Abstract
Research in anatomy, embryology, and developmental biology has largely relied on the use of model organisms. In order to study development in live embryos model organisms, such as the chicken, are often used. The chicken is an excellent model organism due to its low cost and minimal maintenance, however they present observational challenges because they are enclosed in an opaque eggshell. In order to properly view the embryo as it develops, the shell must be windowed or removed. Both windowing and ex ovo techniques have been developed to assist researchers in the study of embryonic development. However, each of the methods has limitations and challenges. Here, we present a simple, optimized ex ovo culture technique for chicken embryos that enables the observation of embryonic development from stage HH 19 into late stages of development (HH 40), when many organs have developed. This technique is easy to adopt in both undergraduate classes and more advanced research laboratories where embryo manipulations are conducted.
Keywords: Developmental Biology, Issue 95, Shell-less culture, microscopy, chicken embryos, manipulations, bead implantation, culturing, methodology
Introduction
Ex ovo culturing has played an important role in the study of development of the chicken1, 2. This culturing method has been used to study neurological diseases, limb development, craniofacial development, and as a model to investigate malformations associated with diabetes 3, 4, 5.
There are many variations to the ex ovo technique. The most common approach is to use a Styrofoam cup6,7,8 or a glass bowl5. In these methods, the cup or bowl is lined with plastic wrap to cradle the embryo, a lid is placed on the cup, and the embryo is then placed in an incubator with appropriate humidity6. This set up however, can be technically challenging. The first challenge is the plastic wrap that is used to cradle the embryo. This wrap is difficult to work with and often does not adhere to the cup very well. To solve this problem, an elastic band is placed around the cup to hold the wrap in place. Despite this, the wrap can still slip, which is fatal to the embryo. The plastic wrap has the potential to tear or get punctured by forceps or needles that may be used during embryo manipulations and observations. Finally, this set-up is not very stable and students can easily knock the cups over. The height of the cups also makes it very difficult to place the embryo under a stereomicroscope, which has a limited objective to stage height. These challenges make it difficult for undergraduate students to work with live chick embryos in teaching labs, such as advanced developmental biology courses.
The above challenges in the ex ovo method has meant that researchers turn to the windowing method 9,10 to view embryonic chick development. In this technique, a hole or “window” is made in the eggshell overlying the embryo. The hole can be re-sealed with tape or wax9 to allow for further embryonic development. Although the windowing method has some advantages, such as the ability to view embryonic development and easy maintenance, this method also has several limitations. The first is that the window needs to be fairly large in order to view the entire embryo (especially at late stages). Secondly, large windows are difficult to seal; an improper seal will lead to sterility and survivability problems. Using molten wax as a sealant adds another inconvenient and messy step to the protocol. Therefore, although the windowing method may be ideal for chick embryos at young stages (HH 11 – HH 27), viewing the entire embryo at late stages is not easily accomplished.
Here, we describe an improved and simple ex ovo culturing technique11 that avoids the need for high tech equipment, is easy to handle under a stereomicroscope, gives the embryo enough support to perform microscopic manipulations, and enables researchers to view the growth of the embryo in its entirety well into the later stages of development (up to HH 40-41). With these advances in the ex ovo technique, individuals gain access to a more complete understanding of embryonic development. For instance, growth into later stages allows individuals to observe developmental processes that do not occur until this time point, such as ossification, feather development, and advanced limb and eye development. The entire embryo and extraembryonic membranes and vasculature are clearly visible. More advanced research can also be performed, such as, embryonic manipulations (i.e., implanting beadssoaked in inhibitors or inserting barriers between tissue layers), and researchers are then able to observe the effect of the manipulations in later stage embryos.
Protocol
Note: All supplies are listed in Table 1.
1. Storing the Chicken Embryos
Incubate chicken eggs of the strain Gallus gallus horizontally at 37oC with approximately 40% humidity and turn eggs once or twice daily. Turning eggs is important to prevent the embryo from adhering to the eggshell.
Do not to turn the egg in the 24 hr prior to setting up the culture as otherwise the embryo will be located ventral to the yolk mass and will be damaged on opening the egg in step 3. In addition, keep the eggs at 4oC degrees for no more than one week before incubation to “halt” development but this is not ideal.
2. Staging Chicken Embryos
Stage the chicken embryos using the Hamburger and Hamilton12 staging table. The ideal stage for setting up the ex-ovo culturing is HH stage 19-20 (around 3- days of incubation) because this is shortly after the head turns at 53 hpf. Note: HH stage 19-20 is characterized by the following morphological traits: somites are extended into the majority of the tail, however the very end of the tail remains unsegmented, the tail bud is curled, the allantois is small and has limited vasculature, the leg-buds are larger than the wing-buds and the eyes are unpigmented or have a grayish hue.
3. Removing the Embryo from the Shell
Before removing the embryo from the shell, spray the shell with 70% ethanol and allow it to dry. Do not turn the egg rapidly as this will damage the embryo.
Being careful not to alter the orientation of the egg, carefully crack the egg on the lower side (i.e., the side that was ventral during incubation) and release the embryo into a sterile weigh boat (88 x 88 x 23 mm). Wipe down weigh boats with 70% ethanol. Inspect the embryo to ensure that the yolk sack is not damaged. If the yolk has broken, discard the embryo, as it will not survive.
Observe the embryo to ensure that it is viable; the heart is beating, vasculature appears normal, and no obvious abnormalities are present. Ensure that the edges of the weigh boat are dry.
Using a pipette, drop 40 μl of penicillin/streptomycin (5,000 units penicillin, 5 mg streptomycin per ml) on top of the albumin to help prevent infection.
4. Preparing the Humidity Chamber
Place a small stack of kim-wipes and/or an absorbent pad made of cotton in the bottom of a sterile plastic container (12 x 12 x 6 cm) wiped with 70% ethanol.
Add sterile, distilled water to moisten the kim-wipes and/or cotton (approximately 150 ml of water).
5. Assembling the Ex Ovo Culture
Place the weigh boat containing the embryo on top of the moist padding. Then, place half of a square sterile petri-dish (9.5 x 9.5 cm) on top of the weigh boat to form a loose lid.
Cover the plastic container with its lid. Press down two corners of the lid, ensuring a partial seal that still allows for good airflow inside the chamber.
Carefully place the setup into the 37oC incubator until desired stage.
Place containers of water in the incubator to help control the humidity, if a controlled humidity incubator is unavailable. Sterilize all water in the humidity chambers and in the incubator and replenish as needed during the incubation period. 40% humidity is ideal.
Representative Results
This ex ovo method allows for the observation of embryos from early stages of development (HH 19/20) to late stages of development (HH 40-41) (Figure 1A and 1B). Setting up the culture at HH 19-20 increases survivability of the embryos in the culture. Prior to the head turning (before 53 hpf) survivability is very low in culture and after stage 21, the embryo tends to stick more to the shell on removal so fewer intact embryos are obtained. In general, survivability of the embryo in ex ovo culture is high up to HH 35-36 (90-100%), but it does drop in the more advanced stages of development (about 40% survive to HH 40-41). Additionally, although it has been shown that development appears normal at these late stages and patterning of the skeleton is unaffected 4,15, we have observed that the limbs are bent at these late stages suggesting that the degree or timing of ossification may be affected. This is not surprising considering the need for calcium from the shell for ossification. A detailed comparison of the development of the embryo compared to controls is underway and is beyond the scope of this methods paper.
Obtaining access to the embryo throughout development is important for two main reasons. First, researchers are able to view development on a daily basis without having to unseal and reseal a window. For example, individuals are able to observe limb and eye development at multiple stages (Figure 1C and 1D). Secondly, ex ovo culturing enables greater (unrestricted) access to the embryo, thereby enabling easier manipulations or surgeries.
Embryonic manipulations most often, need to be performed using a stereomicroscope. Using the ex ovo culturing method described here as opposed to the Styrofoam/plastic wrap method6,7,8 the embryo set-up is easily placed under the microscope, is less deep and will not tip over. This enables the researcher to position the humidity chamber precisely as needed and to rotate it to optimize access to the embryo, since there are no barriers (egg shell) obscuring the embryo and since the chamber is very stable. Overall, the support the embryo receives from the weigh boat allows gentle pressure to be applied to the embryo during manipulation without the set-up toppling over.
Several developmental biology methods can be conducted on the embryos in this culture system. These include detailed observations of organ (e.g., eye, brain etc) or tissue development (e.g., blood vessel growth) or application of teratogenic agents. Manipulations include grafting of tissues onto the chorioallantoic membranes to study the effect of combining different tissue layers or studying tumor growth and metastasis14. Another popular manipulation performed during development is bead or barrier implantation. Bead implantation allows a researcher to soak an inhibitor or factor onto the bead and then implant the bead locally to inhibit or induce genes locally in the area of interest. This is often done using affigel or heparin beads (Figure 2A). For example, bone morphogenetic proteins (BMPs) can be locally inhibited during the induction of neural crest derived bone4, 15, or in the developing limb bud13. After a bead is implanted, the embryo can be returned to the incubator and it will continue to develop. This allows researchers to view the downstream effects of the inhibition of a particular gene (in this case BMP), such as the inhibition of scleral ossicle formation in the sclerotic ring (Figure 2B and 2C)15. Similarly, barriers can easily be inserted between tissue layers in order to study tissue-to-tissue signaling and the microinjection of dyes can also easily be conducted.
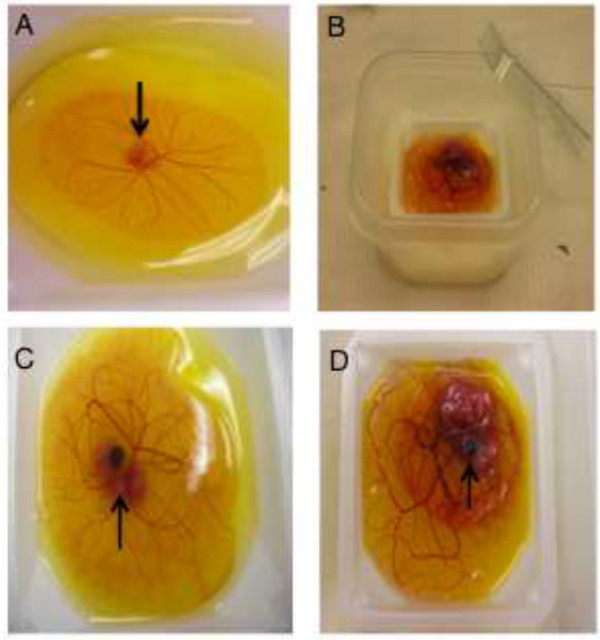 Figure 1. Viewing the embryo ex ovo.
(A) HH stage 20 chick embryo after removal from the shell, arrow points to the embryo with vascularized embryonic membranes. (B) A HH stage 40 embryo in an open humidity chamber. (C) HH stage 35 embryo showing early limb buds (arrow). (D) Stage HH 41 embryo showing later stage development of structures such as the eye (arrow). Please click here to view a larger version of this figure.
Figure 1. Viewing the embryo ex ovo.
(A) HH stage 20 chick embryo after removal from the shell, arrow points to the embryo with vascularized embryonic membranes. (B) A HH stage 40 embryo in an open humidity chamber. (C) HH stage 35 embryo showing early limb buds (arrow). (D) Stage HH 41 embryo showing later stage development of structures such as the eye (arrow). Please click here to view a larger version of this figure.
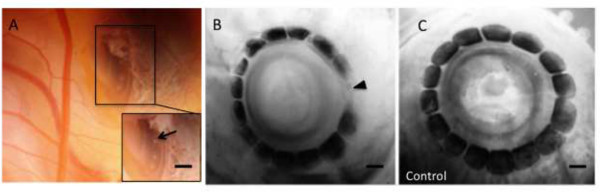 Figure 2. Example of Noggin soaked bead Implantation Experiment4.
(A) Embryo HH stage 35 exposed through a small hole torn in the overlying membranes in order to gain access to the underlying eye for bead implantation. High magnification of the affigel bead adjacent to a conjunctival papilla is shown in inset. Scale bar in C is 75 μm. (B) Alkaline phosphatase stained right eye after Noggin bead implantation (arrow) showing gap in the ring of ossicles, HH 38 (C) AP stained left eye showing no disruption to the ring of ossicles (control), HH 38. Details of this experiment are provided in Duench and Franz-Odendaal4. Please click here to view a larger version of this figure.
Figure 2. Example of Noggin soaked bead Implantation Experiment4.
(A) Embryo HH stage 35 exposed through a small hole torn in the overlying membranes in order to gain access to the underlying eye for bead implantation. High magnification of the affigel bead adjacent to a conjunctival papilla is shown in inset. Scale bar in C is 75 μm. (B) Alkaline phosphatase stained right eye after Noggin bead implantation (arrow) showing gap in the ring of ossicles, HH 38 (C) AP stained left eye showing no disruption to the ring of ossicles (control), HH 38. Details of this experiment are provided in Duench and Franz-Odendaal4. Please click here to view a larger version of this figure.
Discussion
Ex ovo culturing and windowing both have advantages and challenges. Here we compare the advantages and challenges of the Styrofoam cup ex ovo method and the windowing method to our optimized ex ovo method shown here. Our method enables manipulation and easy observation of the chick embryo at late stages of development and our refinements to the traditional ex ovo method1, 2, 3 make it additionally very easy to use in undergraduate teaching laboratory classes.
Although many researchers prefer the windowing method to study chicken development because it is simple to perform and has excellent survivability to late stages of development (HH 41-42), it has limitations. The largest of which is the limited ability to view the entire embryo after HH stage 30. After stage HH 30, the window in the eggshell needs to be very large in order to view the growing, moving embryo. This large window is difficult to seal completely (leading to problems with sterility and survivability), and obtaining good lighting inside the egg can be challenging. In addition, advanced stage embryos are large and difficult to access (or manipulate) through the window.
The ex ovo method described here provides full uninhibited access to the embryo. Once the weigh boat is placed in the humidity chamber, the entire set-up is very stable. The entire humidity chamber can be placed under a dissecting microscope and high magnification can be used to observe the entire embryo. In comparison, the Styrofoam cup method has a humidity chamber that is tall (cup height) and this makes it very difficult to place under the microscope objectives. The cup design is also far less stable than the flat weigh boat and these cups can easily be knocked over. Our improvements to the ex ovo method make this method straightforward to use in a classroom or lab setting and enables the student to have full access to embryos from stage HH 19 through to HH 40; stages when major organogenesis is occurring. The biggest disadvantage of our ex ovo method is sterility. In order to prevent infection, our method includes the addition of antibiotics. Another dose of antibiotic can be given to the embryo at later stages. The antibiotics administered during culture set-up also does not appear to affect development of the embryo. Sterilizing containers with 70% ethanol prior to use can dramatically increase survivability.
Overall, the ex ovo method is ideal for viewing and manipulating embryos without limitations on access or stage of development. Previous research has shown that culturing the embryos ex ovo does not negatively affect patterning4,15. For example, we do not see any changes in the patterning or induction of the skeleton despite the absence of the eggshell. Our improved method solves multiple challenges that exist with the current traditional ex ovo method1,2, 3.
Disclosures
The authors have no competing financial interests in regards to the information presented in this manuscript.
Acknowledgments
We would like to thank Paul Poirier, the Media Producer, at Mount Saint Vincent University for his work in filming and editing the video portion of this manuscript. We acknowledge the Natural Science and Engineering Research Council of Canada for funding.
References
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev. Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- Gennaro LD, Packard DS, Stach RW, Wagner BJ. Growth and differentiation of chicken embryos in simplified shell-less cultures under ordinary conditions of incubation. Growth. 1980;44:343–354. [PubMed] [Google Scholar]
- Tufan CA, Akdogan I, Adiguzel E. Shell-less culture of the chick embryo as a model system in the study of developmental neurobiology. Neuroanat. 2004;3:8–11. [Google Scholar]
- Duench K, Franz-Odendaal TA. BMP and Hedgehog signaling during the development of scleral ossicles. Dev. Biol. 2012;365(1):251–258. doi: 10.1016/j.ydbio.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Datar S, Bhonde RR. Shell-less Chick Embryo Culture as an Alternative in vitro Model to Investigate Glucose-Induced Malformation in Mammalian Embryos. Rev Diabet Stud. 2005;2(4):221–227. doi: 10.1900/RDS.2005.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CJ, editor. Chick embryos in shell-less culture. Tested studies for laboratory teaching. 5th Workshop/Conference of the Association for Biology Laboratory Education (ABLE).1983. [Google Scholar]
- Dunn BE. Technique for shell-less culture of the 72-hour avian embryo) Poultry Science. 1974;53:409–412. doi: 10.3382/ps.0530409. [DOI] [PubMed] [Google Scholar]
- Yalcin H, Shekhar A, Rane AA, Butcher JT. An ex-ovo chicken embryo culture system suitable for imaging and microsurgery applications. J. Vis. Exp. 2010. [DOI] [PMC free article] [PubMed]
- Silver PHS. Special problems of experimenting in ovo on the early chick embryo, and a solution. J Embryol Exp Morph. 1960;8(4):369–375. [Google Scholar]
- Spurlin J, Lwigale P. A technique to increase accessibility to late-stage chick embryos for in ovo manipulations. Dev. Dyn. 2012;242(2):148–154. doi: 10.1002/dvdy.23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, et al. Ex ovo model for directly visualizing chicken embryo development. American Biology Teacher. 2012;74(9) [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Drossopoulou G, et al. A model for anteroposterior patterning of the vertebrate limb based on sequential long-and short-range Shh signaling and Bmp signaling. Development. 2000. pp. 127–1337. [DOI] [PubMed]
- Sys GM, et al. The in ovo CAM-assay as a xenograft model for sarcoma. J. Vis. Exp. 2013. p. e50522. [DOI] [PMC free article] [PubMed]
- Franz-Odendaal T. Towards understanding the development of scleral ossicles in chicken, Gallus gallus. Dev. Dyn. 2008;237:3240–3251. doi: 10.1002/dvdy.21754. [DOI] [PubMed] [Google Scholar]


