Abstract
Isolated hepatic perfusion (IHP) is a procedure where the liver is surgically isolated and perfused with a high concentration of the chemotherapeutic agent melphalan. Briefly, the procedure starts with the setup of a percutaneous veno-venous bypass from the femoral vein to the external jugular vein. Via a laparotomy, catheters are then inserted into the proper hepatic artery and the caval vein. The portal vein and the caval vein, both supra- and infrahepatically, are then clamped. The arterial and venous catheters are connected to a heart lung machine and the liver is perfused with melphalan (1 mg/kg body weight) for 60 min. This way it is possible to locally perfuse the liver with a high dose of a chemotherapeutic agent, without leakage to the systemic circulation.
In previous studies including patients with isolated liver metastases of uveal melanoma, an overall response rate of 33-100% and a median survival between 9 and 13 months, have been reported. The aim of this protocol is to give a clear description of how to perform the procedure and to discuss IHP as a treatment option for liver metastases of uveal melanoma.
Keywords: Medicine, Issue 95, Isolated hepatic perfusion, Melphalan, Surgical technique, Uveal Melanoma, Liver metastases, Regional therapy
Introduction
Uveal melanoma is the most common primary intraocular malignancy in adults, with the incidence being highest in Caucasian populations 1. Local treatment consists of enucleation or local plaque radiation therapy 2,3. Irrespective of local treatment, metastatic disease will ultimately develop in about 35-50% of the patients 4,5. The liver is the most common site of metastases and approximately 50% of the patients present with isolated liver metastases. The median overall survival (OS) for patients with liver metastases is 6-12 months 4. Several different systemic and regional treatment strategies have been explored, but survival rates have not improved 2.
Treatment with systemic chemotherapy, both single substance (Dacarbazine or Temozolomide6) or combination treatments (BOLD regimen7, gemcitabine with treosulfan 8), shows response rates of less then 10 %. Hepatic intra-arterial chemotherapy (HIA) has shown a slightly higher overall response rate compared to systemic chemotherapy, but no increase in overall survival 9. Transarterial chemoembolization (TACE) with cisplatin and carboplatin showed a partial response in 57% of patients with a median survival of 9 months10. Selective internal radiation therapy (SIRT) has shown a partial response in 62% of patients with a median survival of 7 months 11. In a study on liver resection, including 255 patients, an overall survival of 14 months was reported; in 76 of the patients where a microscopically complete resection was possible, the survival was 27 months 12.
Isolated hepatic perfusion (IHP) is a regional treatment option, originally developed by Ryan and Ausman. The rationale behind the technique is to surgically isolate and perfuse the liver with a high dose of a chemotherapeutic agent while avoiding systemic toxicity 13. In addition, concurrent hyperthermia mediates an increased uptake of the chemotherapeutic agent by changes in tumor blood flow and cellular membrane permeability 14. Complete vascular isolation is confirmed with a radioactive tracer technique using Tc-99 labeled human serum albumin 15. A retrospective phase II study has shown a potential survival benefit of 14 months (26 vs. 12 months) for patients that underwent IHP compared to the longest survivors with uveal melanoma liver metastases in Sweden 16.
Here we present the technique of IHP with a short review of current results and future perspectives.
Protocol
Patients provided written informed consent and the study was approved by the Regional Ethical Review Board at the University of Gothenburg.
1. Anesthesia
Set up all the monitoring (ECG, blood pressure cuff) 17.
Insert an epidural catheter in the lower to mid thoracic level 17.
Induce anesthesia with Propofol 2 mg/kg, 150 µg of Fentanyl, and Rocuronium 0.6 mg/kg through a previously inserted peripheral venous catheter.
Preoxygenate and intubate the patient. Maintain anesthesia with Sevoflurane, oxygen and air 17.
Insert a double lumen central venous catheter and an arterial catheter. Monitor CVP and arterial pressure 17.
2. Isolation of the Liver and Cannulation of Vessels
Administer 100 IE/kg of heparin and give antibiotic prophylaxis (e.g., 160 mg of Trimethoprim and 800 mg of Sulfamethoxazole).
Using the percutaneous Seldinger technique, place a 17 Fr catheter in the femoral vein and a 15 Fr catheter in the external jugular vein 18. Verify the positions of the catheters by fluoroscopy.
Perform a laparotomy by an L-shaped incision from the xiphoid process down the midline and then extended to a right subcostal incision 19.
- Verify that no extra-hepatic metastases exist in the abdomen by a complete inspection and palpation.
- If any suspicious lesions are found, verify malignancy by frozen section.
- Terminate the procedure if there is evidence of extrahepatic manifestation (periaortal adenopathy, abdominal metastases). Lymphadenopathy in the porta hepatis is not considered a contraindication.
Mobilize the liver by dividing the falciform ligament, the left and right triangular ligament and the coronary ligament 19.
Dissect the caval vein beginning from the renal veins up to the diaphragm with ligation of retroperitoneal tributary veins.
Identify the right adrenal vein and place a vessel loop around it.
- Identify the right gonadal vein. Dissect it for 5-10 cm. Place a purse-string suture around the entrance to the caval vein.
- Place a ligature distally on the gonadal vein. Using scissors, open the gonadal vein 3-5 cm from the caval vein.
- Insert an 18 Fr wire-reinforced catheter through the right gonadal vein and place the tip retrohepatically above the renal veins.
- Place a ligature around the gonadal vein to secure the position of the catheter.
- Verify the return of blood through the catheter. Heparinize the catheter 18.
- Dissect the hepatoduodenal ligament and visualize the common hepatic artery, the gastroduodenal artery and the proper hepatic artery.
- Place vessel loops around the common hepatic artery and the gastrodoudenal artery.
- Dissect the portal vein and place a vessel loop around it.
- Isolate the rest of the ligament containing the common bile duct and include all of this in a vessel loop.
- Lift the gastroduodenal artery with the vessel loop. After interrupting the arterial inflow, make an incision using scissors.
- Insert an 8-12 Fr catheter with its tip into the proper hepatic artery. Secure the position with a ligature around the gastroduodenal artery.
- Verify blood return in the catheter and then heparinize it 18.
Prime the perfusion system with one unit of packed erythrocytes, 100 ml of albumin (50 g/L), 100 ml of Tribonat (sodium bicarbonate), 2500 units of heparin (5,000 IE/ml) and 250 ml of crystalloids.
Connect the arterial and the caval vein catheters to the perfusion system consisting of a heart lung machine equipped with a roller pump and a heater together with a venous reservoir, oxygenator and tubing systems of disposable type.
Connect the femoral and external jugular catheters to the venovenous bypass pump consisting of a centrifugal pump, along with a heater unit and start bypass.
- Complete the isolation of the liver by tightening a Rumel tourniquet around the caval vein infrahepatically above the renal veins and clamping the caval vein suprahepatically using a Klintmalm vascular clamp.
- Tighten a Rumel tourniquet around the common bile duct, the portal vein and the right adrenal vein and finally clamp the hepatic artery using a vascular clamp.
3. The Perfusion
Start the perfusion and manually monitor the blood level in the venous reservoir. Adjust the flow rate until a steady-state level is reached, usually a flow rate between 500-1,200 ml/min.
Place thermistor probes into the right and left liver lobes and one directly into the arterial catheter.
Heat the perfusate aiming at a target liver temperature of 40 oC. Keep this temperature throughout the perfusion.
Place a scintillation probe over the venovenous bypass pump head.
- Calibrate the leakage monitoring system, by injecting a small dose of 10 MBq 99mTc-albumin (1 ml) into the systemic circulation and register the baseline activity.
- Inject a ten-fold higher dose, 100 MBq 99mTc-albumin (1 ml), into the hepatic perfusion unit.
- Record any leakage from the perfusion system to the systemic circulation as an increase in activity measured by the scintillation probe.
- Record any leakage from the systemic circulation to the perfusion system as an increase in volume in the venous reservoir.
When steady-state of the flow rate is established, no leakage is present and the liver temperature has reached 40 °C, inject melphalan at a dose of 1 mg/kg bodyweight into the perfusion system divided into two doses given 30 min apart.
4. End the Perfusion and Remove Catheters
After 60 min of perfusion, empty the perfusate from the system and irrigate the liver with 1,000 ml of crystalloid.
Add one unit of packed erythrocytes to the perfusion unit, infuse into the liver and then stop the perfusion.
Release the Klintmalm clamp and the Rumel tourniquet from the caval vein.
Release the Rumel tourniquet from the hepatoduodenal ligament.
Remove the ligature from the gastroduodenal artery. Remove the catheter and ligate the vessel.
Release the clamp from the hepatic artery.
Remove the catheter from the gonadal vein and ligate the vessel.
Stop the veno-venous bypass pump and disconnect the tubes.
Close the abdomen with an uninterrupted fascial suture and then an intracutaneous skin suture.
Remove the catheters in the femoral and external jugular veins and apply local pressure for 5 min.
5. Postoperative Care
Extubate the patient and transfer to postoperative care unit for 24 hr. Keep monitoring central venous pressure and arterial blood pressure.
Transfer the patient to a surgical department for a typical of 3-7 days of post-operative care.
Continue with low-molecular weight heparin (LMWH) at a dose of 5,000 IE/day for a total of 4 weeks.
Representative Results
An initial report included 22 patients treated with melphalan with or without TNF-alpha. The results showed an overall response rate of 62%, including two patients with CR (10%), and a median overall survival of 11 months 20. A subsequent study reported on the outcome of 29 consecutive patients treated using melphalan alone. The outcome was very similar with a 62% overall response including 10% complete response and a median survival of 12 months 21.
In a study by Rizell et al., 20 patients with uveal melanoma were included. The patients were divided into three different time eras, and the results showed a decrease in mortality from 27% to 0% due to improved patient selection and technical development 22.
The knowledge about IHP is primarily based on smaller retrospective single institution series, and the published results are listed in Table 1. In summary, the overall response rate is 33-100%, including a 0-20% complete response rate. The post-operative mortality is 6% and the median survival is 12 months.
| Author | n | Type | ORR | CR | Median survival | Mortality |
| Alexander et al. 2000 | 22 | IHP | 62% | 10% | 11 months | 5% |
| Alexander et al. 2003 | 29 | IHP | 62% | 10% | 12 months | 0% |
| Noter et al. 2003 | 8 | IHP | 50% | 0% | 10 months | 0% |
| Pingpank et al. 2005 | 10 | PHP | 50% | 20% | N/A | 0% |
| Rizell et al. 2008 | 27 | IHP | 70% | 7% | 13 months | 22% |
| Verhoef et al. 2008 | 4 | IHHP | 100% | 0% | 9 months | 0% |
| van Iersel et al. 2008 | 12 | IHP | 33% | 0% | 10 months | 0% |
| van Etten et al. 2009 | 8 | IHHP | 37% | 0% | 11 months | 0% |
| Weighted mean | 120 | 59% | 7% | 12 months | 6% | |
| Olofsson et al. | 34 | IHP | 68% | 12% | 24 months | 0% |
Table 1: Results of isolated hepatic perfusion. Publications with clinical outcomes reported for patients with isolated liver metastases treated with different modalities of isolated hepatic perfusion. ORR = overall response rate; CR = complete response; IHP = isolated hepatic perfusion; IHHP = isolated hypoxic hepatic perfusion; PHP = percutaneous hepatic perfusion; N/A = Not available.
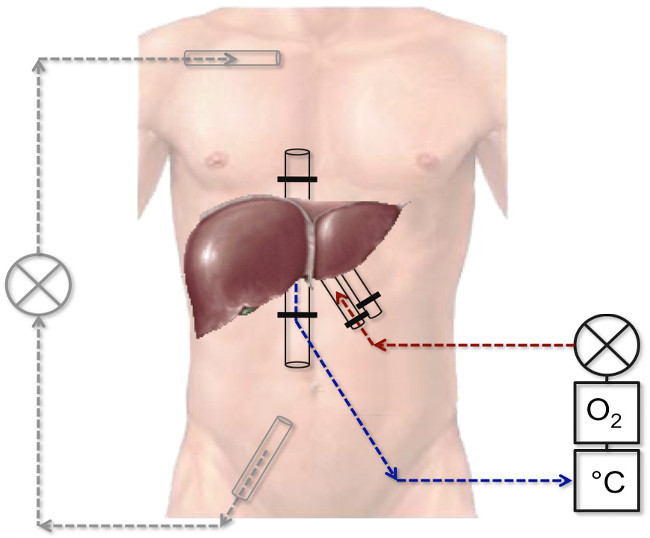 Figure 1: Schematic picture of the veno-venous bypass system and the perfusion system. The red line represents the catheter in the hepatic artery and the blue one represents the catheter in the caval vein. They are connected to a perfusion system consisting of an oxygenator, a roller pump and a heating unit. The black lines represent the veno-venous bypass system connected to a roller pump.
Figure 1: Schematic picture of the veno-venous bypass system and the perfusion system. The red line represents the catheter in the hepatic artery and the blue one represents the catheter in the caval vein. They are connected to a perfusion system consisting of an oxygenator, a roller pump and a heating unit. The black lines represent the veno-venous bypass system connected to a roller pump.
Discussion
Patients with metastatic uveal melanoma have few therapeutic options. Chemotherapy 6, immunotherapy 23 and targeted therapy 24 have not been able to show improved OS. For patients with few liver metastases, liver resection is an option 12. Other treatments include hepatic intra-arterial chemotherapy (HIA) 9, transarterial chemoembolization (TACE) 25 and selective internal radiation therapy (SIRT) 26.
IHP is a major and complex surgical intervention and since the first report by Ausman there have been many developments in the surgical technique. In 2004, van Etten and colleagues reported the first clinical results using the isolated hypoxic hepatic perfusion (IHHP) technique 27. In the early 1990s, three independent groups developed a novel percutaneous hepatic perfusion (PHP) system using extracorporeal chemofiltration 28-30. No studies comparing these techniques to IHP have been performed. The reports so far do not seem to be in favor of any specific technique.
Patient selection is of high importance for this treatment. Since it is a local treatment, there should be no evidence of extrahepatic disease. Due to the risk of developing hepatic failure, no more than 50 % of the liver volume should be replaced by tumour 22. Patients should have a good performance status (ECOG 0-1) and no heart-, lung-, kidney- or liver disease that could increase the risk of severe morbidity after surgery.
In a recent phase II trial from our group, a median survival of 26 months was shown, implying a potential survival benefit of 14 months compared with a control group 16. To verify this finding, a multicenter randomized phase III trial between IHP and Best Alternative Care (BAC) has recently started in Scandinavia (the SCANDIUM trial, ClinicalTrials number: NCT01785316). The BAC treatment may include all other treatment options, either existing or experimental, with the notion that cross-over is not allowed from the BAC to IHP group. Inclusion criteria include biopsy verified liver metastases and no evidence of extra-hepatic tumor manifestations by PET-CT. The primary endpoint is overall survival at 24 months with secondary endpoints including response rate, progression-free survival and quality of life. The planned inclusion is 78 patients during 5 years.
In summary, there still exists no treatment that in phase III trials has shown any prolonged overall survival for patients with uveal melanoma liver metastases. IHP is an interesting and promising regional therapy where potential benefits in survival are currently being investigated in a randomized trial (the SCANDIUM trial).
Disclosures
The authors have nothing to disclose.
Acknowledgments
Lennart Wiman (photographer) is acknowledged for excellent video recording.
References
- Virgili G, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114(12):2309–2315. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Pereira PR, et al. Current and emerging treatment options for uveal melanoma. Clinical Ophthalmology. 2013;7:1669–1682. doi: 10.2147/OPTH.S28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman L, Nilsson B, Lundell G, Lundell M, Seregard S. Ruthenium brachytherapy for uveal melanoma, 1979-2003: survival and functional outcomes in the Swedish population. Ophthalmology. 2005;112(5):834–840. doi: 10.1016/j.ophtha.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Diener-West M, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Archives of Ophthalmology. 2005;123(12):1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Investigative Ophthalmology & Visual Science. 2003;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- Bedikian AY, Papadopoulos N, Plager C, Eton O, Ring S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Research. 2003;13(3):303–306. doi: 10.1097/00008390-200306000-00013. [DOI] [PubMed] [Google Scholar]
- Kivela T, et al. vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. European Journal Of Cancer. 2003;39(8):1115–1120. doi: 10.1016/s0959-8049(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Schmittel A, et al. Phase II trial of cisplatin, gemcitabine and treosulfan in patients with metastatic uveal melanoma. Melanoma Research. 2005;15(3):205–207. doi: 10.1097/00008390-200506000-00010. [DOI] [PubMed] [Google Scholar]
- Leyvraz S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Annals Of Oncology : Official Journal Of The European Society for Medical Oncology / ESMO. 2014;25(3):742–746. doi: 10.1093/annonc/mdt585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert PE, et al. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. European Journal Of Radiology. 2010;74(3):38–44. doi: 10.1016/j.ejrad.2009.03.064. [DOI] [PubMed] [Google Scholar]
- Klingenstein A, Haug AR, Zech CJ, Schaller UC. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovascular and Interventional Radiology. 2013;36(1):158–165. doi: 10.1007/s00270-012-0373-5. [DOI] [PubMed] [Google Scholar]
- Mariani P, et al. Surgical management of liver metastases from uveal melanoma: 16 years' experience at the Institut Curie. European Journal Of Surgical Oncology : The Journal Of The European Society Of Surgical Oncology And The British Association of Surgical Oncology. 2009;35(11):1192–1197. doi: 10.1016/j.ejso.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Ausman RK, Aust JB. Isolated perfusion of the liver with HN2. Surgical Forum. 1960;10:77–79. [PubMed] [Google Scholar]
- Dahl O. Interaction of hyperthermia and chemotherapy. Recent results in cancer research. Fortschritte der Krebsforschung. Progres Dans Les Recherches Sur Le Cancer. 1988;107:157–169. doi: 10.1007/978-3-642-83260-4_23. [DOI] [PubMed] [Google Scholar]
- Barker WC, Andrich MP, Alexander HR, Fraker DL. Continuous intraoperative external monitoring of perfusate leak using iodine-131 human serum albumin during isolated perfusion of the liver and limbs. European Journal Of Nuclear Medicine. 1995;22(11):1242–1248. doi: 10.1007/BF00801607. [DOI] [PubMed] [Google Scholar]
- Olofsson R, et al. Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit. Annals of Surgical Oncology. 2014;21(2):466–472. doi: 10.1245/s10434-013-3304-z. [DOI] [PubMed] [Google Scholar]
- Redai I, Emond J, Brentjens T. Anesthetic considerations during liver surgery. The Surgical Clinics of North America. 2004;84:401–411. doi: 10.1016/S0039-6109(03)00229-9. [DOI] [PubMed] [Google Scholar]
- Sakai T, et al. Insertion and management of percutaneous veno-venous bypass cannula for liver transplantation: a reference for transplant anesthesiologists. Clinical Transplantation. 2010;24(5):585–591. doi: 10.1111/j.1399-0012.2009.01145.x. [DOI] [PubMed] [Google Scholar]
- Skandalakis JE, Skandalakis LJ, Skandalakis PN, Mirilas P. Hepatic surgical anatomy. The Surgical Clinics of North America. 1016;84(2):413–435. doi: 10.1016/j.suc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Alexander HR, et al. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clinical Cancer Research : An Official Journal Of The American Association for Cancer Research. 2000;6(8):3062–3070. [PubMed] [Google Scholar]
- Alexander HR, Jr , et al. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clinical Cancer Research : An Official Journal Of The American Association For Cancer Research. 2003;9(17):6343–6349. [PubMed] [Google Scholar]
- Rizell M, et al. Isolated hepatic perfusion for liver metastases of malignant melanoma. Melanoma research. 2008;18(2):120–126. doi: 10.1097/CMR.0b013e3282f8e3c9. [DOI] [PubMed] [Google Scholar]
- Iersel LB, et al. Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases. Annals of Surgical Oncology. 2008;15(7):1891–1898. doi: 10.1245/s10434-008-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal RD, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA : the Journal Of The American Medical Association. 2014;311(23):2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KV, et al. Hepatic arterial chemoembolization for management of metastatic melanoma. AJR. American Journal Of Roentgenology. 2008;190(1):99–104. doi: 10.2214/AJR.07.2675. [DOI] [PubMed] [Google Scholar]
- Eldredge-Hindy H, et al. Yttrium-90 Microsphere Brachytherapy for Liver Metastases From Uveal Melanoma: Clinical Outcomes and the Predictive Value of Fluorodeoxyglucose Positron Emission Tomography. American Journal Of Clinical Oncology. 2014. [DOI] [PMC free article] [PubMed]
- Etten B, et al. Isolated hypoxic hepatic perfusion with orthograde or retrograde flow in patients with irresectable liver metastases using percutaneous balloon catheter techniques: a phase I and II study. Annals of Surgical Oncology. 2004;11(6):598–605. doi: 10.1245/ASO.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Ku Y, et al. Extracorporeal adriamycin-removal following hepatic artery infusion: use of direct hemoperfusion combined with veno-venous bypass. Nihon Geka Gakkai Zasshi. 1989;90(10):1758–1764. [PubMed] [Google Scholar]
- Curley SA, et al. Hepatic arterial infusion chemotherapy with complete hepatic venous isolation and extracorporeal chemofiltration: a feasibility study of a novel system. Anti-Cancer Drugs. 1991;2(2):175–183. doi: 10.1097/00001813-199104000-00008. [DOI] [PubMed] [Google Scholar]
- Beheshti MV, et al. Percutaneous isolated liver perfusion for treatment of hepatic malignancy: preliminary report. Journal of Vascular And Interventional Radiology : JVIR. 1992;3(3):453–458. doi: 10.1016/s1051-0443(92)71988-5. [DOI] [PubMed] [Google Scholar]


