Abstract
Respirometric profiling of isolated mitochondria is commonly used to investigate electron transport chain function. We describe a method for obtaining samples of human Vastus lateralis, isolating mitochondria from minimal amounts of skeletal muscle tissue, and plate based respirometric profiling using an extracellular flux (XF) analyzer. Comparison of respirometric profiles obtained using 1.0, 2.5 and 5.0 μg of mitochondria indicate that 1.0 μg is sufficient to measure respiration and that 5.0 μg provides most consistent results based on comparison of standard errors. Western blot analysis of isolated mitochondria for mitochondrial marker COX IV and non-mitochondrial tissue marker GAPDH indicate that there is limited non-mitochondrial contamination using this protocol. The ability to study mitochondrial respirometry in as little as 20 mg of muscle tissue allows users to utilize individual biopsies for multiple study endpoints in clinical research projects.
Keywords: Medicine, Issue 96, Respirometry, mitochondria, bioenergetics, skeletal muscle, Vastus lateralis, biopsy
Introduction
Mitochondria are the primary energy production sites in the cell and have important roles in aging as well as various age related disorders such as cardiovascular disease, Alzheimer’s disease, diabetes, cancer, and obesity. Respirometric profiling of isolated mitochondria provides direct analysis of electron transport chain (ETC) function and has contributed significantly to our understanding of mitochondrial biology and its role in health and disease. Isolated mitochondria are used to study various aspects of bioenergetics like, substrate transport, ATP synthase activity, proton leak, etc. The methodology described in this manuscript has been optimized to permit respirometric analysis of mitochondria isolated from skeletal muscle tissue biopsies obtained from human subjects. The biopsy protocol described in this manuscript has been utilized by our staff for the past 12 years. Our group has performed over 700 procedures on adults of various ages, up to 90 years old, and with various chronic disease conditions without any adverse safety issues. A key aspect of this protocol is that it is specifically designed to utilize minimal amounts of tissue, thereby facilitating its use in clinical research studies.
Various protocols have been developed for isolating mitochondria. Fernandez-Vizarra et al.1,2 described a method for isolating mitochondria from various rat tissues as well as cultured cells. Garcia-Cazarin et al.3 have reported a method for isolating mitochondria from skeletal muscles from rat and mouse. A method for isolating mitochondria from rat brain has also been reported by Iglesias-Gonzales et al.4 Gross, et al.5 reported a method of isolating mitochondria using the barocycler and/or the PCT shredder. Recently, Franko et al.6 reported a method of isolating highly enriched mitochondria using anti-TOM22 magnetic beads.
While these protocols yield excellent results, tissue size requirements are high compared to the method described in this manuscript. For example, Gross et al.5 used 1.5-1.8 g of the gastrocnemius muscle, and about 2 g of the kidney tissue. Similarly, Franco et al.6 used 500 mg mouse liver tissue. From our experience, typical yields to be expected from percutaneous needle biopsy of skeletal muscle (Vastus lateralis) range from 100-200 mg. The ability to assess mitochondrial function in 20-50 mg of muscle tissue using the protocol described here permits users to perform multiple assessments per biopsy and to store samples for future use in other molecular biology experiments. This is a critical feature in clinical research and other studies that require diligent use of samples. It should be noted that previously frozen mitochondria are not good for studying coupled respiration due to outer mitochondrial membrane damage and loss of Cytochrome C activity. Our method has been adapted and modified from the method published by Chappell and Perry7.
Using the methods described in this manuscript, we have recently reported that the respirometric profile of mitochondria isolated from human Vastus lateralis is directly correlated with physical ability, measured as gait speed8.
Protocol
NOTE: The protocol described was approved by the Institutional Review Board of Wake Forest School of Medicine. Informed consent was obtained in writing. All participants were healthy older adults (65-79 years) of both genders, with BMIs ranging from 23-35.
1. Skeletal Muscle Biopsy
As previously described, 9 perform all biopsies in the early morning after an O/N fast. Ask the subjects to refrain from taking aspirin, prescription and over-the-counter non-steroidal anti-inflammatory drugs, or other compounds that may affect bleeding, platelets, or bruising for the week prior to the biopsy. Ask the participants to also refrain from any strenuous activity for at least 36 hr prior to the biopsy. NOTE: Muscle is obtained from the Vastus lateralis using the percutaneous needle biopsy technique with a percutaneous needle under local anesthesia with 1% lidocaine. No medical complications or other reported adverse events from the procedure have occurred in our clinical research unit.
- NOTE: The biopsy procedure described is adapted from that of Bergstrom10.
- Briefly, locally administer 1% lidocaine taking care not to infiltrate the muscle. Follow this with a 10 min wait period to permit sufficient numbing.
- Take the biopsies from the belly of the muscle (the middle region of muscle between insertion and origin) avoiding subfascial and myotendonous areas.
- Use percutaneous needle (a suction assisted reusable device with a side cutting window and inner cutting cylinder) and follow a fascial “pop” or resistance of the fascia as a guide.
- Estimate the depth with the anesthesia needle, then again feel it with the narrow scalpel blade and make a 4-5 mm incision through the fascia. Advance the needle through the incision until it is inserted into the muscle.
- Collect multiple samples with the window turned in different directions. Apply continuous suction using a 60 cc syringe while advancing and withdrawing muscle samples into the percutaneous needle two to four times in different directions. Discontinue the suction and remove the needle. NOTE: Each pass (insertion and removal of needle) should take less than a minute.
- Have an assistant apply firm pressure to puncture site for 5 min to establish hemostasis. Disconnect needle from suction tubing and carefully remove muscle samples from the window and barrel.
- Make a second pass if more muscle is needed by repeating the above procedure.
- Remove any visible blood clots from the muscle sample using forceps, weigh the sample, and immediately place in a tube containing ice-cold DPBS. NOTE: The average yield using this methodology is 150 ± 20 mg.
2. Mitochondrial Isolation
Freshly prepare the Chappel-Perry (CP) and Mitochondrial Assay Solution (MAS) buffers (Table 1) on the day of the experiment, or aliquot and store them at -20 °C. Prepare the compounds in dimethyl sulfoxide at a concentration of 2.5 mM and aliquot and store at -20 °C. Use the buffer and compound aliquots stored at -20 °C within 2 months from the day of preparation.
Remove visible connective tissue from the sample using sharp scissors and tweezers; if needed use a dissecting microscope for this step. Thoroughly wash specimens 3-4 times with ice-cold DPBS buffer to remove blood. Keep the samples on ice-cold DPBS and process as soon as possible, taking care not to exceed more than 45 min from biopsy. Take precaution to carefully remove any tendons or adipose tissue from the muscle sample.
Immediately chop the muscle tissue into fine pieces using a sterile pair of scissors and suspend in 500 μl to 1 ml CPI containing proteinase (Nagarse) at a concentration of 0.2 mg/g tissue. Follow this with a 5 min incubation at RT and then transfer to ice.
Homogenize the minced tissues treated with Nagarse using an automated homogenizer. Keep the sample on ice throughout this process. Homogenize each tissue sample four times, each time for a pulse of 2 sec, using the automated homogenizer at a speed setting of 10,000 rpm. Wash the probe with 70% ethanol followed by distilled water between tissues.
Wash the homogenized tissue with an equal volume of CP I (500 μl to 1 ml) and 2x volume of CP II (1 ml to 2 ml), and collect the content in a centrifuge tube and centrifuge at 600 x g, 4 °C for 10 min. Pass the supernatant through a wetted cheese cloth, collect the filtrate and discard the pellet, thus removing the majority of non-mitochondrial fractions.
3. Washing the Mitochondria
Centrifuge the supernatant from the above step at 10,000 x g, 4 °C for 10 min. Suspend the pellet in 4 ml of CP II buffer and further centrifuge at 10,000 x g, 4 °C for 10 min. NOTE: On rare occasions, a thin pellet of blood is formed below the mitochondrial pellet. In that case, remove the mitochondrial pellet by gentle aspiration with CPII buffer. This step can be avoided by thoroughly washing away blood at step 2.
Suspend the pellet obtained in 2ml of CP I buffer. At this stage use a small aliquot from this suspension for protein estimation. Resuspend the remaining sample in CP I buffer and centrifuge as above. Suspend the final pellet in a minimal amount (200 μl) of Mitochondrial Assay Solution (MAS). NOTE: Protein is assayed at this stage because CP I buffer does not have BSA, whereas MAS in which the samples will be later suspended does have BSA in it.
4. Estimate the Mitochondrial Content by Measuring Protein Concentration Using a BCA Protein Assay Kit
NOTE: Use this concentration to calculate the quantity of mitochondria used for loading onto a 24-well microplate for respirometric measurements, or for Western blot experiments. Take into account the dilution factor (10) for calculation of the protein concentration.
5. Perform the XF assays as described by Rogers, GW, et al.12
NOTE: Visualize the O2 consumption rate (OCR) in pMoles O2/min, or absolute levels of O2 and pH in the data output.
Use 1x MAS to prepare compounds to be injected. Add a 10x concentration of the compounds to the ports A-D to give a final concentration as follows: Port A, ADP [Adenosine 5’ –diphosphate, 2 mM, 50 μl]; port B, oligomycin (2 μM, 55 μl); port C, FCCP [carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, 6 µM, 60 μl]; and port D, 2 μM antimycin-A (65 μl). Prepare sufficient volume of compounds for the required number of wells.
Determine the optimum amount of mitochondria by titration. For example, load 1.0 μg, 2.5 μg, and 5.0 μg of mitochondria per well in a 50 μl volume of ice-cold 1x MAS containing substrate. To minimize variability between wells, first dilute 10x mitochondria in cold 1x MAS + substrate. Next, deliver 50 μl of this suspension to each well (except background correction wells).
Centrifuge the plate at 2,000 x g, 20 min at 4 °C. After centrifugation, gently add 450 μl of 1x MAS + succinate (10 mM) and rotenone (2 μM) (initial conditions; see below) to each well. View the mitochondria under the microscope to ensure homogenous adherence to the well prior to transferring the plate to the XF analyzer. NOTE: The initial conditions used will depend on whether the respiration is driven by either ETC complex I or complex II. For complex II-driven respiration, use succinate (10 mM) and rotenone (2 μM) as initial conditions. Rotenone blocks complex I and succinate provides fuel for complex II (succinate dehydrogenase). To study respiration driven by complex I, use either pyruvate and malate, each at a final concentration of 5 mM or glutamate and malate each at a final concentration of 10 mM as initial conditions. The latter can help in distinguishing dysfunction among tricarboxylic acid cycle and substrate transport. To study respiration driven by both complex I and complex II, include pyruvate and succinate without rotenone or malate as initial conditions. Use palmitoyl carnitine as a substrate for β-oxidation.
Sequentially measure the mitochondrial respiration in real time using the respirometer by programming it as previously described 12. Use the settings for the respirometer provided in Table 2. NOTE: A brief explanation of various mitochondrial respiration states are as follows:
State 2 = coupled state with substrate present; State 3 = phosphorylating respiration in the presence of saturating ADP; State 4o = non-phosphorylating respiration induced by oligomycin; State 3u = maximal uncoupled respiration stimulated by the uncoupler FCCP; Residual non-mitochondrial respiration = respiration after complex III inhibition by antimycin-A. It should also be pointed out that, the length of the OCR measurement in combination with the mitochondrial concentration could influence the data by depleting oxygen during the measurement periods.
6. Western Blot
NOTE: Determine the mitochondrial marker COX IV and whole tissue GAPDH by Western blotting to assure enrichment of mitochondria in the final sample
Separate the isolated mitochondria and whole tissue extract by 12% sodium dodecyl sulfate polyacrylamide gels.
Transfer the proteins onto a polyvinylidene fluoride (PVDF) membrane.
Incubate with COX IV antibody (1:20,000), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. For GAPDH determination, strip the blot and probe with a GAPDH monoclonal antibody (1:2,000). NOTE: If differences in ER contamination are of concern, include ER markers such as ERp72, calnexin, or calreticulin in the analysis.
Representative Results
Figure 1 depicts a detailed flowchart of the entire protocol.
Western blot profiles of COX IV/GAPDH (Figure 2) depict the expression of the mitochondrial protein, COX IV, and the non-mitochondrial marker, GAPDH. Expression of both COX IV and GAPDH are evident in the whole muscle lysate. After mitochondria are isolated using the technique described in this protocol, COX IV bands are still evident while GAPDH is absent at the same exposure. Longer exposures may reveal a faint GAPDH band. These blots indicate that the isolated mitochondria have minimal non-mitochondrial contamination. Moreover, COX IV expression in isolated mitochondria is consistent between samples.
Figure 3 shows typical respirometric profiles driven by complex II (succinate and rotenone) using 1.0 μg, 2.5 μg, and 5.0 μg of mitochondria. As expected, overall OCR is increased with higher amounts of mitochondria. The calculated respiratory control ratio (RCR) for this assay is 7.95, indicating that the mitochondrial preparation is of high quality. Furthermore, state 3u OCR is slightly higher than that of state 3, confirming mitochondrial quality.
In order to compare consistency of results when profiling different amounts of mitochondria, we performed ANOVA (analysis of variance) and calculated the sum of squares (SS) as actual values of variance using 1.0 µg, 2.5 µg, and 5.0 µg of mitochondria loaded per well (Figure 4). SS is presented for state 2, state 3, state 3u, antimycin A, and RCR. For state 2 and state 3 measurements, one way ANOVA was statistically significant (p<0.01 and p<0.05, respectively). Similarly, the one way ANOVA was statistically significant for antimycin and RCR (p<0.01 and p<0.0001, respectively. No significant difference was seen for state 3u between groups. These results indicate that 5.0 μg of mitochondria per well gave the lowest SS compared to other concentrations and is the optimal amount to use in the XF 24 system with our population of participants.
Figure 5 serves as a guide to indicate how much mitochondrial protein can be expected based on the initial muscle sample size. As expected there is a strong correlation between the amount of muscle (mg) processed and the total mitochondrial protein content (mg) of the final sample.
| Chappel-Perry buffer I (CPI) | |
| Chemical | Concentration |
| KCl | 100 mM |
| MOPS | 50 mM |
| EDTA | 1 mM |
| MgSO4 | 5 mM |
| ATP | 1 mM |
| pH | 7.4 |
| Chappel-Perry buffer II (CPII) | |
| Chemical | Concentration |
| KCl | 100 mM |
| MOPS | 50 mM |
| EDTA | 1 mM |
| MgSO4 | 5 mM |
| ATP | 0.2 mM |
| Fatty-acid free BSA | 0.50% |
| pH | 7.4 |
| Mitochondrial Assay Solution (MAS) (2X) | |
| Chemical | Concentration |
| Sucrose | 35 mM |
| Mannitol | 110 mM |
| KH2PO4 | 2.5 mM |
| MgCl2 | 2.5 mM |
| HEPES | 1.0 mM |
| EGTA | 0.5 mM |
| Fatty-acid free BSA | 0.10% |
| pH | 7.4 |
| Mitochondrial Assay Solution (MAS) with complex II initial conditions | |
| Chemical | Concentration |
| 1X MAS | |
| Succinate | 10 mM |
| Rotenone | 2 μM |
| pH | 7.4 |
| Mitochondrial Assay Solution (MAS) with complex I initial conditions | |
| Chemical | Concentration |
| 1X MAS | |
| Pyruvate | 5 mM |
| Malate | 5 mM |
| pH | 7.4 |
| *All buffers to be made in deionized water |
Table 1. Solution and buffer recipes.
| Protocol Steps | ||
| StartProtocol | ||
| Command | Time (min) | Port |
| Calibrate | 0.00 | |
| Wait | 10.00 | |
| Mix | 1.00 | |
| Wait | 3.00 | |
| Mix | 1.00 | |
| Wait | 3.00 | |
| Mix | 0.50 | |
| Measure | 3.00 | |
| Mix | 1.00 | |
| Measure | 3.00 | |
| Mix | 0.50 | |
| Inject | A | |
| Mix | 1.00 | |
| Measure | 6.00 | |
| Mix | 1.00 | |
| Inject | B | |
| Mix | 1.00 | |
| Measure | 3.00 | |
| Mix | 1.00 | |
| Inject | C | |
| Mix | 1.00 | |
| Measure | 3.00 | |
| Mix | 1.00 | |
| Inject | D | |
| Mix | 1.00 | |
| Measure | 3.00 | |
| EndProtocol |
Table 2. Mix, measure, and mix cycle setting for the respirometer.
 Figure 1.
Flowchart of the entire protocol.
Please click here to view a larger version of this figure.
Figure 1.
Flowchart of the entire protocol.
Please click here to view a larger version of this figure.
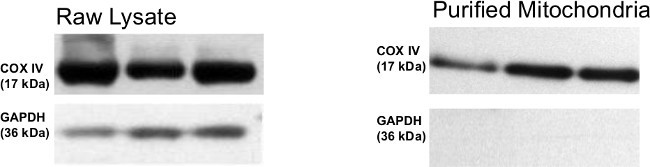 Figure 2. A representative Western blot for whole skeletal muscle tissue as well as isolated mitochondria. Whole tissue extract as well as isolated mitochondria were immunoblotted with COX IV antibody as mitochondrial marker and GAPDH antibody for non-mitochondrial control. No GAPDH band was observed in the isolated mitochondria indicating little or no contamination from non-mitochondrial sources. Please click here to view a larger version of this figure.
Figure 2. A representative Western blot for whole skeletal muscle tissue as well as isolated mitochondria. Whole tissue extract as well as isolated mitochondria were immunoblotted with COX IV antibody as mitochondrial marker and GAPDH antibody for non-mitochondrial control. No GAPDH band was observed in the isolated mitochondria indicating little or no contamination from non-mitochondrial sources. Please click here to view a larger version of this figure.
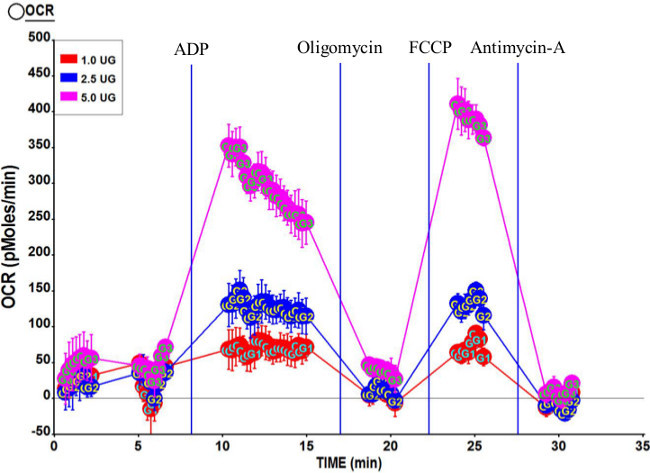 Figure 3.Representative respirometric profile of mitochondria isolated from human Vastus lateralis. Three concentrations of isolated mitochondria, 5.0 μg, 2.5 μg, and 1.0 μg were used in this assay. Final concentrations of compounds after port injections were 2 mM ADP (port A); 2 μM oligomycin (port B); 6 μM FCCP (port C); and 2 μM antimycin A (port D). Calculated RCR for this run was 7.95. Please click here to view a larger version of this figure.
Figure 3.Representative respirometric profile of mitochondria isolated from human Vastus lateralis. Three concentrations of isolated mitochondria, 5.0 μg, 2.5 μg, and 1.0 μg were used in this assay. Final concentrations of compounds after port injections were 2 mM ADP (port A); 2 μM oligomycin (port B); 6 μM FCCP (port C); and 2 μM antimycin A (port D). Calculated RCR for this run was 7.95. Please click here to view a larger version of this figure.
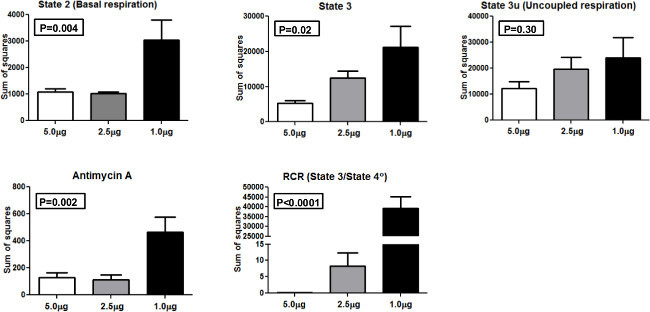 Figure 4.Sum of squares. Sum of squares for the different mitochondrial respiration states and RCR using 5.0 μg, 2.5 μg, and 1.0 μg mitochondria. Please click here to view a larger version of this figure.
Figure 4.Sum of squares. Sum of squares for the different mitochondrial respiration states and RCR using 5.0 μg, 2.5 μg, and 1.0 μg mitochondria. Please click here to view a larger version of this figure.
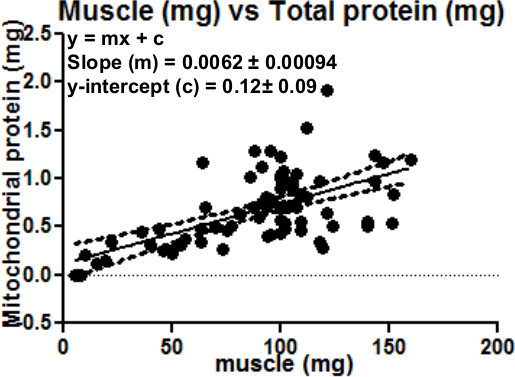 Figure 5.This can be used as a guide to estimate the amount of mitochondrial protein that can be expected based on the initial muscle sample weight. Regression analysis: of amount of muscle (mg) and total mitochondrial protein yield (mg). As expected, there is a direct positive correlation between the amount of muscle and the total mitochondrial protein obtained.
Figure 5.This can be used as a guide to estimate the amount of mitochondrial protein that can be expected based on the initial muscle sample weight. Regression analysis: of amount of muscle (mg) and total mitochondrial protein yield (mg). As expected, there is a direct positive correlation between the amount of muscle and the total mitochondrial protein obtained.
Discussion
Isolated mitochondria are often utilized in studies that examine the role of ETC function, as well as other mitochondrial activities, including substrate transport and TCA cycle function. Respirometric assays using isolated organelles permit direct examination of basic processes of oxidative phosphorylation and intrinsic properties of the ETC. Respirometric profiling of isolated mitochondria in comparison to whole cells or permeabilized muscle fibers has the advantages of relatively easy data interpretation and the absence of “interference” from non-mitochondrial processes or changes in mitochondrial mass/biogenesis. Normalization of data is based on mitochondrial protein content, thereby allowing straightforward cross-comparison of mitochondria between samples. Respirometric profiling of isolated mitochondria is a preferred approach when the aim of the study is to determine underlying mechanisms and identifying specific targets such as ETC components/complexes, or mitochondrial transport mechanisms.
Described is a protocol for muscle biopsy and isolation of functional mitochondria from small tissue samples. This method yields reproducible results between users due to utilization of an automated homogenizer versus hand operated dounce homogenizers. Isolation of mitochondria can be performed with as little as 20 mg of muscle tissue. The amount of isolated mitochondria that can be obtained from this sample size is sufficient to run Seahorse plate-based respirometry while leaving surplus mitochondria for other experiments and storage for further molecular analyses. It may be noted that this method can be translated to the XF 96, where even smaller amounts of mitochondria can be used (1-2 μg per well).
Several protocols for isolating mitochondria rely on dounce homogenizers for initial tissue disruption. A drawback of this method is the hands-on nature of the initial tissue homogenization. The force and speed of the pestle in the homogenizer can vary significantly between operators 6. This can result in experiment-to-experiment variation, as well as lab-to-lab variation, and leads to difficulty in comparing data between experiments. This is of particular concern in human intervention studies when data from participants are collected at separate time points, typically before and after treatment, and potentially at multiple sites. We use an automated homogenizer for a more consistent approach that yields more reproducible results with limited person-to-person variation. The speed of preparation also makes this approach suitable for handling multiple samples at the same time. Typically, up to three experiments can be performed in a single day.
Potential limitations of the technique described here arise from the use of isolated organelles and the use of a plate-based format. For example, Picard et al. have demonstrated that isolated mitochondria possess functional characteristics that differ fundamentally from those of intact mitochondria in permeabilized myofibers. They proposed that mitochondrial isolation techniques result in altered bioenergetic function, such as significantly increased RCR compared to permeabilized myofibers accompanied by greater reactive oxygen species production 13. Compared to permeabilized muscle fibers, isolation of mitochondria does require longer preparation time. Also, loss of cellular content diminishes physiological relevance, something that is retained in whole cells and even permeabilized fibers. The use of plate-based respirometry with the described technique permits replicate runs per sample. However, mitochondria must adhere to the bottom of each well. This configuration is different from their normal environment and may affect functional characteristics. In addition, it should be noted that using this protocol for mitochondrial isolation, there may still be contamination from endoplasmic reticulum (ER) in the mitochondrial preparation. Differences in ER contamination may affect the determination of mitochondrial yield and influence results.
In conclusion, this study presents data that confirms that mitochondria isolated from tissues using this procedure are functionally active and can be used for studies/applications that require high-quality isolated mitochondria from minimal amount of skeletal muscle samples. The advantage of this method is that: i) it is possible to isolate mitochondria from minimal quantities of skeletal muscle, ii) the procedure is quick, iii) with the plate based technology, it is possible to run multiple samples at the same time, and iv) there is enough surplus tissue and isolated mitochondria after the bioenergetic assay for sample storage and other molecular biological investigations.
Disclosures
The author, George Rogers, is an employee of Seahorse Bioscience that produces the instrument used in this article. Open Access fees were supported by Seahorse Biosciences.
Acknowledgments
We would like to thank Dr. Marc Liesa, Boston University School of Medicine, helpful discussions; Ms. Karin Murphy, Ms. Heather Gregory, and Mr. John Stone, all from Wake Forest School of Medicine, for helpful technical assistance in the development of this protocol.
References
- Fernandez-Vizarra E, et al. Isolation of mitochondria for biogenetical studies: An update. Mitochondrion. 2010;10:253–262. doi: 10.1016/j.mito.2009.12.148. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vizarra E, Lopez-Perez MJ, Enriquez JA. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods. 2002;26:292–297. doi: 10.1016/S1046-2023(02)00034-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazarin ML, Snider NN, Andrade FH. Mitochondrial isolation from skeletal muscle. Journal of Visualized Experiments : JoVE. 2011. [DOI] [PMC free article] [PubMed]
- Iglesias-Gonzalez J, Sanchez-Iglesias S, Beiras-Iglesias A, Soto-Otero R, Mendez-Alvarez E. A simple method for isolating rat brain mitochondria with high metabolic activity: effects of EDTA and EGTA. Journal of Neuroscience Methods. 2013;213:39–42. doi: 10.1016/j.jneumeth.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Gross VS, et al. Isolation of functional mitochondria from rat kidney and skeletal muscle without manual homogenization. Analytical Biochemistry. 2011;418:213–223. doi: 10.1016/j.ab.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko A, et al. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PloS One. 2013;8:e82392. doi: 10.1371/journal.pone.0082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell JB, Perry SV. The respiratory and adenosinetriphosphatase activities of skeletal-muscle mitochondria. The Biochemical Journal. 1953;55:586–595. doi: 10.1042/bj0550586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell DJ, et al. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2014. [DOI] [PMC free article] [PubMed]
- Nicklas BJ, et al. Relationship of physical function to vastus lateralis capillary density and metabolic enzyme activity in elderly men and women. Aging Clinical and Experimental Research. 2008;20:302–309. doi: 10.1007/bf03324860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scandinavian Journal of Clinical and Laboratory Investigation. 1975;35:609–616. [PubMed] [Google Scholar]
- Rogers GW, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PloS One. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, et al. Mitochondrial structure and function are disrupted by standard isolation methods. PloS One. 2011;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]


