Abstract
Acupuncture has been a powerful clinical tool for treating chronic diseases. However, there is currently no appropriate method to clarify the therapeutic effect of acupuncture. Here, we use photoacoustic tomography (PAT) to study the effect of acupuncture on mouse brain blood vessels. Ten healthy mice were stimulated with acupuncture needles on two acupoints. PAT images were obtained before and after acupuncture. We report that stimulation of certain acupoints resulted in changes in hemodynamics/blood flow at these points. The results demonstrate that PAT can non-invasively detect blood flow changes in mouse brain under acupuncture. This pilot study shows the potential of PAT as a visualization tool for illuminating the mechanism of acupuncture and promoting its clinical applications.
OCIS codes: (110.5120) Photoacoustic imaging, (170.3880) Medical and biological imaging
1. Introduction
Acupuncture is one of the essences of Chinese traditional medicine and has two-thousand years of application history. In recent years, progresses in the research of clinical acupuncture and meridian theory have driven acupuncture to an elevated stage in the international arena. In 1996, the world health organization (WHO) initially confirmed 64 kinds of diseases indicated by acupuncture. Although the mechanism of acupuncture is not clear, several hypotheses have been proposed. A research proposed that the neural system generates neural electrical signals evoked by acupuncture [1]. Another study indicated that acupuncture produces an effect by regulating the nervous system [2]. Hui observed that acupuncture can activate the limbic system and produce relevant responses with the sensorimotor system [3]. When acupuncture of Yongquan was applied to small animals, Yang et al. discovered some activated areas included bilateral frotal, lobe cortex, primarily section 1, 2;cingulated gyrus, primarily section 1, 2; contralateral caudate nucleus, insula, backlimb representation cortex and parietal lobe cortex and primarily section 1 [4]. Overall, acupuncture is regarded as an exogenous stimulation, and the mechanism is likely linked to the neuroendocrine system. Acupuncture primarily influences the neural system, respiratory system and cardiovascular systems.
During acupuncture, the brain’s cortical area is activated while the subject receives various sensory stimulations. This is correlated with increased blood flow, increased oxyhemoglobin levels and increased metabolic rates. Studies [5–17] using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) have attempted to quantitate the brain effects of acupuncture. In addition, near-infrared spectroscopy [18] has been used to study acupuncture effects. In fMRI, signals are influenced by the oxygen level of blood. The reconstructed fMRI image reflects the corresponding nerve activity by measuring changes in oxygen levels of blood. Although fMRI provides the simultaneous structural and functional images, it is expensive and has low spatial and temporal resolution. Likewise, although PET records glucose metabolism and blood flow, it has low spatial and temporal resolution, and is invasive as patients need to be injected with tracer isotopes before imaging. Therefore, it is urgent to find a replacement which is affordable and noninvasive. Furthermore, high spatial-temporal resolution is required for studying temporal and spatial characteristics of the brain under acupuncture.
In this report, photoacoustic tomography (PAT) is used in brain imaging during acupuncture. PAT employs short laser pulses to generate ultrasonic waves from biological tissues with frequencies raging from 20KHz to 20MHz [19]. As the light is absorbed by tissue, it produces heat bilges, cold shrinks and subsequent ultrasonic waves [20]. The optical absorption coefficient will vary with different biological tissues, and the absorption distribution can be reconstructed with algorithms. In short, PAT combines high ultrasonic resolution and strong optical contrast in a single modality, capable of providing both structural and functional images [21]. In addition, parameters such as oxygen hemoglobin, blood flow, oxygen saturation and metabolic rate are accessible using PAT. Compared with fMRI and PET, PAT can identify the cerebrovascular state and provide an ideal method to image the change of blood flow during acupuncture.
In this study, we developed a PAT system to examine the change in blood flow in mouse brain in response to needle simulation on Yongquan acupoints. The blood vessels were imaged using the PAT system before and after acupuncture, and the one-dimensional and two-dimensional distributions of optical absorption were analyzed.
To our best knowledge, this work provides the first study using PAT to study brain blood vessel changes during acupuncture. Our work shows the potential of PAT as a visualization tool for studying the mechanism of acupuncture.
2. Materials
2.1 Experimental setup
The schematic of experimental setup was discussed in our previous work [22], presented as Fig. 1 in this article. A Q-switched Nd: YAG laser light was used at a wavelength of 532 nm with duration of 6.5 ns and a repetition rate of 1 Hz. A mirror was used to change the light path by 90 degrees. The laser beam was expanded by a concave lens. To obtain a uniform laser beam, ground glass was used. A hole was placed at the bottom of water tank with a diameter of 6 cm. A polytetrafluoroethylene film, transparent to light and ultrasound, was employed as a separation layer between water and mouse brain tissue. During the experiment, the homogeneous beam illuminated the intact mouse head. The energy density on the brain surface was below 15mJ/cm2. An ultrasonic transducer with a central frequency of 5MHz and a diameter of 0.8mm was motor driven by a computer. During the experiment, the transducer rotated around the mouse brain 360 degrees with a step size of 2 degrees at the scanning position. The detector received the photoacoustic signals generated by the brain tissues at each step. The signal was then sent to an amplifier and transmitted into a data acquisition card. After the scanning, the delay and sum algorithm was adopted for reconstructing the distribution of the optical absorptions within the tissues.
Fig. 1.
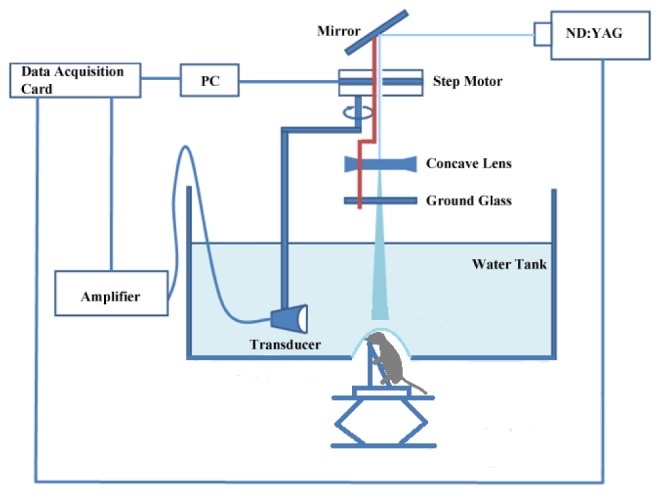
Setup for photoacoustic imaging of the mouse brain
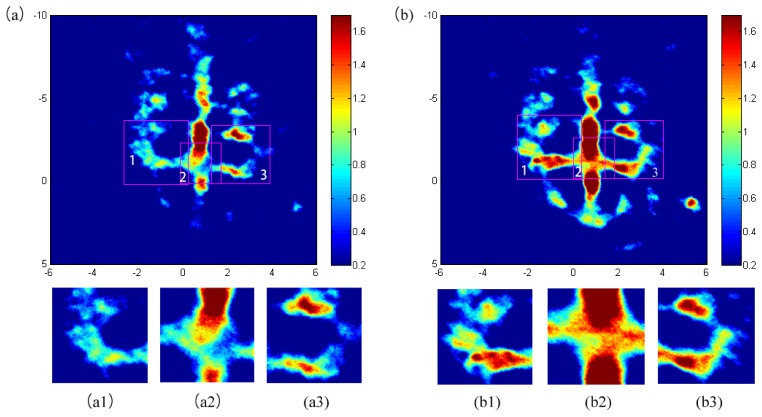
We also conducted a group of control experiments on mouse brain without acupuncture where the time interval between each experiments were the same as that used in the experiments with acupuncture (see below). The results from the control experiments are given in Fig. 2 . We can see from Fig. 2 that the images at different time points are almost the same from the control group.
Fig. 2.
PAT images from control experiments at the 3, 8, 13 minutes respectively . (a1)-(a3) is the magnified image of box 1,2 and 3 in (a). (b1)-(c3) are acquired similarly in (b) and (c).
From the above images, we can get a conclusion that experimental results at different time points are almost same. To better understand the results, we marked three boxes located in different blood vessels in figure (a)-(c) in the same place and applied magnification. From (a1)-(c1), (a2)-(c2) and (a3)-(c3), the light absorption distribution are consistent basically.
2.2 Animal model
Ten healthy Kunming mice, weighing 32.9 ± 8.4g, were used for imaging. Before the experiment, the mice were not exposed to any procedures that would affect their mental or physical health. In addition the mice were not treated with any drugs prior to this study. The mice were exposed to adaptive feeding from birth and adapted to the living environment for one month. Before imaging, the mice were anesthetized by intraperitoneal injection of chloral hydrate solution. The hair on the mouse head was removed using depilatory paste and the head was coated with a layer of ultrasound coupling agent. The mouse was placed in a homemade mounting bracket and then was adjusted so the head was consistent to the transducer scanning plane. After the data acquisition for PAT, each mouse was sacrificed with overdasage.
2.3 Acupoints selection
We chose Yongquan acupoints located in the middle of the hind paw as the stimulated position. In Chinese traditional medicine, Yongquan is related to insomnia, irritability and eclampsia. The acoupoints were selected based on published data [23]. The selected acupoints, labeled LYA and RYA are shown in Fig. 3 .
Fig. 3.
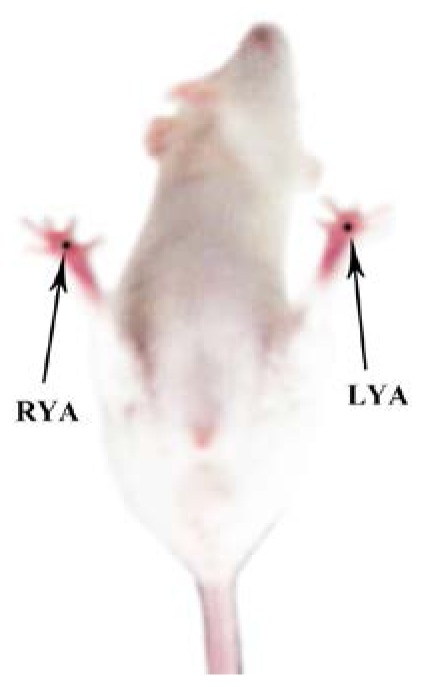
Selected acupoints of a mouse photographed before the PAT experiment. RYA: Right Yongquan acupoint. LYA: Left Yongquan acupoint.
2.4 Methods
Ten mice were imaged using PAT before and after acupuncture on both RYA and LYA, respectively. We conducted acupuncture on the mouse for 2 minutes and obtained images with 3 min following.
2.5 Statistical analysis
Data were analyzed after the experiment on ten mice using a t-test. The criterion for significance was p < 0.05.
3. Results
3.1 Acupuncture on RYA
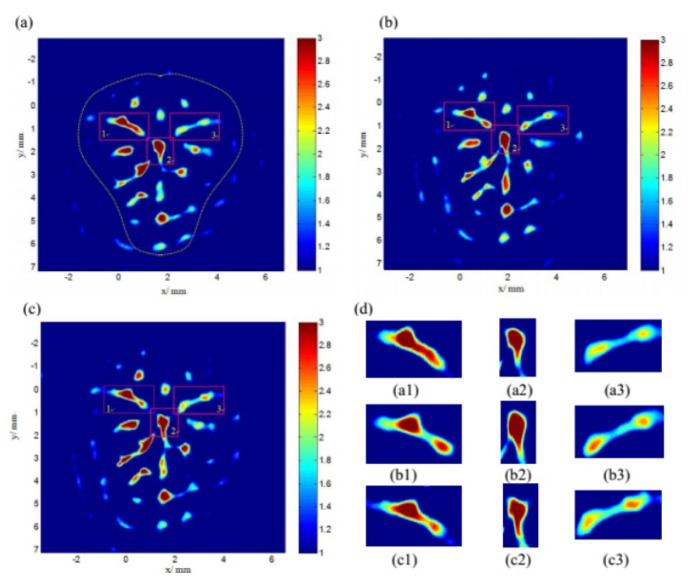
Figure 4 shows the cerebral hemodynamic changes in response to acupuncture in the right hind paw. Figure 4(a) shows the image before acupuncture, while Fig. 4(b) and Fig. 4(c) were obtained after acupuncture and needle rotation, respectively. Our current PAT system has limitations in resolution, which results in low clarity of some blood vessels. However, the images of Figs. 4(a)-4(c) were matched with the anatomical photographs obtained after imaging shown in Fig. 4(d). To better understand the results, we marked three boxes located in different blood vessels in Figs. 4(a)-4(c) in the same place and applied magnification.
Fig. 4.
Noninvasive PAT imaging of a mouse brain at a wavelength of 532nm with skin and skull intact. Photoacoustic image acquired (a) before acupuncture (at the 3 minutes), (b) after acupuncture (at the 8 minutes), (c) after rotation of the needle (at the 13 minutes). (a1)-(a3) is the magnified image of box 1,2 and 3 in (a). (b1)-(c3) are acquired similarly in (b) and (c). (d) Photograph of the small mouse brain obtained after the PAT experiment. MCA, middle cerebral artery. BL, branch vessel.
Longitudinally, Figs. 4(a1)-4(a3) and Figs. 4(b1)-4(b3) indicate that there was significant difference between the non-stimulation periods and the stimulation phases. Blood flow changed more intensively after needle rotation as shown in Figs. 4(c1)-4(c3). The distribution of blood flow showed significant changes between the middle cerebral artery and branch vessel. The increased blood flow occurred at different positions, indicating that changes in response to acupuncture are dynamic.
Figure 5 shows the relative light absorption distribution of the middle cerebral artery in Fig. 4 along the same MCA position. . It reveals that the maximum values of the relative light absorption are located in different place before and after acupuncture, indicating dynamic changes in blood flow. The dashed line is a reference for comparison. In Fig. 5(a) to Fig. 5(b), the value of relative absorption is about 2.5 before acupuncture with increment increases, with a maxim value of 2.876 after acupuncture. Figure 5(c) shows no significant increase in the maxim value compared with Fig. 5(b). Instead, a slight decrease appears after needle rotation which does not represent an experimental trend. In summary, mice received needle stimulation followed by brain cortex response and increases in blood flow.
Fig. 5.
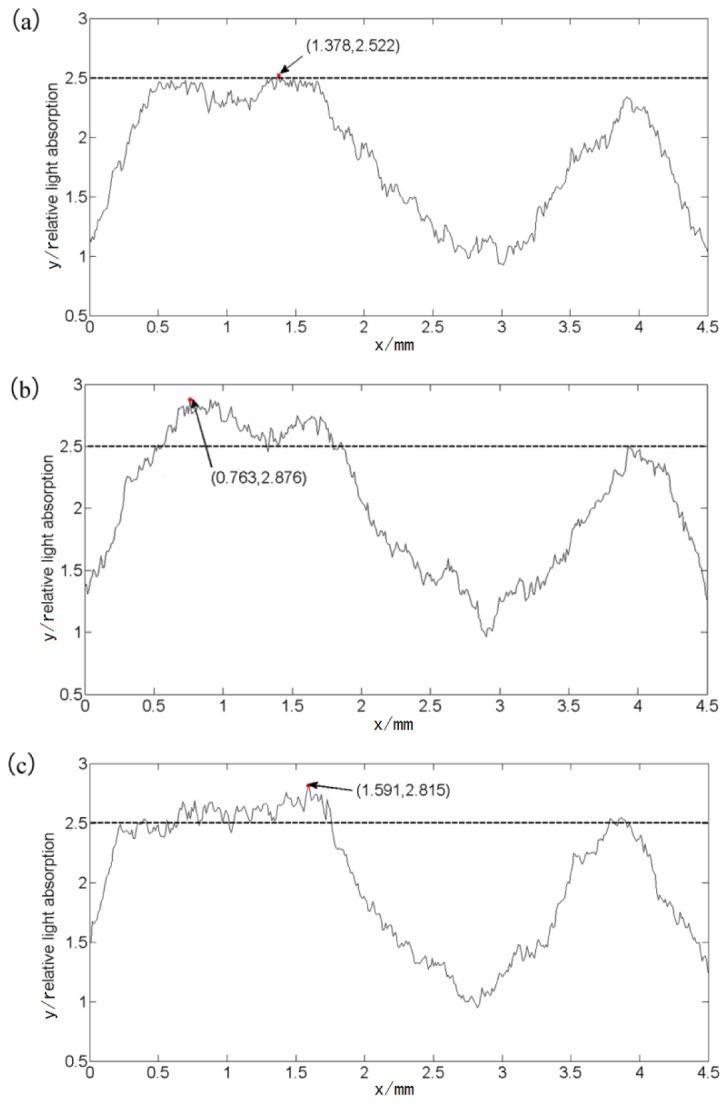
(a) before acupuncture. (b) After acupuncture. (c) After rotation of the needle. The horizontal axis represents the distance along MRC in Fig. 4, and the vertical axis represents the relative light absorption.
After obtaining three sets of reconstruction data before acupuncture, after acupuncture and after needle rotation, subtraction is performed among the three sets of data to intuitively observe the blood flow changes before and after acupuncture. The results were shown in Fig. 6 . Significant changes could be perceived in branch vessels after acupuncture as Fig. 6(a) shows and is more obvious after needle rotation shown in Fig. 6(b).
Fig. 6.
Subtraction between images in Fig. 4. (a) Before acupuncture and after acupuncture. (b) Before acupuncture and after needle rotation
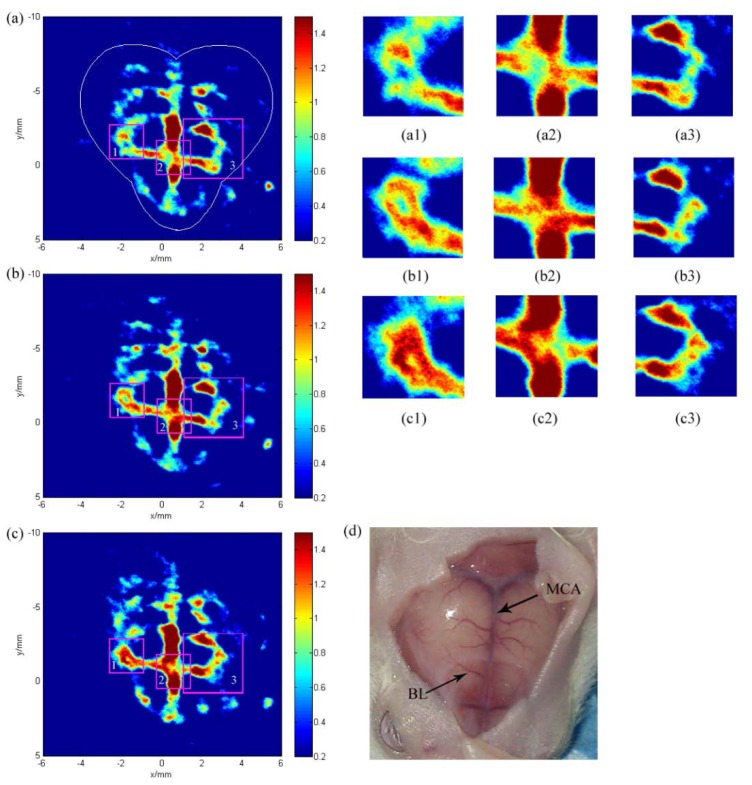
3.2 Acupuncture on LYA
Figure 7 shows the cerebral hemodynamic changes in response to acupuncture in the left hind paw. The hemodynamic changes in the blood vessels in the superficial cortex are apparent after acupuncture in Fig. 7(b) compared with Fig. 7(a) obtained before acupuncture. To better understand the results, we marked three boxes located in different blood vessels in Fig. 7(a) and Fig. 7(b) in the same place and applied magnification. Significant changes can be seen in the magnified figures, shown as Figs. 7(a1)-7(b3). These results indicate that visual change regions can be monitored using PAT. The increased light absorption represent photoacoustic signal enhancement, which are likely induced by acupuncture.
Fig. 7.
Noninvasive PAT imaging of a mouse brain at a wavelength of 532nm with skin and skull intact. Photoacoustic image acquired (a) before acupuncture. (b) After acupuncture. (a1)-(a3) Is the magnified image of box 1,2 and 3 in (a). (b1)- (b3) Are acquired similarly in (b).
Figure 8 shows the relative light absorption distribution of the middle cerebral artery in Fig. 7 along the same position. The relative value was roughly 2.5 before acupuncture and rose to 2.8 after simulation, indicating that acupuncture leads to increased blood flow.
Fig. 8.
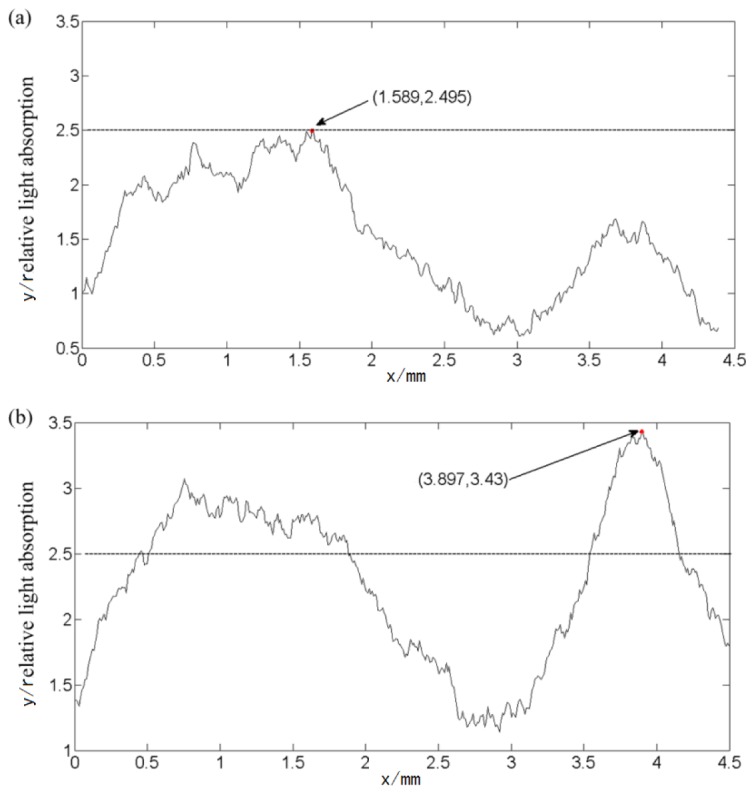
(a) Before acupuncture. (b) After acupuncture. The horizontal axis represents the distance along the middle cerebral artery in Fig. 7, and the vertical axis represents the relative light absorption.
Similarly to the previous experiment, we conducted acupuncture on nine mice and used PAT to detect the blood change before and after acupuncture on both RYA and LYA, respectively. The data were processed using the following method: assuming that number ‘1’ represented an effect of acupuncture on Yongquan to cause changes in blood flow of brain, while number ‘0’ indicates no effect. The statistical analysis on changes in blood flow from ten mice was ere summarized in Table 1 .A t-test was used after obtaining reconstruction data from ten mice received acupuncture. The results were shown in Table 1. It reveals that acupuncture on Yongquan acupoints had a significant effect on mice cerebral blood flow (p<0.05). The results verified the ability of PAT to monitor the effect of acupuncture.
Table 1. Measure of changes under acupuncture on the right and left hind paw.
| Acupoint | N = 10
|
p- value | t-value | |
|---|---|---|---|---|
| + * | -* | |||
| RYA | 8 | 2 | 6.851E-4 | 6.324 |
|
| ||||
| LYA | 9 | 1 | 2.77E-5 | 9.486 |
+ *: Number of samples with changes in blood flow after acupuncture.
-*: Number of samples without changes in blood flow after acupuncture.
4. Discussion
Because anesthesia impacts the nervous system of mice, we conducted an experiment to find the most appropriate dose of anesthesia to ensure that mice would not display movement but could respond to acupuncture. To do this, 5 mice were anesthesitized with different dose of chloral hydrate via intraperitoneal injection. One mouse could move freely. Three mice lacked autonomous movement after 2 minutes but responded to needle rotation. One mouse lost consciousness after one minute. In summary, mice injected with 0.0027 ± 0.00017ml/g chloral hydrate did not experience abnormal movements and could effectively respond to acupuncture.
Due to the use of a single ultrasonic transducer, the temporal resolution of the PAT system could not be performed in real-time. Because the image acquisition time is 3 minutes, we can currently observe the average change of blood flow over time. However, the system can be improved by using an ultrasonic transducer array to reduce the imaging time from minutes to milliseconds, which enable us to capture real-time blood flow changes in mouse brain following acupuncture.
Acupuncture points have different distinct structures compared with other acupoints. However, compared with non-acupoints, the structural distinction is vague. The mission of research on the mechanism of acupuncture is to examine the relationship between active brain regions and acupuncture, to verify the exact effect of acupuncture and to study the specificity of acupoints. Applying PAT to study the effect of acupuncture represents new method for measuring the effects of acupuncture.
Diagnosis and treatment of cerebrovascular disease has been the focus of medical research. A large number of studies have shown that acupuncture has therapeutic effects on cerebrovascular disease, such as stroke. Acupuncture can enhance blood flow in the brain and relieve the ischemia and edema. Photoacoustic imaging of the brain is beneficial to locate brain vascular lesions. Hence PAT could potentially be used to perform targeted acupuncture. This work provides the first demonstration of using PAT to study acupuncture at the micron level. This study suggests that PAT can be applied to cerebrovascular disease detection and clinical acupuncture treatment monitoring.
5. Conclusions
With this development a non-invasive and visual tool for acupuncture monitoring, we have presented preclinical results using the PAT system. Photoacoustic imaging was conducted before and after acupuncture using mice. Our results show that the cerebral hemodynamic changes can be detected non-invasively using PAT. The blood flow of mouse brain increased after the mouse received acupuncture and the effect was more pronounced after needle rotation. This represents a step toward developing a visualization tool for acupuncture. Future work will improve the quality of reconstruction images and measure the effects of acupuncture in real-time. This pilot study shows the potential of PAT as a visualization tool for illuminating the mechanism of acupuncture and promoting its clinical use.
Ethics statement
This study was approved by the Research Ethics Board at the University of Electronic Science and Technology of China (UESTC). All protocols and procedures have been approved by the Animal Care Guide for the care and use of experimental animals in the UESTC.
Acknowledgments
The authors would like to thank Dr Ji Shu for his contribution and Dr Yang Xin for assistance with acupoints selection.
References and links
- 1.Han C. X., Wang J. D. B., Guo Y., Liu Y. Y., “Cluster analysis of electrical signals from dorsal spinal nerve root evoked by different acupuncture manipulations at Zusanli point,” J. Tianjin University 44(5), 412–416 (2011). [Google Scholar]
- 2.Han J. S., Terenius L., “Neurochemical basis of acupuncture analgesia,” Annu. Rev. Pharmacol. Toxicol. 22(1), 193–220 (1982). 10.1146/annurev.pa.22.040182.001205 [DOI] [PubMed] [Google Scholar]
- 3.Hui K. K., Napadow V., Liu J., Li M., Marina O., Nixon E. E., Claunch J. D., LaCount L., Sporko T., Kwong K. K., “Monitoring acupuncture effects on human brain by FMRI,” J. Vis. Exp. 8(38), 1190–1197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L., Gao X. L., Yang X. L. L. X., Li H., Li C. X., Lei H., Tian J., Yang D. P., “Effects of TENS of Yongquan on Functional Magnetic Resonance Imaging and on Pain Threshold in Rats,” Radiol. Prat. 20(12), 1087–1089 (2005). [Google Scholar]
- 5.Hui K. K., Liu J., Marina O., Napadow V., Haselgrove C., Kwong K. K., Kennedy D. N., Makris N., “The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI,” Neuroimage 27(3), 479–496 (2005). 10.1016/j.neuroimage.2005.04.037 [DOI] [PubMed] [Google Scholar]
- 6.Napadow V., Dhond R. P., Purdon P., Kettner N., Makris N., Wong K. K., Hui K. K., “Correlating acupuncture FMRI in the human brainstem with heart rate variability,” in Proceedings of IEEE Conference on Medicine and Biology Society, (Institute of Electrical and Electronics Engineers, Shanghai, 2005), pp. 4496–4499 10.1109/IEMBS.2005.1615466 [DOI] [PubMed] [Google Scholar]
- 7.Napadow V., Makris N., Liu J., Kettner N. W., Kwong K. K., Hui K. K., “Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI,” Hum. Brain Mapp. 24(3), 193–205 (2005). 10.1002/hbm.20081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napadow V., Kettner N., Liu J., Li M., Kwong K. K., Vangel M., Makris N., Audette J., Hui K. K., “Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome,” Pain 130(3), 254–266 (2007). 10.1016/j.pain.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh J. C., Tu C. H., Chen F. P., Chen M. C., Yeh T. C., Cheng H. C., Wu Y. T., Liu R. S., Ho L. T., “Activation of the hypothalamus characterises the acupuncture stimulation at the analgesic point in the human: a PET Study,” Neurosci. Lett. 307, 105–108 (2001). 10.1016/S0304-3940(01)01952-8 [DOI] [PubMed] [Google Scholar]
- 10.Yoo S. S., Teh E. K., Blinder R. A., Jolesz F. A., “Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study,” Neuroimage 22(2), 932–940 (2004). 10.1016/j.neuroimage.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 11.Haker E., Egekvist H., Bjerring P., “Effect of sensory stimulation (acupuncture) on sympathetic and parasympathetic activities in healthy subjects,” J. Auton. Nerv. Syst. 79(1), 52–59 (2000). 10.1016/S0165-1838(99)00090-9 [DOI] [PubMed] [Google Scholar]
- 12.Wu M. T., Hsieh J. C., Xiong J., Yang C. F., Pan H. B., Chen Y. C., Tsai G., Rosen B. R., Wong K. K., “Central nervous system pathway for acupuncture stimulation: localisation of processing with fMRI of the brain – preliminary experience,” Radiology 212, 133–141 (1999). 10.1148/radiology.212.1.r99jl04133 [DOI] [PubMed] [Google Scholar]
- 13.Zhang W. T., Jin Z., Cui G. H., Zhang K. L., Zhang L., Zeng Y. W., Luo F., Chen A. C., Han J. S., “Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study,” Brain Res. 982(2), 168–178 (2003). 10.1016/S0006-8993(03)02983-4 [DOI] [PubMed] [Google Scholar]
- 14.Qin W., Tian J., Bai L. J., Pan X. H., Yang L., Chen P., Dai J. P., Ai L., Zhao B. X., Gong Q. Y., Wang W., von Deneen K. M., Liu Y. J., “FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network,” Mol. Pain 4(1), 55 (2008). 10.1186/1744-8069-4-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pariente J., White P., Frackowiak R. S., Lewith G., “Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture,” Neuroimage 25(4), 1161–1167 (2005). 10.1016/j.neuroimage.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 16.Siedentopf C. M., Koppelstaetter F., Haala I. A., Haid V., Rhomberg P., Ischebeck A., Buchberger W., Felber S., Schlager A., Golaszewski S. M., “Laser acupuncture induced specific cerebral cortical and subcortical activations in humans,” Lasers Med. Sci. 20(2), 68–73 (2005). 10.1007/s10103-005-0340-3 [DOI] [PubMed] [Google Scholar]
- 17.Quah-Smith I., Sachdev P. S., Wen W., Chen X. H., Williams M. A., “The brain effects of laser acupuncture in healthy individuals: An FMRI Investigation,” PLoS ONE 5(9), e12619 (2010). 10.1371/journal.pone.0012619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litscher G., Bauernfeind G., Gao X. Y., Putz G. M., Wang L., Anderle W., Gaischek I., Litscher D., Neuper C., Niemtzow R. C., “Battlefield acupuncture and near-Infrared spectroscopy–miniaturized computer-triggered electrical stimulation of battlefield ear acupuncture points and 50-channel near-infrared spectroscopic mapping,” Med. Acupuncture 23(4), 263–270 (2011). 10.1089/acu.2011.0809 [DOI] [Google Scholar]
- 19.Tan Y., Xing D., Wang Y., Zeng Y. G., Yin B. Z., “Influence of Bandwidth of Ultrasonic Transducer on Photoacoustic Imaging,” Acta Opt. Sin. 25(1), 40–44 (2005). [Google Scholar]
- 20.Wang L. H. V., “Prospects of photoacoustic tomography. Medical Physicals,” Am. Assoc. Phys. Med 35(12), 5758–5767 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X. D., Pang Y. J., Ku G., Xie X. Y., Stoica G., Wang L. V., “Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain,” Nat. Biotechnol. 21(7), 803–806 (2003). 10.1038/nbt839 [DOI] [PubMed] [Google Scholar]
- 22.Li T. T., Rong J., Huang L., Chen B. Z., Jin X. Y., Zhong X. C., “Photoacoustic imaging of mouse brain using ultrasonic transducer with different central frequencies,” in Proceedings of International Conference on Information Technology and Computer Application Engineering, Liu H. C., ed. (CRC Press, New York, 2013) 10.1201/b15936-153 [DOI] [Google Scholar]
- 23.Yu S. G., “Commonly used acupuncture points in mice and map,” in Experimental Acupuncture, Xu B., ed. (People's Medical Publishing House, Beijing, 2012). [Google Scholar]