Abstract
Cytomegalovirus (CMV) colitis usually occurs in immunocompromised patients after undergoing organ transplantation or chemotherapy. We report the case of a 60-year-old immunocompetent Japanese woman who presented with abdominal pain and bloody diarrhea. She was initially diagnosed as having ischemic colitis with pseudomembranous colitis on the basis of her symptoms, Clostridium difficile antigen positivity, and colonoscopic findings, which showed ulcer formation from the sigmoid colon to rectum. In spite of bowel rest and administration of metronidazole, her symptoms did not improve. On follow-up colonoscopy, ulcerations remained unchanged. Biopsy of the ulceration revealed CMV-infected cells leading to a diagnosis of CMV colitis. CMV colitis is a rare but possible differential diagnosis in immunocompetent patients. We recommend endoscopic biopsy in a case of refractory abdominal pain and bloody diarrhea.
Keywords: cytomegalovirus, colitis, immunocompetent, enterocolitis
Introduction
Cytomegalovirus (CMV) is classified as a herpes virus. The proportion of humans with evidence of prior CMV infection varies throughout the world, with seroprevalence rates ranging between 40% and 100% of the adult population. CMV infection in immunocompetent hosts is generally asymptomatic or may present as a mononucleosis syndrome. Gastrointestinal involvement with CMV is uncommon in immunocompetent hosts, but can cause significant morbidity and mortality. Symptomatic CMV colitis is a well known entity in immunocompromised patients undergoing immunosuppressive chemotherapy, those with human immunodeficiency virus (HIV), and transplant recipients. While CMV colitis is almost always secondary to reactivation of latent infection in immunosuppressed patients, CMV colitis in the immunocompetent host can occur in the setting of primary infection. We report a case of CMV colitis in an immunocompetent Japanese woman with no history of HIV infection, organ transplant, or malignancy.
Case report
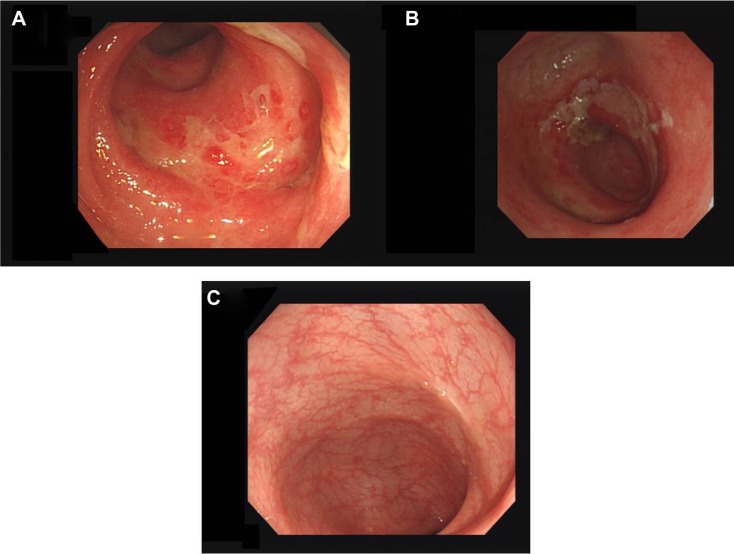
A 60-year-old Japanese woman presented with watery diarrhea. She denied nausea, vomiting, or abdominal pain. She noted a 4-week history of extremity weakness. Her past medical history involved schizophrenia, treated with paliperidone 6 mg and flunitrazepam 2 mg per day. She had no history of diabetes, malignancy, or steroid use. On admission, her physical findings included a masked face and slight rigidity. No electrolyte abnormalities were detected and magnetic resonance imaging showed no abnormal findings. Her cerebrospinal fluid revealed no inflammatory changes and albuminocytologic dissociation was not detected. Her family doctor had increased her dose of paliperidone 1 month prior. We suspected her weakness might be caused by drug-induced parkinsonism. On admission, her white blood cell count was 22,500/mm3 and C-reactive protein was 1.91mg/dL. Aspartate transaminase was slightly elevated (66 U/L; normal reference 9–32 U/L), compared with normal alanine transaminase (28 U/L; normal reference 3–38 U/L). Urine white blood cells and nitrites were positive. We suspected concomitant urinary tract infection and administered cefotaxime. Nine days after admission, her weakness improved gradually, but she developed abdominal pain and bloody diarrhea. She had no fever, nausea, or vomiting. On abdominal computed tomography, bowel wall thickening, and nonspecific inflammatory changes of surrounding fat tissue were seen from the sigmoid colon to rectum. Firstly, we suspected ischemic colitis and treated her with bowel rest. Four days after bowel rest, her symptoms did not improve. We performed colonoscopy, which revealed mucosal erosion and ulceration from the sigmoid colon to rectum (Figure 1A). Clostridium difficile was detected from culture of bowel lavage. We suspected pseudomembrane colitis and treated her with a 10-day course of metronidazole 250 mg four times daily. After treatment, her symptoms persisted and we performed follow-up colonoscopy, which showed only minimally improved ulceration (Figure 1B). Hematoxylin-eosin stained biopsy specimens of ulceration showed no inclusion bodies (Figure 2A), but immunostained specimens revealed CMV-infected cells (Figure 2B). Laboratory findings showed elevated CMV-immunoglobulin M (0.87; normal reference 0.00–0.79). We made a diagnosis of CMV colitis. HIV serology was negative and upper gastrointestinal endoscopic examination revealed that she had no malignancy, in addition to the result of the colonoscopy described above. She was considered as immunocompetent. By the time of diagnosis, her symptoms had substantially improved. Two months later, we performed follow-up colonoscopy, which showed complete resolution of ulceration (Figure 1C); biopsy demonstrated no CMV-infected cells.
Figure 1.
Endoscopic findings.
Notes: (A) Mucosal erosion and ulceration in rectum. (B) The lesion got little improvement after 10-day course of metronidazole. (C) The lesion showed complete recovery of the ulceration.
Figure 2.
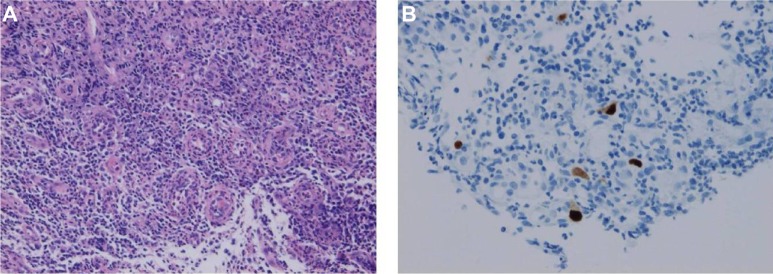
Histopathological findings. (A) Hematoxylin-eosin stained, 100×. (B) Immunohistochemistry for cytomegalovirus, 200×.
Discussion
We found 12 case reports involving CMV colitis in an immunocompetent host over the past 16 years1–10 (Table 1). The age of onset ranged from 29 to 85 years. Presenting symptoms included fever, diarrhea, gastrointestinal bleeding, and abdominal pain. Patel et al11 reported frequent involvement of the sigmoid colon and rectum in CMV colitis occurring in immunocompetent patients. Endoscopic findings often show ulceration. Occasionally pseudomembrane and pseudopolyps may be detected, findings which can be pitfalls in the misdiagnosis of CMV colitis as pseudomembranous colitis. In this case, we used antibiotics to treat urinary tract infection, leaving the patient at risk for pseudomembranous colitis. Coinfection of CMV and C. difficile sometimes occurs in immunocompromised hosts such as post-transplant recipients and patients undergoing immunosuppressive chemotherapy.12,13 With regards to immunocompetent patients, the concomitant infection is rare. We found one case report of coinfection in an immunocompetent patient over the past 10 years.14 However, bloody diarrhea and a poor response to metronidazole is not characteristic of pseudomembranous colitis. Additionally, the poor response to bowel rest is not typical in the time course of ischemic colitis. CMV colitis, a rare but possible disease in immunocompetent hosts, may cause bowel perforation, leading to a poor prognosis. We recommend endoscopic biopsy and measurement of CMV immunoglobulin M antibody level in cases of refractory abdominal pain and bloody diarrhea, in consideration of CMV colitis. In immunocompromised patients, a viral antibody test is usually insufficient to diagnosis CMV colitis, because their ability to produce antibodies is often suppressed. In such cases, detection of the antigen DNA and RNA is helpful for correct diagnosis of CMV colitis. However, in immunocompetent patients, such advanced tests are not always necessary, considering their costs.
Table 1.
Case reports on cytomegalovirus colitis in immunocompetent hosts
| Age | Sex | Comorbidity | Initial presentation | Treatment | Reference |
|---|---|---|---|---|---|
| 75 | F | Achalasia, gallstone | Fever, watery diarrhea | Gancyclovir | 1 |
| 61 | F | None | Abdominal pain, fever | Supportive | 2 |
| 29 | M | Unknown | Altered mental status | Ganciclovir | 2 |
| 56 | M | CRF, DM | Abdominal pain, watery diarrhea | Gancyclovir | 3 |
| 33 | M | None | Fever, bloody stool | Supportive | 4 |
| 78 | F | None | Abdominal pain, bloody stool | Colectomy | 5 |
| 76 | F | DM, HTN | Abdominal pain, watery diarrhea | Gancyclovir | 6 |
| 64 | M | Cerebral infarction | Nausea, diarrhea | Gancyclovir | 7 |
| 82 | M | None | Epigastric pain, diarrhea | Gancyclovir | 8 |
| 60 | F | None | Fever, abdominal pain, bloody stool | Enterectomy | 9 |
| 85 | M | Pneumonia | Abdominal pain, diarrhea | Colectomy | 10 |
| 76 | M | Parkinson’s disease, peptic ulcer | Diarrhea | Gancyclovir | 10 |
Abbreviations: CRF, chronic renal failure; DM, diabetes mellitus; HTN, hypertension.
Galiatsatos et al15 performed a meta-analysis of CMV colitis in immunocompromised hosts, reporting that advancing age (>55 years), male sex, and immunomodulating conditions (pregnancy, chronic renal failure, untreated nonhematological malignancy, and diabetes) may adversely influence survival, and patients with such risk factors should be treated with antiviral therapy. Our case had the poor prognostic factor of advanced age. Nonetheless, the colon ulceration and erosions improved and CMV was eradicated without use of antiviral drugs. Indication for antiviral drugs warrants further discussion.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Seminari E, Fronti E, Contardi G, et al. Colitis in an elderly immunocompetent patient. J Clin Virol. 2012;55(3):187–190. doi: 10.1016/j.jcv.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Momin N, Telisinghe PU, Chong VH. Cytomegalovirus colitis in immunocompetent patients. Singapore Med J. 2011;52(9):170–172. [PubMed] [Google Scholar]
- 3.Kim SH, Kim YS, Kim HW, et al. A case of cytomegalovirus colitis in an immunocompetent. Hemodialysis patient. Hemodial Int. 2011;15(2):297–300. doi: 10.1111/j.1542-4758.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen E, Grønbaek K, Linnemann D. Cytomegalovirus colitis in immunocompetent young male. Ugeskr Laeger. 2009;171(15):1298. Danish. [PubMed] [Google Scholar]
- 5.June L, Chin N, Chatterjee D. Cytomegalovirus colitis presenting as massive lower gastrointestinal bleeding in an immunocompetent patient. Indian J Surg. 2008;70(1):28–31. doi: 10.1007/s12262-008-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter D, Olchovsky D, Pokroy R, Ezra D. Cytomegalovirus-associated colitis causing diarrhea in an immunocompetent patient. World J Gastroenterol. 2006;12(42):6898–6899. doi: 10.3748/wjg.v12.i42.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockwood MR, Liddle J, Kitsanta P. Cytomegalovirus colitis – an unusual cause for diarrhoea in an elderly woman. Age Ageing. 2006;35(2):198–200. doi: 10.1093/ageing/afj038. [DOI] [PubMed] [Google Scholar]
- 8.Siegal DS, Hamid N, Cunha BA. Cytomegalovirus colitis mimicking ischemic colitis in an immunocompetent host. Heart Lung. 2005;34(4):291–294. doi: 10.1016/j.hrtlng.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Petrogiannopoulos CL, Kalogeropoulos SG, Dandakis DC, et al. Cytomegalovirus enteritis in an immunocompetent host. Chemotherapy. 2004;50(6):276–278. doi: 10.1159/000082625. [DOI] [PubMed] [Google Scholar]
- 10.Klauber E, Briski LE, Khatib R. Cytomegalovirus colitis in the immunocompetent host: an overview. Scand J Infect Dis. 1998;30(6):559–564. doi: 10.1080/00365549850161098. [DOI] [PubMed] [Google Scholar]
- 11.Patel SM, Cohen P, Pickering MC, Gazzard BG, Andreyev J. Successful treatment of acute haemorrhagic cytomegalovirus colitis with ganciclovir in an individual without overt immunocompromise. Eur J Gastroenterol Hepatol. 2003;15(9):1055–1060. doi: 10.1097/00042737-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 12.John SG, Dominguez C, Chandiramani V, Vemulappalli T. A rare case intractable diarrhea secondary to Clostridium difficile and cytomegalovirus coinfection. Am J Case Rep. 2013;14:498–501. doi: 10.12659/AJCR.889700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahman M, Krell R, Brayman K, et al. Simultaneous Clostridium difficile-associated colitis and late-onset intestinal cytomegalovirus disease in a renal transplant recipient. Ann Transplant. 2010;15(4):72–76. [PubMed] [Google Scholar]
- 14.Kurtz M, Morgan M. Concomitant Clostridium difficile colitis and cytomegalovirus colitis in an immunocompetent elderly female. BMJ Case Rep. 2012;2012:pii:bcr2012007273. doi: 10.1136/bcr-2012-007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiatsatos P, Shrier I, Lamoureux E, Szilagyi A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci. 2005;50(4):609. doi: 10.1007/s10620-005-2544-6. [DOI] [PubMed] [Google Scholar]