 0.05 U bleomycin instilled into the trachea and studied 2 weeks after this insult.
Figure 2 shows a typical PV curve from a control mouse. From such a PV curve, measure variables that are easy to quantify, reproducible from mouse to mouse, and representative of structural changes that occur in lung disease. These are listed in Table 1 and shown graphically in Figure 2. Table 1 lists these variables, and Figure 2 illustrates how they are measured from the PV curve. The rationale behind each is discussed later.
Figure 3 shows typical PV curves from representative control, emphysematous, and fibrotic mice, respectively. Variables measured from the curves generated in female control and fibrotic mice are presented in Figure 4. Variables measured from the curves generated in male control mice and those with 2 degrees of emphysema severity are presented in Figure 5. Statisticalcomparisons between groups were analyzed with either an unpaired t-test (fibrosis model) or a one-way ANOVA and significance level assessed with Tukey’s correction for multiple comparisons (emphysema model). A p <0.01 was considered significant.
These results show that the methods used here to obtain measurement of lung PV curves are useful in being able to detect changes in the distensibility of the lung in different pathologies where such structural changes have been clinically described. The approach and analysis generates several variables that characterize different aspects of the PV curve. The interpretation of what each of these measured variables signifies is discussed in more detail in the next section.
 Figure 1: Experimental set up showing syringe pump with volume and pressure transducers.
Please click here to view a larger version of this figure.
Figure 1: Experimental set up showing syringe pump with volume and pressure transducers.
Please click here to view a larger version of this figure.
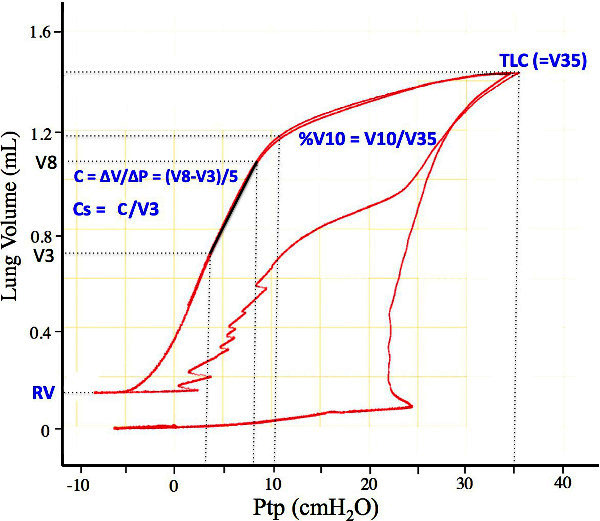 Figure 2: Representative PV curve showing how the different variables in Table 1 are measured. V3, V8, and V10 are the lung volumes on the first deflation limb at 3, 8, and 10 cm H2O, respectively. V35 is the volume 35 cm H2O and is defined as the total lung capacity (TLC). RV is the residual volume, defined as the volume of trapped gas at the en of the first deflation curve. Please click here to view a larger version of this figure.
Figure 2: Representative PV curve showing how the different variables in Table 1 are measured. V3, V8, and V10 are the lung volumes on the first deflation limb at 3, 8, and 10 cm H2O, respectively. V35 is the volume 35 cm H2O and is defined as the total lung capacity (TLC). RV is the residual volume, defined as the volume of trapped gas at the en of the first deflation curve. Please click here to view a larger version of this figure.
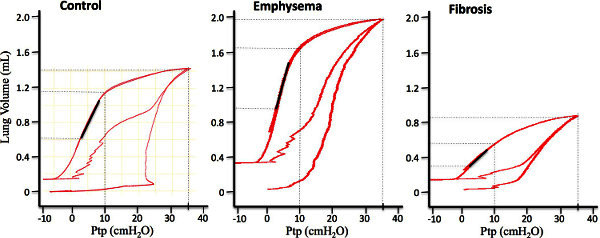 Figure 3: Representative mouse PV curves from control, emphysematous, and fibrotic lungs. The slope of the dark line segment indicates the deflation limb compliance, C. Please click here to view a larger version of this figure.
Figure 3: Representative mouse PV curves from control, emphysematous, and fibrotic lungs. The slope of the dark line segment indicates the deflation limb compliance, C. Please click here to view a larger version of this figure.
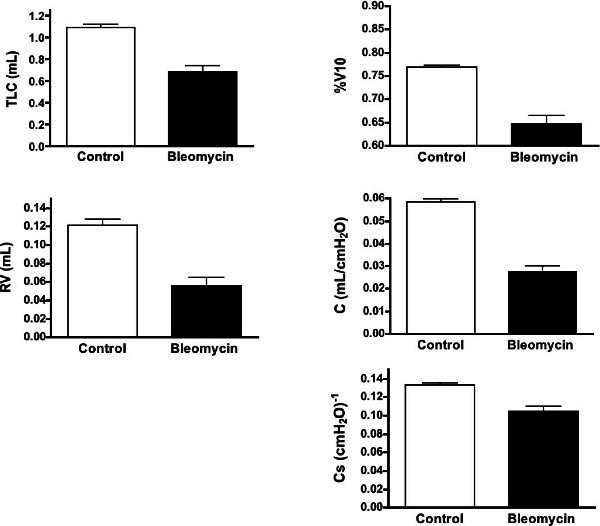 Figure 4: Changes in the variables measured from the PV curves in the control and fibrotic mice. Shown are means ± SEM, n = 9 for each group. All variables in the fibrotic lungs were significantly different from control lungs with P <0.01. Please click here to view a larger version of this figure.
Figure 4: Changes in the variables measured from the PV curves in the control and fibrotic mice. Shown are means ± SEM, n = 9 for each group. All variables in the fibrotic lungs were significantly different from control lungs with P <0.01. Please click here to view a larger version of this figure.
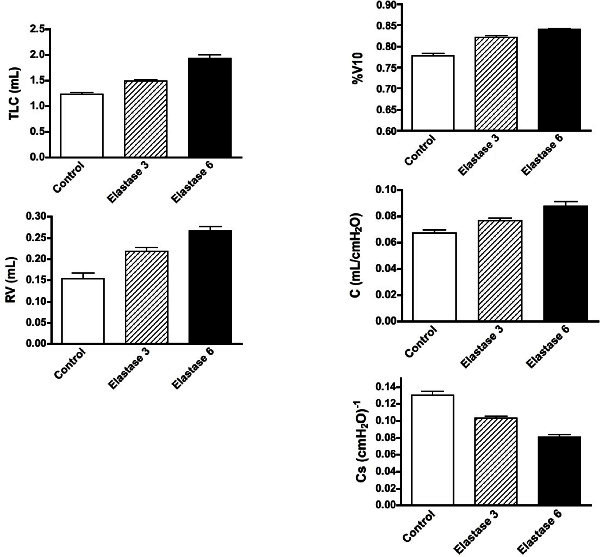 Figure 5: Changes in the variables measured from the PV curves in the control and emphysematous mice. Shown are means ± SEM, n = 9 for each group. All variables in the emphysematous lungs at either degree of severity were significantly different from control lungs and each other with P <0.01. Please click here to view a larger version of this figure.
Figure 5: Changes in the variables measured from the PV curves in the control and emphysematous mice. Shown are means ± SEM, n = 9 for each group. All variables in the emphysematous lungs at either degree of severity were significantly different from control lungs and each other with P <0.01. Please click here to view a larger version of this figure.
| Measurement | What it quantifies | Changes with pathologies |
| TLC | "Maximal" inflation; defined in mice as the lung volume at 35 cm H2O | Increases in emphysema; Decreases in fibrosis |
| RV | Trapped air volume after airway collapse on deflation | Increases in emphysema; Decreases in fibrosis |
| %V10 | Shape of the deflation limb | Increases with lung development; Decreases with surfactant inhibition; Increases in emphysema; Decreases in fibrosis |
| C | The quasistatic slope of the deflation limb | Increases in emphysema; Decreases in fibrosis |
| Cs | Specific compliance from the deflation limb = C/V3 | Decreases in emphysema; Decreases in fibrosis |
Table 1: Listing of the different variables measured from mouse PV curves.
Discussion
In this paper a straightforward reproducible method has been described to measure in mice a classical method of phenotyping lung elasticity, the total lung PV curve. Such curves were instrumental in the discovery of pulmonary surfactant and its importance in providing lung stability. Here it is shown how the PV curve is also useful in providing a means to measure several variables related to lung elasticity in adult mouse lungs. There were highly significant changes in all variables in two commonly used mouse models to generate pathologic changes in mouse lungs. The following section briefly discusses the significance of the changes in each the measured variables.
The TLC is a measure of the maximal lung volume, or more accurately, the volume at a defined maximal pressure, where the inflation limb begins to flatten. As already noted, the inflation limb of a PV curve never really flattens, and the mouse is particularly extreme in this behavior10. Although the TLC is defined in humans as the volume at the end of a maximal voluntary inspiratory effort, in every animal model it is defined as the volume at some arbitrary user-defined pressure. With the pathologic models shown in this paper, a progressive increase in TLC with increasing emphysema was observed as well as a decrease with fibrosis. These observations reflect clinical manifestations with each of these conditions and are thus what would be expected in a useful mouse model.
The RV is a variable that reflects the residual air trapped in alveoli as the airways close on a maximal expiration. This variable thus reflects the same phenomenon in humans and animal models. The RV is known to increase in humans in asthma and COPD14,15. This increase in RV is related to the fact that the small airways close sooner on the deflation limb with either increased smooth muscle tone or the loss of tethering support from the surrounding lung parenchyma16. In the two pathologic models used here, opposite effects were found. There was a significant increase in RV with increasing emphysematous injury, but with fibrosis, there was a decreased RV, since the stiffer airways and surrounding lung closed at lower lung volumes on deflation.
The %V10 is a shape factor that has been used to reflect the stability of the lung on deflation, and was initially used to reflect the maturation of the surfactant system17. As the fetal lung matures, the deflation limb changes from a relatively straight curve to one that is convex toward the volume axis, with a concomitant increase in %V1018. The final shape in adults varies considerably among mammalian species, with %V10 varying between 75 and 90%19. The %V10 is also known to decrease progressively as the pulmonary surfactant becomes less effective20,21. In the pathologic models studied here, major changes in surfactant were not expected, but the shape of the curve is also dependent on the lung tissue elasticity. The fact there were significant increases in %V10 with emphysema and significant decreases with fibrosis likely reflects these structural changes. Although this metric is not normally measured in human subjects, it may be very useful in animal models as a phenotypic variable related to specific pathologic changes in lung structure.
The compliance (C) is a metric that can be obtained from any linearized region of the nonlinear PV curve. In the mouse, the deflation limbs from most strains are quite linear between 3 and 8 cm H2O, and for this reason it is easy to define a reproducible C over that range. One of the critical issues in using any slope measurement from the PV curve is that the value is highly dependent on both the range of pressure over which it is measured and the preceding volume history (i.e., how the section of curve was generated), so consistency is critically important if comparisons are going to be made between control and pathologic models. In the two pathologic models used in this study, significant increases in C in emphysema and significant decreases in fibrosis were observed; findings that mimic what are observed clinically in humans.
The specific compliance, Cs has classically been used to correct for the fact that a larger lung with the same structure as a smaller lung will have a bigger change in lung volume over the same change in pressure, thereby resulting in a bigger compliance22. The Cs is also equivalent to the inverse of the bulk modulus of elasticity of the lung. Clinically it is measured as the compliance divided by FRC, but since in the mouse we don’t know the FRC, we have chosen to use the volume at 3 cm H2O. By normalizing to the volume at 3 cm H2O (i.e., using the fractional change in volume), one would then calculate the same specific compliance in a big or small lung, if the big lung simply consisted of more of the same small lung. Results in the present paper show that there was a decrease in Cs in the fibrosis model, indicating that the measured change in C was not simply due to the lung volume being smaller. Rather, the lung parenchyma itself was significantly stiffer. In the emphysema model, however, the Cs also decreased, which is opposite to the increase in C. This calculated decrease in Cs occurred because the increase in lung volume was larger than the increase in C. However, this mathematical fact doesn’t provide any insight into the structural changes that led to these changes. At the present time, additional pathologic insights are not clear, and further experimental work is beyond the scope of this methods paper.
The reasons underlying these changes in PV variables are dependent on the pathologic changes in the different models. In emphysema, the loss of alveolar walls decreases the overall tissue recoil and increases peripheral airspace size. This enlargement of residual airspaces would increase radius of curvature of the airspace surface, further decreasing the elastic recoil due to surface tension. Both of these factors would lead to the increase in TLC observed. In the fibrotic model, the deposition of collagen and other matrix elements leads to a stiffening as well as a thickening of all tissues that is detectable clinically and in mice as a reduced diffusing capacity13,23. These pathologic changes are reflected in significantly lowered TLC. The increase in RV seen in the emphysema model likely results from a decrease in the tethering support of the airways, which manifests itself by an earlier airway closure on the expiratory limb. In fibrosis, the stiffer airways resist collapse until a lower pressure is reached on expiration, thereby decreasing the residual volume. The compliance changes reflect similar pathologies that impact the lung volumes. A loss of elastic elements in the parenchymal walls will result in an increased compliance, where as collagen deposition in airways and parenchyma will lead to a stiffer lung with decreased compliance. The slight increase in %V10 in the emphysema model and the decrease with fibrosis are not as easy to explain. There are no comparable studies in the literature with which to compare these results. Since the elastic recoil with emphysema is lower, the maximal lung volume is apparently able to stay higher than normal even as the pressure decreases, and this is manifested by the increased %V10. With fibrosis, the elastic recoil remains high even at high pressures, so the volume falls more quickly as the pressure decreases from TLC. This would also be consistent with a degradation of pulmonary surfactant, but there is no literature where this was assessed in fibrosis. Thus, although the %V10 has not been used to phenotype adult human lungs, the results presented here suggest it may be a sensitive variable that can monitor progressive changes at least in the two pathologies studied. Until more complete studies are done, however, where the dose-response relations with elastase or bleomycin are done, the sensitivity of this variable will remain speculative.
The importance of correcting for gas compression cannot be overemphasized. This is a critical initial step in the set up, since as the pressure increases, the gas volume decreases, and thus the volume of air delivered to the mouse will be increasingly less than the displacement of the syringe barrel. The procedure to empirically correcting for this was shown in he protocol above. It is worth noting that if the PV setup volume doesn’t change, then this empirical correction procedure need only be done once. And if the coefficient is written down, it can be entered in manually if ever needed. It should be emphasized, however, that this method only works since the lung is starting from a degassed state. If the PV curve were started from a normal end-expiratory lung volume (FRC), it would not be possible to correct for the gas compression unless one knew the magnitude of that volume. In addition, the shape of a PV curve will be quite dependent on the starting lung volume, so if there were changes observed in a pathologic lung starting from FRC, it would not be possible to interpret those changes until the control and pathologic FRCs were known. This is another advantage of always starting from zero lung volume. Finally, it is worth noting that the PV curves were done in mice with an intact chest wall. This simplifies the entire procedure and greatly reduces the possibility of errors due to distorted lung shape or surgical errors. Fortunately, the presence of a normal chest wall has a negligible effect on the PV curve9, so the PV curve done with an intact chest provides a straightforward and reliable means to assess the distensibility of the lung.
In conclusion, this paper shows how to simply perform a reproducible measurement of the lung PV curve in mice. The PV curve has a unique ability to document structural change in the lung both in animals with genetically altered lung as well as with other environmental insults. Thus, as shown here, this measurement can provide phenotypic insight into the manifestation of specific structural changes in the lung with emphysema and fibrosis, and it can similarly be used too assess any other pathologies that might affect lung elasticity.
Disclosures
None of the authors have any financial interests that would be in conflict with the material presented in this paper.
Acknowledgments
This work has been supported by NIH HL-1034.
References
- Neergaard Kv. Neue Auffasungen über einn Grundbergriff der Atemtechnik. Die Retraktionskraft der unge, abhangig von den Oberflachenspannung in den Alveolen. (New interpretations of basic concepts of respiratory mechanics. Correlation of pulmonary recoil force with surface tension in the alveoli.) Zeitschrift Fur Gesamte Experi Medizin. 1929;66:373–394. [Google Scholar]
- Hildebrandt J. Pressure-volume data of cat lung interpreted by a plastoelastic, linear viscoelastic model. J. Appl. Physiol. 1970;28:365–372. doi: 10.1152/jappl.1970.28.3.365. [DOI] [PubMed] [Google Scholar]
- Hoppin FG, Hildebrandt J. West JB. Bioengineering Aspects of the Lung. New York, NY: Marcel Dekker; 1977. pp. 83–162. [Google Scholar]
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease) AMA. J. Dis. Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Clements JA, Hustead RF, Johnson RP, Gribetz I. Pulmonary surface tension and alveolar stability. Tech Rep CRDLR US Army Chem. Res. Dev. Lab. 1961;3052:1–24. [PubMed] [Google Scholar]
- Radford EP. In: Tissue Elasticity. Remington JW, editor. Bethesda, MD: American Physiological Society; 1957. pp. 177–190. [Google Scholar]
- Mitzner W, Johnson JWC, Scott R, London WT, Palmer AE. Effect of betamethasone on the pressure-volume relationship of fetal rhesus monkey lung. Journal of Applied Physiology. 1979;47:377–382. doi: 10.1152/jappl.1979.47.2.377. [DOI] [PubMed] [Google Scholar]
- Smaldone GC, Mitzner W, Itoh H. The role of alveolar recruitment in lung inflation: Influence on pressure-volume hysteresis. Journal of Applied Physiology. 1983;55:1321–1332. doi: 10.1152/jappl.1983.55.4.1321. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. Journal of Applied Physiology. 1999;86:1764–1769. doi: 10.1152/jappl.1999.86.6.1764. [DOI] [PubMed] [Google Scholar]
- Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J. Appl. Physiol. 2004;96:1658–1664. doi: 10.1152/japplphysiol.01098.2003. [DOI] [PubMed] [Google Scholar]
- Stengel PW, Frazer DG, Weber KC. Lung degassing: an evaluation of two methods. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1980;48:370–375. doi: 10.1152/jappl.1980.48.2.370. [DOI] [PubMed] [Google Scholar]
- Limjunyawong N, Mitzner W, Horton M. A mouse model of chronic idiopathic pulmonary fibrosis. Physiol Rep. 2014;2:e00249. doi: 10.1002/phy2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallica J, Das S, Horton MR, Mitzner W. Application of Carbon Monoxide Diffusing Capacity in the Mouse Lung. J. Appl. Physiol. 2011;110:1455–1459. doi: 10.1152/japplphysiol.01347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, et al. The structural basis of airways hyperresponsiveness in asthma. J. Appl. Physiol. 2006;101(1):30–39. doi: 10.1152/japplphysiol.01190.2005. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, et al. Ultrasonographic Assessment of the Diaphragm in Chronic Obstructive Pulmonary Disease Patients: Relationships with Pulmonary Function and the Influence of Body Composition - A Pilot Study. Respiration: International Review of Thoracic Diseases. 2014;87(5):364–371. doi: 10.1159/000358564. [DOI] [PubMed] [Google Scholar]
- Mitzner W. Airway-parenchymal interdependence. Comprehensive Physiol. 2012;2:1921–1935. doi: 10.1002/cphy.c110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Permutt S, Sipple JH, Salem ES. Effect of Intra-Alveolar Fluid on Pulmonary Surface Tension Properties. J. Appl. Physiol. 1964;19:769–777. doi: 10.1152/jappl.1964.19.4.769. [DOI] [PubMed] [Google Scholar]
- Palmer S, Morgan TE, Prueitt JL, Murphy JH, Hodson WA. Lung development in the fetal primate, Macaca nemestrina. II. Pressure-volume and phospholipid changes. Pediatr. Res. 1977;11:1057–1063. doi: 10.1203/00006450-197710000-00006. [DOI] [PubMed] [Google Scholar]
- Lum H, Mitzner W. A species comparisonof alveolar size and surface forces. Journal of Applied Physiology. 1987;62:1865–1871. doi: 10.1152/jappl.1987.62.5.1865. [DOI] [PubMed] [Google Scholar]
- Faridy EE. Effect of distension on release of surfactant in excised dogs' lungs. Respir. Physiol. 1976;27:99–114. doi: 10.1016/0034-5687(76)90021-9. [DOI] [PubMed] [Google Scholar]
- Faridy EE, Permutt S, Riley RL. Effect of ventilation on surface forces in excised dogs' lungs. J. Appl. Physiol. 1966;21:1453–1462. doi: 10.1152/jappl.1966.21.5.1453. [DOI] [PubMed] [Google Scholar]
- Comroe JH, Forster RE, Dubois AB, Briscoe WA, Carlsen E. The Lung: Clinical Physiology and Pulmonary Function Tests. New York, NY: Year Book Medical Publishers, Inc; 1962. [Google Scholar]
- Martinez FJ, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann. Intern. Med. 2005;142:963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]


