Abstract
Illumination engineering is critical for obtaining high-resolution, high-quality images in microscope settings. In a typical microscope, the condenser lens provides sample illumination that is uniform and free from glare. The associated condenser diaphragm can be manually adjusted to obtain the optimal illumination numerical aperture. In this paper, we report a programmable condenser lens for active illumination control. In our prototype setup, we used a $15 liquid crystal display as a transparent spatial light modulator and placed it at the back focal plane of the condenser lens. By setting different binary patterns on the display, we can actively control the illumination and the spatial coherence of the microscope platform. We demonstrated the use of such a simple scheme for multimodal imaging, including bright-field microscopy, darkfield microscopy, phase-contrast microscopy, polarization microscopy, 3D tomographic imaging, and super-resolution Fourier ptychographic imaging. The reported illumination engineering scheme is cost-effective and compatible with most existing platforms. It enables a turnkey solution with high flexibility for researchers in various communities. From the engineering point-of-view, the reported illumination scheme may also provide new insights for the development of multimodal microscopy and Fourier ptychographic imaging.
OCIS codes: (170.2945) Illumination design, (170.0180) Microscopy, (170.3010) Image reconstruction techniques, (100.3190) Inverse problems
1. Introduction
The condenser lens system is a ubiquitous component of conventional microscope platforms for uniform sample illumination. It typically consists of a high numerical aperture (NA) condenser lens and a condenser diaphragm placed at the back focal plane of the lens [1]. This condense diaphragm allows for manual adjustment of the optimal illumination aperture, which defers with different microscopy techniques. In bright-field microscopy, the illumination NA needs to be matched to the collection NA by adjusting the size of the condenser diaphragm. In dark-field microscopy, an aperture stop is placed at the condenser diaphragm to ensure the illumination NA is larger than the collection NA. In phase-contrast microscopy, a ring aperture is placed at the condenser diaphragm to match to the ring-shape phase plate of the objective lens. In short, each microscopy technique requires vastly different condenser illumination. Currently, these requirements are met by physical adjustment of condenser diaphragms and, in some cases, a need for specialized condenser apertures. However, with the maturity of liquid crystal display in consumer electronics, there exists an opportunity for cost-effective, active digital control of the illumination system.
In this paper, we report the use of a $15 liquid crystal display to achieve programmable condenser illumination control. In our prototype setup, we placed the display at the back focal plane of the condenser lens. By setting different binary patterns on the display, we can actively control the illumination and the spatial coherence of the microscope platform. To demonstrate the versatility of the reported scheme, we use the prototype platform for multimodal microscopy imaging, including bright-field microscopy, darkfield microscopy, polarization microscopy, phase-contrast microscopy [2, 3], 3D tomographic imaging [4], and super-resolution Fourier ptychographic imaging [5]. Essentially, the liquid crystal display (with the back light removed) severs as a transparent spatial light modulator (SLM) in the reported scheme. The use of SLM in microscopy has drawn much attention in recent years [6]. However, in these techniques, the SLMs are placed in the detection path to modulate the pupil function or to project intensity patterns onto the sample. To the best of our knowledge, this is the first report of the use of an SLM for the modulation of the condenser illumination. Although the active illumination control for microscopy setting using an LED array have been reported before [4, 5, 7–10], the technique we describe here has some important advantages over the previous demonstrations: 1) it is cost-effective and is compatible with most existing compound microscopes. The only modification required is the addition of a low-cost liquid crystal display at the condenser diaphragm. 2) The liquid crystal display provides a large degree of freedom for illumination engineering. As a reference, a typical liquid crystal display used for consumer electronics provides more than 400 pixels per inch, which is the equivalent of 800 by 800 pixels over a condenser diaphragm of ~2 inches. This provides orders of magnitudes improvement in degrees of freedom, over the previously demonstrated LED array approach, for controlling spatial coherence and microscope illumination. 3) The illumination intensity of the reported scheme is determined by the light source of the microscope platform. We can use one or multiple high-power light sources to increase the photon budget. For the LED array approach, it is difficult to increase the illumination power since it scales with the size of LED elements. 4) In the reported scheme, the illumination from the condenser lens is a plane wave modulated by the active liquid-crystal-display aperture. In contrast, the previously demonstrated LED approach essentially provides an array of spherical wave illumination, necessitating a plane wave approximation of splitting the entire image into small tiles [5]. 5) Finally, the intensity of the light source in the reported scheme does not fluctuate as we set different patterns on the display. For the LED array approach, one needs to calibrate for the intensity differences between LED elements and the intensity fluctuations over time. In addition, engineering the condenser aperture using a liquid crystal display is more efficient when illuminating the sample at a large incident angle. For the LED array approach, no lens is placed between the LED array and the sample, and as such, less than 8% of the LED emission from the edge of the array can be delivered to the sample.
In summary, the reported illumination-engineering scheme provides a turnkey solution with high flexibility for researchers in various communities. From the engineering point-of-view, it may also provide new directions for the development of multimodal microscopy including the recently developed Fourier ptychographic imaging approach. This paper is structured as follows: in section 2, we will present the prototype setup of the reported illumination scheme. In section 3, we will demonstrate the use the reported scheme for multimodal microscopy. Finally, we will summarize the results and discuss future directions.
2. Illumination engineering using a liquid crystal display
The reported illumination-engineering scheme is shown in Fig. 1(a) , where a low-cost liquid crystal display is used as a transparent SLM and placed at the back focal plane of the condenser lens. By showing different binary patterns on the display, we can achieve different microscopy imaging modalities, as shown in Fig. 1(b).
Fig. 1.
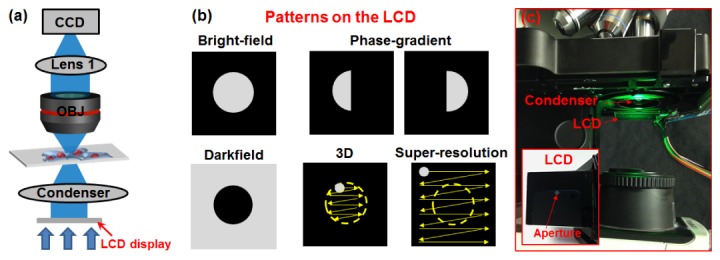
(a) The reported scheme using a low-cost liquid crystal display at the condenser diaphragm. (b) Different patterns can be displayed for achieving different microscopy modalities. (c) The experimental setup with a green LED as the light source. A $15 liquid crystal display (with back light removed) is placed at back-focal plane of the condenser lens. Media 1 (463.3KB, MP4) shows the different patterns on the display.
For bright field microscopy, we can display a circular pattern as shown in Fig. 1(b), where the pixel transmission is turned off outside the circle. The diameter of the pattern can be adjusted to match to different NAs of the objective lenses. Such an adjustment process is similar to adjusting the size of condenser diaphragm in conventional microscope platforms. However, in the reported scheme, this process is performed without any mechanical switching. Similar to the bright-field microscopy, we can also display a complementary pattern for darkfield microscopy. In this case, the pixel transmission was turned off within the circle. As such, no direct transmission light is able enter the objective lens. This darkfield imaging process is similar to adding a darkfield aperture stop at the condenser diaphragm. We also note that, due to the use of liquid crystal display, the illumination is polarized in the reported platform. We can, therefore, place another polarizer with a different orientation at the detection path to achieve polarization imaging modality.
A more interesting microscopy modality is the phase contrast (or phase gradient) imaging. In the reported scheme, we can display two complementary semicircular patterns at the liquid crystal display (Fig. 1(b)) and capture two images I1 and I2 using conventional objective lenses. The phase contrast image of the sample can then be recovered by 2(I1-I2)/(I1 + I2) [3, 7]. This phase-contrast imaging modality is similar to the scanning differential phase contrast system reported in [11] where a split-detector or a quadrant diode is placed in the Fourier plane of the collector and the image is formed by subtracting intensities recorded by two halves of the detector. The phase-contrast imaging scheme demonstrated here is a reciprocal system by placing the semicircular aperture stop in the condenser diaphragm instead of the Fourier plane. We also note that, in conventional phase contrast microscopy, one needs to place a ring-aperture at the condenser diaphragm to match the phase plate ring in the phase contrast objective lens. In the reported scheme, we can simply show a ring pattern on the liquid crystal display where the pixel transmission is turned off outside the ring pattern.
The reported scheme can also be used to perform 3D tomographic imaging, which was previously demonstrated by our group [4]. In the reported scheme, we can simply set a scanning aperture pattern on the liquid crystal display (Fig. 1(b)). For each position of the aperture, the illumination is a plane wave with an oblique incident angle. Therefore, by showing a scanning aperture on the display, we effectively illuminate the sample with different incident angles. With the captured images, we can perform 3D tomographic reconstruction to recover images at different sections. We note that, this imaging modality requires the direct transmission light enters the collection optics. Thus, the scanning aperture is restricted within the NA of the collection optics, i.e., the yellow circle in Fig. 1(b).
Lastly, we can also use the reported scheme for super-resolution Fourier ptychographic imaging, a recently developed computational imaging approach [5]. In brief, FP illuminates the sample with different oblique incident angles and captures the corresponding intensity images using a low-NA objective lens. The captured images are then judicially combined in the Fourier domain to recover a high-pixel-count sample image that surpasses the diffraction limit of the employed optics [5, 12–18]. The recovery process of FP switches between the spatial and the Fourier domain. In the spatial domain, the captured images are used as the intensity constraint for the solution. In the Fourier domain, the confined pupil function of the objective lens is used as the support constraint for the solution. This pupil function constraint is digitally panned across the Fourier space to reflect the angular variation of its illumination. In the reported scheme, we can simply show a scanning aperture across the liquid crystal display (Fig. 1(b)). In contrast to the 3D imaging case, the illumination NA here is larger than the collection NA to enable super resolution imaging. Therefore, the scanning aperture is not restricted by the NA of the objective lens, as shown in Fig. 1(b).
The experimental setup of reported scheme is shown in Fig. 1(c). In this platform, we used a conventional microscope platform (Olympus CX41) with a low-cost liquid crystal display (1.8 inch, 160 by 128 pixels, Amazon, $15). We removed the backlight of the display and used it as a transparent SLM. As shown in Media 1 (463.3KB, MP4) , we used a micro-controller for showing different binary patterns on the display. To build the prototype platform, we only need to place the display at the back focal plane of the condenser lens, as shown in Fig. 1(c). No other modification is needed. Therefore, the reported platform provides a turnkey solution for microscopy users in different communities and settings.
3. Multimodal imaging demonstration using the reported platform
Here, we demonstrate the versatility of the reported scheme for multimodal microscopy imaging. Figure 2(a) and 2(b) show the bright-field and dark-field images of a starfish embryo sample. We note that, for the dark-field image in Fig. 2(b), we capture a reference image by setting the display to the ‘off state’ and subtract this reference image to enhance the contrast. Figure 2(a1)-2(a3) shows bright field images with different illumination NAs, corresponding to different degrees of the spatial coherence. Figure 2(c1)-2(c2) show the phase gradient (contrast) images along different directions for the same sample. For each of these phase contrast images, we captured two raw images corresponding to the two complementary half-circular patterns at the display, and processed them as discussed in the previous section. Figure 2(d) (cotton fibers) show the polarization microscopy images by adding a polarizer at the detection path. In Fig. 2(d1), the orientation of the added polarizer is the same as the liquid crystal display. In Fig. 2(d2), we rotated the polarizer by 90 degree and the sample contrast comes from the rotation of the polarized light. We used a 10X, 0.25 objective lens for Fig. 2.
Fig. 2.
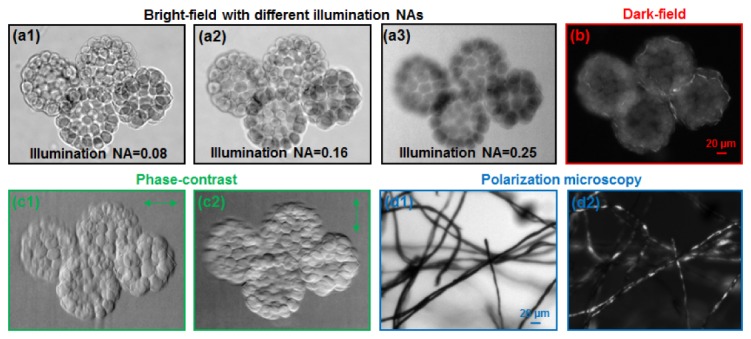
(a) Bright-field, (b) Dark-field, (c) Phase-contrast imaging using reported scheme. (c1) and (c2) shows the phase gradient images along two different directions. (d) Polarization microscopy images using an added polarizer at the detection path.
Figure 3 shows the 3D tomographic imaging capability of the reported platform. In this experiment, we captured 49 images by showing a scanning aperture pattern on the display. We used a 10X, 0.25 objective lens in this demonstration. We then used the captured images to recover images at different sections. The reconstruction process is the same as tomographic reconstruction reported before [4]. From Fig. 3, we can see that different parts of the starfish embryo sample are in-focus at different recovered sections. Media 2 (4.8MB, MP4) shows the entire digital refocusing process from −40 µm to + 40 µm.
Fig. 3.
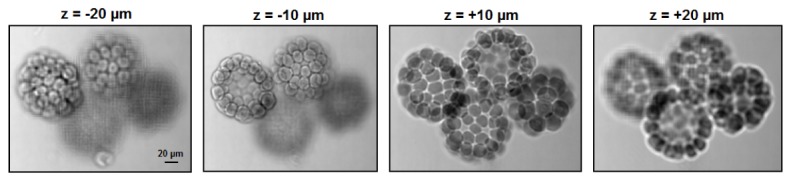
3D tomographic reconstruction using the reported scheme. We captured 49 images by presenting a scanning aperture at the transparent liquid crystal display. These images are used to recover sample images at different sections. Media 2 (4.8MB, MP4) shows the entire digital refocusing process from −40 µm to + 40 µm.
Lastly, we also tested the reported platform for super-resolution Fourier ptychographic microscopy. The image acquisition process is similar to that of the 3D tomographic imaging case. However, in this case, the illumination NA needs to be larger than the collection NA to achieve the super-resolution imaging capability. In our implementation, we captured 121 raw images corresponding to a scanning aperture pattern at different positions on the display. We used a 4X, 0.1 NA objective in the acquisition process and the captured images were then synthesized in the Fourier domain to increase the synthetic NA to ~0.5. Figure 4 (a1) shows the raw image of an USAF resolution target and Fig. 4(a2) shows the recovered image with a synthetic NA of 0.5. We also tested the reported platform for biological samples. Figure 4(b1) and 4(c1) show the raw images of a pathology slide and a mouse brain section. The corresponding super-resolution recoveries are shown in Fig. 4(b2) and 4(c2). Media 3 (1MB, AVI) shows the 121 raw images of the mouse brain section (raw data available for downloads in our website). This super-resolution imaging experiment demonstrated the high flexibility of the reported illumination-engineering scheme.
Fig. 4.
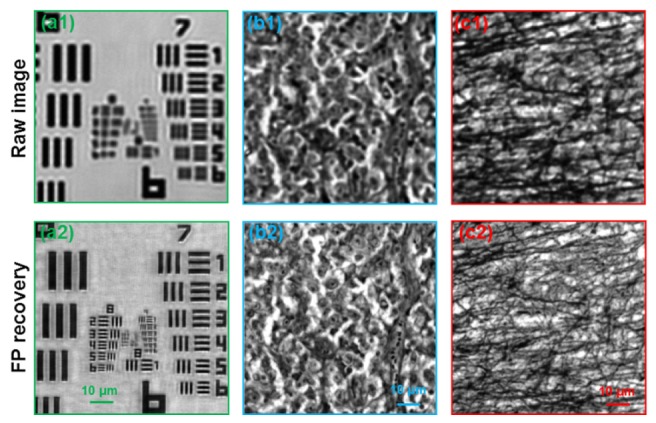
Super-resolution imaging using the reported scheme. We captured 121 images by presenting a scanning aperture at the transparent liquid crystal display. These images are used to recover super-resolution images using the Fourier ptychographic algorithm. (a1)-(c1) Raw images for a USAF resolution target, a pathology slide, and a mouse brain section. (a2)-(c2) Recovered super-resolution images of the samples. Also refer to Media 3 (1MB, AVI) .
4. Summary and discussion
We have demonstrated a simple and effective approach for microscopy illumination engineering. The reported approach is cost-effective and compatible with most existing platforms. On the application front, we have demonstrated the versatility of our platform for multimodal imaging of biological samples. By simply presenting different patterns on the liquid crystal display, we are able to perform bright-field microscopy, darkfield microscopy, phase-contrast microscopy, polarization microscopy, 3D tomographic imaging, and super-resolution Fourier ptychographic imaging. The reported scheme may further find applications in phase tomography, where angle-varied plane waves are used for sample illumination [19]. It can also be used in field-portable Fourier ptychographic microscope for active illumination control [13]. With further modification, the liquid crystal display can also be placed at the Fourier plane of a 4f system to perform aperture-scanning Fourier ptychographic imaging for 3D holography and aberration correction [14, 20].
One limitation of the current prototype platform is the low extinction ratio of the liquid crystal display. This ratio is about 300 in our prototype setup, and thus, the ‘on-state’ transmission is only 300 times higher than that of the ‘off-state’. This relative low extinction ratio leads to a residue background of the captured image, especially for images with large incident angles. Although we can subtract this background from the measurements, the noise would remain in the images. One of the future directions is to increase the extinction ratio by putting two displays together. In that case, the extinction ratio would be ~100,000 instead of 300. Finally, we can also use multiplexing scheme to improve the light delivering efficiency. For example, we can scan multiple apertures and/or turn on multiple wavelengths at the same time to increase the photon budget [15, 16].
References and links
- 1.Boas D. A., Pitris C., Ramanujam N., Handbook of Biomedical Optics (CRC press, 2012). [Google Scholar]
- 2.Yi R., Chu K. K., Mertz J., “Graded-field microscopy with white light,” Opt. Express 14(12), 5191–5200 (2006). 10.1364/OE.14.005191 [DOI] [PubMed] [Google Scholar]
- 3.Mehta S. B., Sheppard C. J. R., “Quantitative phase-gradient imaging at high resolution with asymmetric illumination-based differential phase contrast,” Opt. Lett. 34(13), 1924–1926 (2009). 10.1364/OL.34.001924 [DOI] [PubMed] [Google Scholar]
- 4.Zheng G., Kolner C., Yang C., “Microscopy refocusing and dark-field imaging by using a simple LED array,” Opt. Lett. 36(20), 3987–3989 (2011). 10.1364/OL.36.003987 [DOI] [PubMed] [Google Scholar]
- 5.Zheng G., Horstmeyer R., Yang C., “Wide-field, high-resolution Fourier ptychographic microscopy,” Nat. Photonics 7(9), 739–745 (2013). 10.1038/nphoton.2013.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer C., Jesacher A., Bernet S., Ritsch-Marte M., “What spatial light modulators can do for optical microscopy,” Laser Photon. Rev. 5(1), 81–101 (2011). 10.1002/lpor.200900047 [DOI] [Google Scholar]
- 7.Tian L., Wang J., Waller L., “3D differential phase-contrast microscopy with computational illumination using an LED array,” Opt. Lett. 39(5), 1326–1329 (2014). 10.1364/OL.39.001326 [DOI] [PubMed] [Google Scholar]
- 8.Zheng G., Lee S. A., Antebi Y., Elowitz M. B., Yang C., “The ePetri dish, an on-chip cell imaging platform based on subpixel perspective sweeping microscopy (SPSM),” Proc. Natl. Acad. Sci. U.S.A. 108(41), 16889–16894 (2011). 10.1073/pnas.1110681108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z., Tian L., Liu S., Waller L., “Real-time brightfield, darkfield, and phase contrast imaging in a light-emitting diode array microscope,” J. Biomed. Opt. 19(10), 106002 (2014). 10.1117/1.JBO.19.10.106002 [DOI] [PubMed] [Google Scholar]
- 10.Zheng G., “Microscopy-Programmable LED array makes microscopes more versatile,” Laser Focus World 48, 66 (2012). [Google Scholar]
- 11.Hamilton D. K., Sheppard C. J. R., “Differential phase contrast in scanning optical microscopy,” J. Microsc. 133(1), 27–39 (1984). 10.1111/j.1365-2818.1984.tb00460.x [DOI] [Google Scholar]
- 12.Ou X., Horstmeyer R., Yang C., Zheng G., “Quantitative phase imaging via Fourier ptychographic microscopy,” Opt. Lett. 38(22), 4845–4848 (2013). 10.1364/OL.38.004845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong S., Guo K., Nanda P., Shiradkar R., Zheng G., “FPscope: a field-portable high-resolution microscope using a cellphone lens,” Biomed. Opt. Express 5(10), 3305–3310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong S., Horstmeyer R., Shiradkar R., Guo K., Ou X., Bian Z., Xin H., Zheng G., “Aperture-scanning Fourier ptychography for 3D refocusing and super-resolution macroscopic imaging,” Opt. Express 22(11), 13586–13599 (2014). 10.1364/OE.22.013586 [DOI] [PubMed] [Google Scholar]
- 15.Dong S., Shiradkar R., Nanda P., Zheng G., “Spectral multiplexing and coherent-state decomposition in Fourier ptychographic imaging,” Biomed. Opt. Express 5(6), 1757–1767 (2014). 10.1364/BOE.5.001757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian L., Li X., Ramchandran K., Waller L., “Multiplexed coded illumination for Fourier Ptychography with an LED array microscope,” Biomed. Opt. Express 5(7), 2376–2389 (2014). 10.1364/BOE.5.002376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams A., Chung J., Ou X., Zheng G., Rawal S., Ao Z., Datar R., Yang C., Cote R., “Fourier ptychographic microscopy for filtration-based circulating tumor cell enumeration and analysis,” J. Biomed. Opt. 19(6), 066007 (2014). 10.1117/1.JBO.19.6.066007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng G., “Breakthroughs in Photonics 2013: Fourier Ptychographic Imaging,” IEEE Photonics J. 6(2), 1–7 (2014). 10.1109/JPHOT.2014.2317977 [DOI] [Google Scholar]
- 19.Choi W., Fang-Yen C., Badizadegan K., Oh S., Lue N., Dasari R. R., Feld M. S., “Tomographic phase microscopy,” Nat. Methods 4(9), 717–719 (2007). 10.1038/nmeth1078 [DOI] [PubMed] [Google Scholar]
- 20.Horstmeyer R., Ou X., Chung J., Zheng G., Yang C., “Overlapped Fourier coding for optical aberration removal,” Opt. Express 22(20), 24062–24080 (2014). 10.1364/OE.22.024062 [DOI] [PMC free article] [PubMed] [Google Scholar]


