Abstract
Cancer progression (initiation, growth, invasion and metastasis) occurs through interactions between malignant cells and the surrounding tumor stromal cells. The tumor microenvironment is comprised of a variety of cell types, such as fibroblasts, immune cells, vascular endothelial cells, pericytes and bone-marrow-derived cells, embedded in the extracellular matrix (ECM). Cancer-associated fibroblasts (CAFs) have a pro-tumorigenic role through the secretion of soluble factors, angiogenesis and ECM remodeling. The experimental models for cancer cell survival, proliferation, migration, and invasion have mostly relied on two-dimensional monocellular and monolayer tissue cultures or Boyden chamber assays. However, these experiments do not precisely reflect the physiological or pathological conditions in a diseased organ. To gain a better understanding of tumor stromal or tumor matrix interactions, multicellular and three-dimensional cultures provide more powerful tools for investigating intercellular communication and ECM-dependent modulation of cancer cell behavior. As a platform for this type of study, we present an experimental model in which cancer cells are cultured on collagen gels embedded with primary cultures of CAFs.
Keywords: Medicine, Issue 96, Three-dimensional co-culture, cancer, fibroblast, invasion, tumor stroma, collagen
Introduction
Cancer tissue can be perceived as a type of organ, which evolves through close interactions between the cancer and the tumor stromal microenvironment, composed of cancer-associated fibroblasts (CAFs), immune cells, tumor vessels and the extracellular matrix (ECM). CAFs are the major source of soluble factors (cytokines, growth factors and chemokines) that exert mitogenic, pro-migratory and pro-invasive effects on cancer cells. They also stimulate tumor vessel formation and recruit precursor cells, such as bone marrow-derived cells (BMDC). Activated CAFs are involved in the production and remodeling of the ECM, thereby promoting the growth and spread of cancer cells1. CAFs also provide a niche that facilitates tumor cell colonization and metastasis and are capable of conferring stem cell phenotypes onto neighboring cancer cells. Pathological observations suggest that stromal reactions or fibrotic changes in cancer tissues are indicative of a poor prognosis. Recent studies have also demonstrated that tumor stromal features, such as the gene signature, can predict patient prognosis. Furthermore, CAF-derived factors can modulate sensitivity to chemotherapy, highlighting the role of CAFs in determining drug sensitivity and resistance2.
As CAFs play a multifaceted role in the promotion of tumor progression through signaling pathways that mediate interactions between CAFs and different cell types within the tumor microenvironment, they have attracted increasing attention as novel targets for cancer therapies. The heterogeneity of the cell populations within the cancer microenvironment presents an obstacle for targeting CAFs. Several markers for CAFs have been proposed, such as α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP), and fibroblast specific proten-1 (FSP-1: also called S100A4); however, these molecular markers are not specific for distinguishing CAFs from other cells present in non-cancerous tissues3. Therefore, further studies are needed to obtain more knowledge about the specific properties of CAFs. To this end, it is informative to characterize primary cultured CAFs compared with patient-matched normal fibroblasts.
Recently, analyses on patient-derived CAFs have been reported in several cancer types, revealing unique gene expression patterns and cell behaviors compared with fibroblasts derived from non-cancerous tissues. Using isolated CAFs from human lung cancer tissues, we developed a three-dimensional co-culture method, enabling us to evaluate the properties of lung CAFs. In this model, we investigated the effects of the CAFs on lung cancer cell invasion, proliferation and collagen gel contraction, which experimentally recapitulated the tumor-promoting roles of lung CAFs4.
Protocol
NOTE: This study was approved by the appropriate Ethics Committees.
1. Primary Culture of Human Lung Fibroblasts
- Collection of lung tissue:
- Obtain human lung tissue samples from the surgical operating room directly. Collect approximately 1 cm3 blocks from cancerous and non-cancerous lung tissue, with the non-cancerous sample collected as far away from the tumor as possible.
- Suspend the samples in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (serum-free medium). Maintain the suspended samples in sterile 50 ml tubes and transfer to the laboratory. NOTE: Fibroblasts are highly migratory and proliferative compared with other cell types, such as epithelial, endothelial and smooth muscle cells. The outgrowth method takes advantage of these features of fibroblasts, and outgrowing cells from tissue sections are abundant in fibroblasts. After several cell passages, other cell types fail to survive and propagate in DMEM with 10% fetal bovine serum (FBS), resulting in purified fibroblast cell cultures. The cells could be used 3-7 passages following primary culture.
- Explant culture and conditioning for cell outgrowth:
- Perform primary culture of fibroblasts using a previously reported protocol with modifications5.
- In the laboratory, place the tissue sample on a 10 cm tissue culture dish without culture medium and cut into small sections ~2-3 mm in size using sterile forceps and a scalpel. During this process, avoid making the section dry, otherwise the efficiency of cell outgrowth is impaired.
- Separation of the epithelial cell layer and connective tissue:
- Soak the cut tissue sections in culture medium containing 2,000 PU/ml dispase I and culture for 16 hr at 4 °C.
- Transfer the tissue sections to a dish, and separate the epithelial cell layer from the adjacent connective tissue. Process the sections in a clean bench to avoid contamination of microorganisms. NOTE: If primary culture of epithelial cells is needed, perform step 1.3. If only fibroblasts are to be isolated, omit step 1.3 because the subsequent explant culture and cell passages are unfavorable for epithelial cells, which yield purified fibroblast populations.
- Attachment of tissue sections:
- Mince the tissues into 1 mm pieces using two scalpels. If the pieces are not small enough, they detach more easily from the dish, and the cells will fail to outgrow on the dish surface.
- Place small lung tissue sections apart from each other to maintain an empty area surrounding each piece. Make scratches on the surface of the tissue culture dish using a scalpel blade to facilitate attachment of tissue sections. NOTE: Scratching also appears to facilitate cell outgrowth, providing tracks along which cells can migrate.
- Alternatively, place minced tissue pieces individually into each well of a 6-well plate, and cover them with a cover slip. Attach the cover slip to the surface of the plate using silicone grease.
- Place silicone grease at two points 1.5 cm apart in each well using a sterile pin. Coverage of tissue pieces enhances the efficacy of cell outgrowth and cell propagation following several passages.
- Subsequent explant culture:
- Gently add culture medium to the dish before the tissue sections dry out, as efficient cell outgrowth is lost for the most part if the samples dry out. Do not pour the medium too rapidly as the tissue sections would detach.
- Culture cells in DMEM with 10% FBS. Use enough medium to just cover the tissue, but not too much that it allows floating, as additional buoyancy promotes detachment from the dish.
- Cell passage:
- Handle the culture medium with care at all times as repeated movements of the culture dish can lead to detachment of the tissue sections.
- Place the culture dishes in an incubator at 37 °C for 5-7 days. Refresh the medium every other day, and handle the culture dishes minimally during the medium changes. Fibroblasts outgrow from the edge of the tissue sections over the next few weeks. NOTE: After ~2-3 weeks, outgrowing fibroblasts almost reach confluence, depending on the amount of surface area.
- Wash cells with 1x PBS, and add 1 ml of trypsin I to the plate.
- After trypsinization, detach the cells using 9 ml of DMEM supplemented with 10% FBS. Collect cells suspended in the medium in a 10 ml tube, together with the tissue sections. Following centrifugation to form cell pellets, resuspend the resulting pellets in the new medium.
- Seed the cells and tissue sections onto a new culture dish, at which point the tissues fail to attach to the dish while the cells adhere and grow. The following day, remove the floating tissue pieces by aspiration, and change the medium. Attached fibroblasts propagate with subsequent cultures.
2. Three-dimensional Co-culture of Human Lung Fibroblasts and Cancer Cells
- Preparation of cells and reagents:
- Approximately use 2 x 105 epithelial cells and 2.5 x 105 fibroblasts per well. Prepare collagen type IA (3 mg/ml, pH 3), FBS (100%), reconstitution buffer (50 mM NaOH, 260 mM NaHCO3, 200 mM HEPES), 5x DMEM, DMEM supplemented with 10% FBS for fibroblast culture, and a 3D co-culture medium (a 1:1 mixture of fibroblast culture medium and culture medium for cancer cells).
- Culture A549 lung adenocarcinoma cells, the fibroblasts and the A549 3D co-culture in DMEM supplemented with 10% FBS.
- Collagen gel formation:
- Wash fibroblasts cultured on 10 cm dish with 1x PBS, and add 1 ml of trypsin I to the plate. After trypsinization for ~5 min at 37 °C, detach the cells using 9 ml DMEM supplemented with 10% FBS. Collect cells suspended in the medium in a 10 ml tube, and centrifuge them at 200-300 x g to form cell pellets.
- Resuspend the resulting pellets in 100% FBS at a density of 5 x 105/ml.
- Prepare the collagen gel on ice. Cool the reagents, pipettes and tubes in a refrigerator prior to the following procedure. Even on ice, the mixture solidifies to some extent, and the total volume is less than the sum of all constituents.
- In each well, prepare the collagen gel by mixing 0.5 ml of fibroblast suspension (2.5 x 105 cells) in FBS, 2.3 ml type IA collagen, 670 μl 5x DMEM, and 330 μl reconstitution buffer. Allow the gels to set readily at room temperature.
- Vigorously pipette to ensure a homogenous mixture. Avoid creating bubbles during the pipetting process.
- Add the mixture (3 ml) to each well of a 6-well plate and allow to gelatinize in the incubator at 37 °C without disturbance for 30-60 min.
- Cancer cell culture on the collagen gel:
- Perform three-dimensional co-culture using a previously reported protocol with modifications6.
- Resuspend cancer cells in the 3D co-culture medium (or in DMEM with 10% FBS in the case of A549 cells) at a density of 1 x 105/ml.
- Pour 2 ml of the resuspended cell solution (2 x 105 cells) onto the surface of each gel. Modify cell number of cancer cells or fibroblasts in co-culture depending on experimental conditions.
- Gel contraction:
- Incubate gels overnight at 37 °C so the cancer cells can adhere to the collagen gel. Detach each gel, and generate a ‘floating culture’ in each well of the 6-well plate. NOTE: By using a floating culture, various changes in gel size are induced by mechanical tension and collagen degradation, which are mediated by cellular interaction between co-cultured cancer cells and fibroblasts.
- Use an angled 21 G needle or small spatula to separate each gel from the edge of the well. Every 2-3 days, refresh the wells with 2 ml of 3D co-culture medium. Measure the size of each gel every day for 5 days.
- Analysis of collagen gels:
- Measure the collagen content of each gel using collagen quantitation methods, such as the Sircol assay. Perform transcriptomic analysis or quantitative RT-PCR after collecting RNA from the gels5.
3. Air-liquid Interface Culture and Invasion Assay
After culturing the collagen gels at 37 °C for 5 days, expose the contracted gels to air by placing them on a mesh (70 μm pore size) in new 6-well plates. Make the mesh from commercially available cell strainers used for flow cytometry.
Gently fill each well with 11 ml of 3D co-culture medium.
Position the gels onto the mesh using a sterile spoon, and remove any remaining medium from the gels. On this condition, submerge gels in the medium while expose the upper surface of the gel to air. Define this culture condition as air-liquid interface. NOTE: After 5-7 days of maintaining the air-liquid interface culture at 37 °C in the incubator, invasive cancer cells migrate into the gel. Depending on the cell type and as a result of cellular interactions, variable degrees of cell morphological change in the cancer cells are induced by the air-liquid interface culture.
For histological evaluation, fix the gel in formalin solution overnight at room temperature and embed in paraffin. Stain vertical sections (4 μm) with hematoxylin and eosin (H&E). Perform immunohistochemical analysis on the sections4.
Representative Results
This co-culture method, mimicking the tumor microenvironment, is a useful tool to investigate the interactions between cancer cells and fibroblasts embedded in collagen gels. In the previous study, three parameters were evaluated in this experimental model: collagen gel contraction, cancer cell invasion and morphological change. Cancer cell proliferation was also estimated using Ki67 immunostaining4. Lung tissue samples were collected from cancerous and non-cancerous sections of a resected lung lobe (Figure 1A). Samples were minced into small pieces and cultured in DMEM supplemented with 10% FBS (Figure 1B). Fibroblasts growing out of the tissue section were observed under the microscope (Figure 1C). Primary cultured lung fibroblasts were embedded into a collagen gel (Figure 2A, a) and A549 lung cancer cells were co-cultured on the gel (Figure 2A, b). After floating culture in the medium (Figure 2A, c), the gel was further cultured under the condition of air-liquid interface (Figure 2A, d). The gel was fixed in formalin and used for immunohistochemical analyses (Figure 2A, e). For air-liquid interface culture, the gel was placed on a mesh in a well of 6-well plate (Figure 2B). Histological analyses of the gel revealed invasion of A549 cells into the collagen gel embedded with lung cancer-associated fibroblasts (Figure 3).
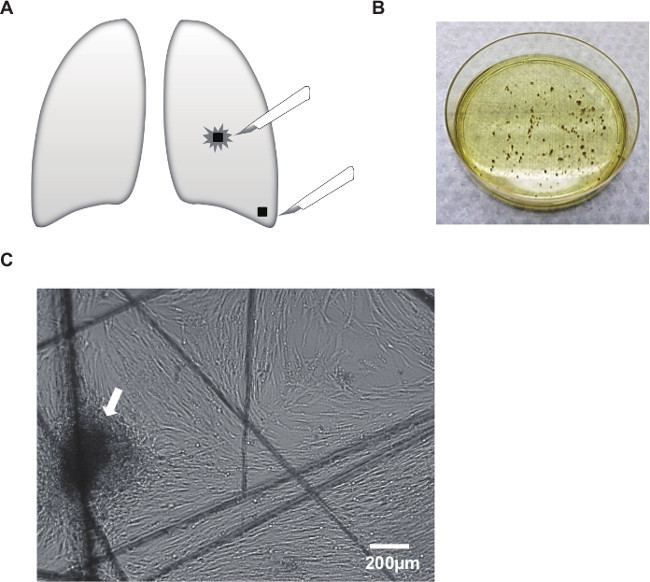 Figure 1: Outgrowth of fibroblasts from surgically resected human lung tissue. (A) Samples were collected from cancerous and non-cancerous sections of a resected lung lobe. (B) Samples were minced into small pieces and cultured in DMEM supplemented with 10% FBS. (C) Fibroblasts grew out of the tissue section (arrow). Note that the cells migrated along the scratches made by the scalpel blades on the culture dish. Scale bar, 200 μm.
Figure 1: Outgrowth of fibroblasts from surgically resected human lung tissue. (A) Samples were collected from cancerous and non-cancerous sections of a resected lung lobe. (B) Samples were minced into small pieces and cultured in DMEM supplemented with 10% FBS. (C) Fibroblasts grew out of the tissue section (arrow). Note that the cells migrated along the scratches made by the scalpel blades on the culture dish. Scale bar, 200 μm.
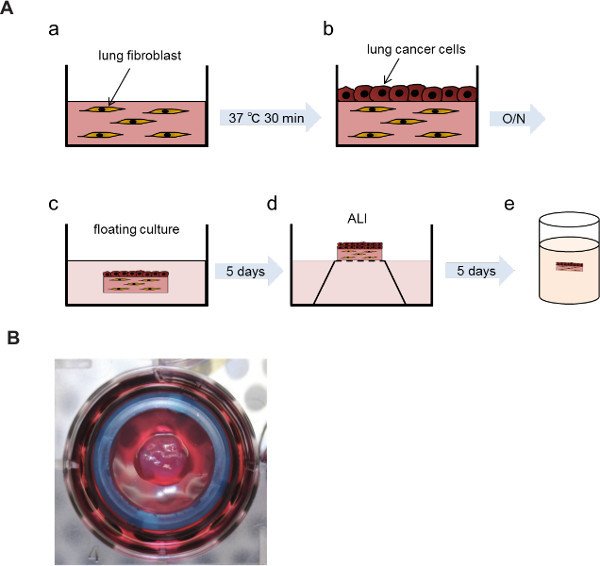 Figure 2: Air-liquid interface culture of the collagen gel. A549 cells were co-cultured on a collagen gel embedded with the fibroblasts, and the layer of A549 cells was exposed to air by placing the gel onto a mesh in medium. (A) Experimental procedures of three-dimensional co-culture and air-liquid interface. (a) Collagen gel embedded with lung fibroblasts. (b) A549 cells co-cultured on the collagen gel. (c) Floating culture. (d) Air-liquid interface (ALI). (e) Fixation of the gel in formalin. (B) Representative picture of the gel placed on the mesh in a well of 6-well plate. O/N: overnight.
Figure 2: Air-liquid interface culture of the collagen gel. A549 cells were co-cultured on a collagen gel embedded with the fibroblasts, and the layer of A549 cells was exposed to air by placing the gel onto a mesh in medium. (A) Experimental procedures of three-dimensional co-culture and air-liquid interface. (a) Collagen gel embedded with lung fibroblasts. (b) A549 cells co-cultured on the collagen gel. (c) Floating culture. (d) Air-liquid interface (ALI). (e) Fixation of the gel in formalin. (B) Representative picture of the gel placed on the mesh in a well of 6-well plate. O/N: overnight.
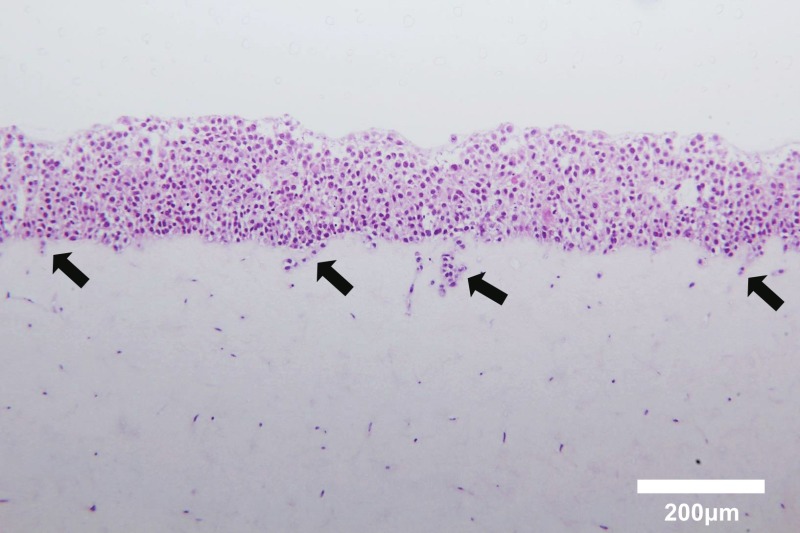 Figure 3. Hematoxylin and eosin staining of cross-sections of a three-dimensionally cultured gel composed of A549 cells and human lung fibroblasts. Note that A549 cells cultured on the collagen gel invaded the gel embedded with fibroblasts (arrows). Scale bar, 200 μm. Please click here to view a larger version of this figure.
Figure 3. Hematoxylin and eosin staining of cross-sections of a three-dimensionally cultured gel composed of A549 cells and human lung fibroblasts. Note that A549 cells cultured on the collagen gel invaded the gel embedded with fibroblasts (arrows). Scale bar, 200 μm. Please click here to view a larger version of this figure.
Discussion
CAFs form a major component of the ECM surrounding cancer cells and not only provide a scaffold for the tumor, but also actively participate in tumor development7. Accumulating evidence unravels the impact of CAFs or their related molecules on the eventual prognosis, highlighting the critical roles of CAF-mediated tumor progression8.
In the previous study, we employed outgrowth method to isolate lung CAFs4. In this experiment, the maintenance of tissue section adhesion onto the dish surface is critical. Cells are unable to migrate out from tissue sections floating in the culture medium, and once the tissue sections become detached from the surface, they can no longer be used for cell outgrowth on the same culture dish. Cutting of the tissue yields exudates, which serve as a glue for attachment to the dish surface. When fixing the tissue section with a cover slip, the amount of grease used is also critical. Small amounts of grease fail to keep the cover slip attached, while excessive grease obscures the cell culture area. Attachment of tissue sections using a cover slip and the appropriate pressure is also a key to facilitating cell outgrowth.
The experimental models for cancer cell proliferation and invasion have mostly relied on two-dimensional monolayer tissue cultures or Boyden chamber assays. To better understand intercellular communication and ECM-dependent modulation of cancer cell behavior, three-dimensional co-cultures are powerful tools. Organotypic cultures are also useful for investigating multicellular interactions, though they need higher technical skills and experimental conditions are limited.
Our co-culture model serves as a platform to evaluate tumor-stromal interactions under conditions mimicking the tumor microenvironment. Cells with specific gene overexpression or knockdown are easily applicable in our culture system, which is an advantage over organotypic culture. Notably, our previous observations suggested that co-cultured fibroblasts actively modulate cancer cell differentiation and invasion4. It would be of interest to test possible multicellular interactions in our experimental setting, such as endothelial cells, inflammatory cells and non-cancerous epithelial cells.
Distinct characteristics of CAFs have been demonstrated in co-culture studies or by gene expression profiling, using primary CAF cultures and their normal counterparts9. On the other hand, these studies have also shown intra-tumoral or inter-individual heterogeneity of CAFs10, part of which might be attributable to different levels of growth factor signalling11 or transcription factor activation12. Thus, it is essential to further characterize heterogeneous CAFs derived from cancer patients13. Following repeated cell passages, the characteristics of primary cultured CAFs may be modified. Therefore, it would be better to characterize CAFs at early passages.
CAFs contribute to ECM deposition and remodeling, which in turn, may activate CAFs through tension-triggered cell signaling. Such a mechanotransduction-mediated feed-forward loop of CAF activation has been suggested14, and our collagen gel contraction model may provide insights into these mechanisms. The mechanisms of collagen gel contraction include cell contraction, proliferation, migration, differentiation, and collagen remodeling15. Detailed histological studies and experiments using ligands, inhibitors, or RNAi may be helpful to gain a mechanistic insight into tumor-stromal interactions.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) (26461185 and 25460137).
References
- Strell C, Rundqvist H, Ostman A. Fibroblasts-a key host cell type in tumor initiation, progression, and metastasis. Ups. J. Med. Sci. 2012;117(2):187–195. doi: 10.3109/03009734.2012.654859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsten M, Hägglöf C, Peña C, Ostman A. A digest on the role of the tumor microenvironment in gastrointestinal cancers. Cancer Microenviron. 2010;3(1):167–176. doi: 10.1007/s12307-010-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsten M. Cancer-Associated Fibroblasts as Another Polarized Cell Type of the Tumor Microenvironment. Front. Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, et al. Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem. Biophys. Res. Commun. 2012;423(1):158–163. doi: 10.1016/j.bbrc.2012.05.104. [DOI] [PubMed] [Google Scholar]
- Ohshima M, et al. TGF-β signaling in gingival fibroblast-epithelial interaction. J. Dent. Res. 2010;89(11):1315–1321. doi: 10.1177/0022034510378423. [DOI] [PubMed] [Google Scholar]
- Ikebe D, Wang B, Suzuki H, Kato M. Suppression of keratinocyte stratification by a dominant negative JunB mutant without blocking cell proliferation. Genes Cells. 2007;12(2):197–207. doi: 10.1111/j.1365-2443.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- Paulsson J, Micke P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin. Cancer Biol. 2014;25:61–68. doi: 10.1016/j.semcancer.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Navab R, et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc. Natl. Acad. Sci. U S A. 2011;108(17):7160–7165. doi: 10.1073/pnas.1014506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M, et al. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin. Cancer Res. 2013;19(21):5914–5926. doi: 10.1158/1078-0432.CCR-13-0694. [DOI] [PubMed] [Google Scholar]
- Hägglöf C, et al. Stromal PDGFRbeta expression in prostate tumors and non-malignant prostate tissue predicts prostate cancer survival. PLoS One. 2010;5(5):e10747. doi: 10.1371/journal.pone.0010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito RA, et al. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res. 2010;70(7):2644–2654. doi: 10.1158/0008-5472.CAN-09-3644. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15(6):637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, et al. Histamine induces human lung fibroblast-mediated collagen gel contraction via histamine H1 receptor. Exp. Lung Res. 2014;40(5):222–236. doi: 10.3109/01902148.2014.900155. [DOI] [PubMed] [Google Scholar]


