Abstract
Percutaneous interventions including balloon angioplasty and stenting have been used to restore blood flow in vessels with occlusive vascular disease. While these therapies lead to the rapid restoration of blood flow, these technologies remain limited by restenosis in the case of bare metal stents and angioplasty, or reduced healing and possibly enhanced risk of thrombosis in the case of drug eluting stents. A key pathophysiological mechanism in the formation of restenosis is intimal hyperplasia caused by the activation of vascular smooth muscle cells and inflammation due to arterial stretch and injury. Surgeries that induce arterial injury in genetically modified mice are useful for the mechanistic study of the vascular response to injury but are often technically challenging to perform in mouse models due to the their small size and lack of appropriate sized devices. We describe two approaches for a surgical technique that induces endothelial denudation and arterial stretch in the femoral artery of mice to produce robust neointimal hyperplasia. The first approach creates an arteriotomy in the muscular branch of the femoral artery to obtain vascular access. Following wire injury this arterial branch is ligated to close the arteriotomy. A second approach creates an arteriotomy in the main femoral artery that is later closed through localized cautery. This method allows for vascular access through a larger vessel and, consequently, provides a less technically demanding procedure that can be used in smaller mice. Following either method of arterial injury, a degradable drug delivery patch can be placed over or around the injured artery to deliver therapeutic agents.
Keywords: Medicine, Issue 96, vascular injury, neointimal hyperplasia, perivascular drug delivery, wire injury, mouse surgical model of restenosis
Introduction
Arterial injury and inflammation caused by angioplasty and stent implantation can induce neointimal hyperplasia that contributes to the thickening of the arterial wall, a process known as restenosis.1,2 The formation of restenosis is major mode of failure for interventions such as angioplasty and stenting with bare metal stents.3 Due to recent concerns with the inhibition of vascular healing in arteries treated with drug eluting stents, there is also a need to find compounds that can inhibit restenosis while maintaining vascular healing and re-endothelialization.4-7 In addition, while stents have had success in the coronary vasculature, percutaneous interventions of all types in the peripheral arteries continue to fail at a higher rate due to restenosis.8-10 Mouse models of surgical interventions allow the use of powerful genetic manipulations that can provide mechanistic insight into the mechanisms underlying the failure of clinical therapies and can provide an initial test bed for compounds to inhibit intimal hyperplasia.
Here, we describe a mouse model of vascular injury that allows the testing of therapeutic compounds to inhibit neointimal hyperplasia and assess whether their effects on re-endothelialization following endothelial denudation. A key challenge in executing vascular injury in mice is the technical skill needed to obtain vascular access and to restore flow to the injured artery following the wire injury. For this reason, simple arterial ligation models have been used to study neointimal hyperplasia in mice that do not require endovascular manipulations but are easier to implement.11 However, this type of surgical model differs substantially from the mechanical and biological aspects of a percutaneous intervention, lacking key aspects including arterial wall stretch, endothelial denudation and luminal blood flow following injury.
We present two methods for obtaining and closing vascular access for wire injury of the femoral artery in mice. The first technique is the conventional method described by several groups previously and uses vascular access through a side branch of the femoral artery.12-14 This method requires older, larger mice and more surgical skill to implement the endoluminal access through the smaller artery. It also requires the ligation of the muscular branch of the femoral artery following the procedure. The second method we describe uses an arteriotomy in the branch point of the main and side branch and thereby allows for a larger access to the artery for performing wire injury. In this method, the arteriotomy is closed using controlled local cauterization that leaves both branches with blood flow following the procedure. The conventional method is applicable to mice of at least 20 weeks of age while the alternative method can be used in mice of at least 15 weeks of age. In both methods, the wire creates arterial stretch and abrasion leading to injury and endothelial denudation. Following either procedure a perivascular drug delivery patch can be implanted that allows the delivery of compounds to alter the response to injury. The use of the drug delivery patch allows mice to be used as a test bed for new compounds to inhibit restenosis through perivascular therapies.15,16
Protocol
NOTE: All methods shown in this protocol have been approved by the Institutional Animal Care and Use Committee.
1. Preparation of Surgical Table
Set up the heating pad with recirculating warm water on the surgical table, under the dissection microscope. Place the stainless steel base plate onto the heating pad. Place a sterile absorbent pad on the base plate and heating pad.
Arrange sterile surgical tools on an adjacent sterile absorbent pad. Gather two pairs of angled fine-tipped forceps, two pairs of angled forceps, three hemostatic forceps, two retractors wires, small surgical scissors, micro-scissors, one angioplasty wire (0.15 inch diameter) and three 6-inch segments of 6.0 silk suture.
Bend the round end of the angioplasty wire to match the curvature of the femoral artery. This will make it easier to advance the wire during the surgery.
2. Preparation of Mouse for Surgery
Anesthetize a mouse by continuous inhalation of 2.5% isoflurane. Be sure to monitor the animal’s status throughout the procedure. Perform a pinch test of the mouse’s foot to confirm that it is fully anesthetized. Ensure the animal does not move when the pinch test is administered.
Apply a lubricating ointment to the animal’s eyes to prevent desiccation. Secure the animal in the supine position to the absorbent pad using surgical tape. Continue administering 2.5% isoflurane via nose cone.
Using a depilatory cream, remove fur from the leg and abdomen to the mid-line. Rinse the skin thoroughly with water. Depilation can be performed one day in advance of surgery. Note that excessive treatment with the cream may lead to skin irritation.
Immediately before surgery, apply povidone-iodine to the depilated area with a cotton-tipped applicator to sterilize the skin. Rinse the depilated skin with 70% ethanol and dry with a sterile cotton-tipped applicator. Repeat 3 times.
Administer a pre-operative dose of 5 mg/kg of carprofen via subcutaneous injection.
3. Isolation of the Femoral Artery
Using small surgical scissors, make a curved incision in the skin over the femoral artery. Bluntly dissect and secure surrounding tissues using retractors magnetic retractor fixators to locate the femoral artery. Moisten the tissues periodically using saline for irrigation Apply saline using a sterile cotton-tipped applicator.
Isolate the femoral artery using forceps. Gently separate the nerve from the vascular bundle using fine-tipped forceps. Avoid puncturing the vein, and do not damage the nerve. Push the nerve away from the bundle to avoid stimulating it.
Gently separate the femoral vein from the femoral artery, locating the femoral bifurcation. The region of the bifurcation is especially difficult to dissect.
Posterior to the bifurcation, loop a 6.0 silk suture under the femoral artery and secure with a hemostat. This proximal suture will be used to restrict blood flow in the artery. NOTE: There is a slight variation in the ties when performing the ligation method versus the cauterization method (see Figure 1 and Figure 2).
Distal to the bifurcation, loop 6.0 silk suture under the femoral artery and secure with a hemostat. This distal suture aids in the positioning of the artery.
Loop two sutures under the muscular branch of the femoral artery, pre-tie them and secure with a hemostat. Remember to moisten the tissues with saline. If performing the cautery method of wire injury, only one looped suture is necessary on the muscular branch.
4. Performance of Femoral Artery Wire Injury
Restrict blood flow into the femoral artery by pulling the proximal suture. Slightly pull the distal hemostat and hemostat securing the branch to expose the site for the arteriotomy. Ligate the muscular branch by tying the suture around it. NOTE: Lifting the artery upward will more effectively restrict blood flow than pulling horizontally alone.
Sever the small branch with the cautery between the two sutures. Using micro-scissors, perform an arteriotomy in the side branch of the bifurcation. Use forceps balanced on a roll of surgical tape to stabilize the micro-scissors.
Confirm the presence of the arteriotomy using fine-tipped forceps. Gently lift the opening of the arteriotomy with the forceps. Introduce the rounded end of the wire into the arteriotomy using forceps. To ease advancement of the wire, add one or two drops of lidocaine to the region using a syringe.
When the wire reaches the proximal suture, release the suture and adjust it so that it cannot impede the advancement of the wire. Insert the wire until it cannot advance further. The tip of the wire should stop in the region of the inguinal ligament.
Allow the wire to remain in the femoral artery for one minute. After one minute, retract and advance the wire in a sawing motion ten times to injure and denude the endothelium of the femoral artery. For an injury of lesser severity, decrease the number of times the wire is pulled in and out of the artery or leave the wire in the artery for 1 min only.
Retract the wire slowly. When the round end of the wire has passed the proximal suture, restrict flow into the artery by pulling the proximal suture. Retract the wire completely.
5. Ligating the Muscular Branch
Tighten the remaining suture on the muscular branch. This will prevent bleeding from the arteriotomy. Return flow to the muscular branch, and confirm that blood is not leaking from it. Trim the ends from the sutures on the muscular branch.
6. Alternative Method: Localized Cauterization of the Arteriotomy
NOTE: An alternative approach can be taken to avoid ligation of the muscular branch and allow vascular access through the larger main femoral artery.
Starting from 4.1, restrict flow to the femoral artery by pulling on the proximal suture. Pull the distal suture and the suture securing the muscular branch slightly to expose the site for the arteriotomy (Figure 1 and Figure 2).
Using micro-scissors, perform an arteriotomy at the branch point of the femoral artery and the muscular branch. If the incision is made in the side of the artery, it may be easier to cauterize. Use forceps balanced on a roll of surgical tape to stabilize the micro-scissors.
To test the success of cauterization, restore flow to the femoral artery by loosening the proximal suture. If bleeding occurs from the arteriotomy, repeat the cautery. If the cautery is successful, blood flow will be restored distal to the arteriotomy.
Introduce the wire and perform the wire injury as described in section 4.
Continue restricting blood flow into the femoral artery. Heat a fine-tipped cautery at least 6 inches away from the mouse. As the cautery tip cools, apply it lightly side of the arteriotomy to close the incision.
To test the success of cauterization, restore flow to the femoral artery by loosening the proximal suture. If bleeding occurs from the arteriotomy, repeat the cautery. If the cautery is successful, blood flow will be restored distal to the arteriotomy. Remove the temporary proximal and distal ties.
7. Implantation of Perivascular Drug Delivery Patch
Create a perivascular drug patch can be created as described in previous studies11 or by using similar methods. Blunt the corners of the barrier region of the drug delivery patch using sterile scissors
Place the drug delivery patch onto the injured femoral artery with the drug-releasing side facing the artery. As necessary, use forceps to improve the position of the patch.
8. Wound Closure and Recovery
Close the wound with a simple interrupted suture consisting of square knots. A discontinuous suture prolongs wound closure in case the animal tries to remove the suture.
Turn off the anesthesia and remove the animal from the set up. Allow the animal to recover on the warming pad.
Continue to monitor the mouse’s recovery. Check the incision site each day to ensure that it remains closed. For treatment of post-surgical pain, administer a subcutaneous injection of 5 mg/kg of carprofen every 12 hr after surgery and then every 12 hr for 2 days. If pain continues beyond the initial 2 days, consult a veterinarian for directions on further pain medication.
9. Harvesting Femoral Arteries for Histology
At 28 days post-surgery, perform carbon dioxide euthanasia on the mouse. NOTE: The appropriate flow rate of carbon dioxide should displace 10-30% of the chamber per minute and will vary depending on the size of the chamber used. Cutting of the diaphragm and cardiac puncture should be performed as a secondary method of euthanasia.
Secure the mouse in the supine position using surgical tape. Make an incision over the femoral artery, where the initial surgical incision was made.
For both the injured artery and the uninjured artery from the contralateral leg, bluntly dissect and secure surrounding tissues using retractors and magnetic retractor fixators to locate the femoral artery. Moisten the tissues periodically using saline for irrigation. Apply saline using a sterile cotton-tipped applicator. Take care to not damage the artery.
After the femoral artery has been isolated from the site of the original arteriotomy to the abdominal aorta, tie a segment of silk suture near the original site of the arteriotomy. This suture will help identify the most distal end of the femoral artery and ease handling of the sample.
Using the micro-dissection scissors to excise the femoral artery. Make one incision distal to the suture. Make the other incision on the opposite end of the femoral artery, beside the abdominal aorta.
Transfer the excised artery to a glass petri dish containing saline. Dissect the artery further to remove excess connective tissue or fat. Gently remove blood from the lumen by rinsing with saline.
Transfer the artery to a vial of 10% buffered formalin. Store the vial at 4 °C with gentle rocking for 48 hr.
Transfer the fixed artery to 70% ethanol, to store until it is processed for histology.
Embed the artery in paraffin blocks and section the blocks for staining.
Perform histochemical and immunochemical staining to assess the extent of injury and intimal hyperplasia. NOTE: For the representative results, we used Hematoxylin and Eosin to visualize the nuclei and overall morphology or a Movat’s Pentachrome stain to visualize the elastic lamellae and other arterial components.
Representative Results
Following wire injury, neointimal hyperplasia develops over time and is typically examined after 14 to 28 days. The techniques described in this work lead to robust generation of intimal hyperplasia in mice as shown the histological results in Figure 3. An uninjured femoral artery will demonstrate intact elastic lamellae and a normal thickness and circumference. An injured femoral artery will show intimal hyperplasia, degraded elastic lamellae and demonstrate re-endothelialization at later time points. Re-endothelialization is typically complete at around 21 days but this depends on the background strain of the mice used and can be assessed using immunostaining for endothelial markers on sections or, preferably, measured on en face preparations using the Evan’s blue dye, scanning electron microscopy or immunostaining with confocal microscopy on en face preparations of the artery.17,18 For quantification of intimal hyperplasia it is best to measure the intimal area or intimal-to-media ratio at three sites along the injured artery to account for potential variations along the length of the injured region of the artery.
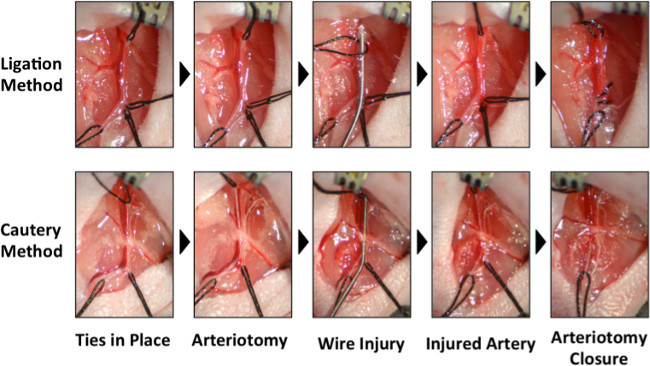 Figure 1: Photographs of the critical steps of the two methods for wire injury in the femoral artery. A comparison of the steps for the ligation method (top) to the cautery method (bottom) for performing wire injury. In the ligation method, the two sutures securing the muscular branch are pre-tied so they can be tightened to ligate the muscular branch. The more distal of the two muscular branch sutures is tightened. The arteriotomy and wire insertion are performed in the muscular branch. After the injury is performed, the remaining suture on the muscular branch is tightened and trimmed. In the cautery method, only one suture is looped under the muscular branch. The arteriotomy and wire insertion are performed at the branch point of the muscular branch from the femoral artery. After the injury is performed, the incision is cauterized without ligating either the femoral artery or muscular branch. Please click here to view a larger version of this figure.
Figure 1: Photographs of the critical steps of the two methods for wire injury in the femoral artery. A comparison of the steps for the ligation method (top) to the cautery method (bottom) for performing wire injury. In the ligation method, the two sutures securing the muscular branch are pre-tied so they can be tightened to ligate the muscular branch. The more distal of the two muscular branch sutures is tightened. The arteriotomy and wire insertion are performed in the muscular branch. After the injury is performed, the remaining suture on the muscular branch is tightened and trimmed. In the cautery method, only one suture is looped under the muscular branch. The arteriotomy and wire insertion are performed at the branch point of the muscular branch from the femoral artery. After the injury is performed, the incision is cauterized without ligating either the femoral artery or muscular branch. Please click here to view a larger version of this figure.
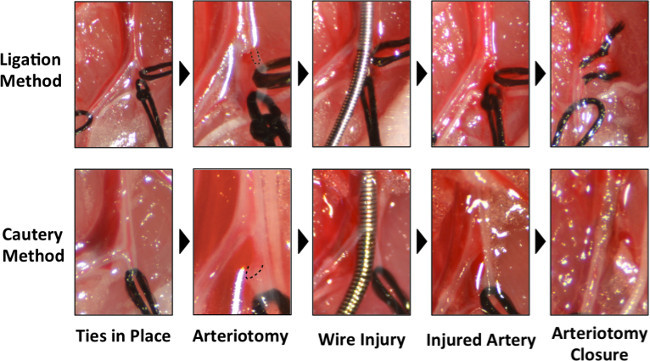 Figure 2: Higher magnification photographs of the critical steps of the two techniques for wire injury. Close-up comparison of steps for the ligation method (top) to the cautery method (bottom). The location of the arteriotomy is demarcated with the dashed line. Please click here to view a larger version of this figure.
Figure 2: Higher magnification photographs of the critical steps of the two techniques for wire injury. Close-up comparison of steps for the ligation method (top) to the cautery method (bottom). The location of the arteriotomy is demarcated with the dashed line. Please click here to view a larger version of this figure.
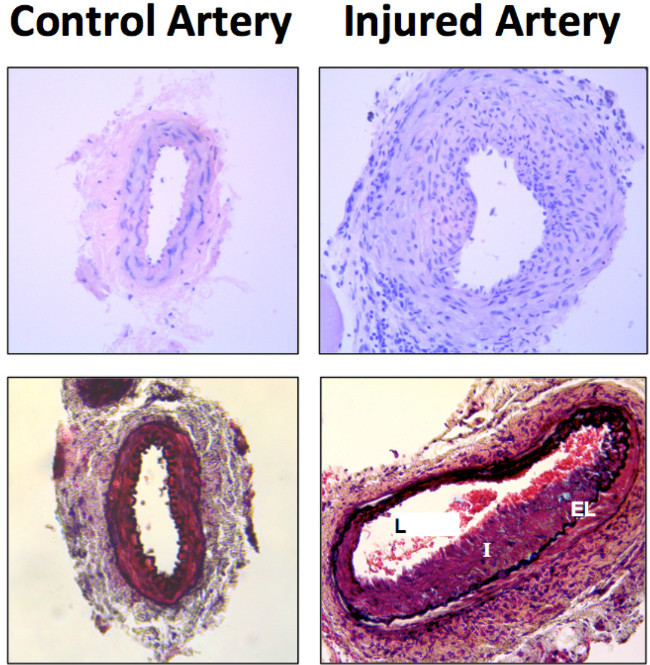 Figure 3: Representative histological results of wire injury 14 days after the procedure. Histological from the arteries sections were stained with Hematoxylin and Eosin (top) and Movat’s pentachrome staining (bottom). Labeled in the image are the lumen (L), intima (I) and elastic lamina (EL).
Figure 3: Representative histological results of wire injury 14 days after the procedure. Histological from the arteries sections were stained with Hematoxylin and Eosin (top) and Movat’s pentachrome staining (bottom). Labeled in the image are the lumen (L), intima (I) and elastic lamina (EL).
Discussion
We have presented a method for performing vascular injury in mice and delivery therapeutic compounds to the injured region through a perivascular cuff. The ligation method for femoral and carotid arteries has been described in conventional methods papers and characterized extensively11-14,19-23 and we present an alternative method for achieving the same vascular injury that is less technically demanding procedure that can often be used in younger mice. One of the chief advantages of the using a mouse wire injury model with a perivascular patch is that it allows the use of genetically modified mice or murine models of disease. The endovascular manipulation of small arteries is technically demanding and this aspect has motivated the creation of models that create injury through a number of extravascular methods including ligation,11,24,25 perivascular cuffing26 and electric shock.27 In our procedures, a contralateral sham surgery can be used as a control by performing the protocol without the wire injury or, alternatively, non-operated control arteries can be used. The critical steps within the protocol are the following: (1) the surgical cut down and dissection of the femoral artery from surrounding structures, (2) the formation of the arteriotomy, (3) performance of the wire injury, (4) closure of the arteriotomy and (5) implantation of the perivascular cuff. Below we will review the major steps and address potential pitfalls in their performance.
For the surgical cut down, the major challenge is the separation of the femoral artery from the femoral vein. Care should be taken at this stage, as it is easy to cause bleeding during the separation and the vein tears easily in comparison to the artery. Using forceps to blunt dissect and remove adventitia surround the artery and vein can help this process (see techniques shown in the video). Also, the artery may have branches underneath that can be torn if an overly aggressive technique is used.
For the formation of the arteriotomy and wire injury, it is critical to use high quality microsurgical scissors. It is often helpful to cut at a 45-degree angle to the axis of the artery and to stabilize the cut with forceps as shown in the video. Too much or too little tension on the artery can make cutting difficult and it is sometimes helpful pull the distal tie before the proximal tie to fill the artery with blood to aid in the performing the arteriotomy. After the cut, opening the hole with the closed point of the scissors or forceps can aid in allowing entry of the wire. The size of the arteriotomy should cut approximately half way through the artery. Again, too much tension on the artery will make insertion of the wire difficult and may tear the vessel. A low to moderate level of tension should be used with the ties. When inserting the wire a slight twisting motion can be helpful but too much twisting will cause the artery to seize, preventing advancement of the wire.
A key step in performing our alternative method is the controlled local cautery that seals the vessel without blocking flow. The cautery should be heated away from the tissue and then touched to the side of the artery. Heating with the cautery while touching the vessel will burn surrounding tissues including the vein and femoral artery branches. One potential limitation of this technique is that if the arteries are not consistently cauterized there may be variations in the flow following the surgery or in the amount of inflammation caused by the burn. Analysis of the artery away from this region and consistent technique can minimize the potential impact of these limitations. We hope this alternative method will make the wire injury surgery more accessible and reduce the need for older mice for vascular injury studies.
Disclosures
None.
Acknowledgments
The authors would like to acknowledge support through the American Heart Association (10SDG2630139), the Welch Foundation and through the NIH Director’s New Innovator Grant (1DP2 OD008716-01). The authors would like to thank the services provided by the ICMB (Institute of Cellular and Molecular Biology) core facility and TherapeUTex at University of Texas at Austin.
References
- Hoffmann R, Mintz GS. Coronary in-stent restenosis - predictors, treatment and prevention. Eur Heart J. 2000;21(21):1739–1749. doi: 10.1053/euhj.2000.2153. [DOI] [PubMed] [Google Scholar]
- Erbel R, et al. Coronary-artery stenting compared with balloon angioplasty for restenosis after initial balloon angioplasty. Restenosis Stent Study Group. N Engl J Med. 1998;339(23):1672–1678. doi: 10.1056/NEJM199812033392304. [DOI] [PubMed] [Google Scholar]
- Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv. 2011;4(2):195–205. doi: 10.1161/CIRCINTERVENTIONS.110.959882. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. Br Med Bull. 2013;106:193–211. doi: 10.1093/bmb/ldt009. [DOI] [PubMed] [Google Scholar]
- Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(10 Suppl):S1–S42. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kim YH. Current status of percutaneous coronary intervention with drug-eluting stents in Asia. Circulation. 2008;118(25):2730–2737. doi: 10.1161/CIRCULATIONAHA.107.743450. [DOI] [PubMed] [Google Scholar]
- Alfonso F. Treatment of drug-eluting stent restenosis the new pilgrimage: quo vadis. J Am Coll Cardiol. 2010;55(24):2717–2720. doi: 10.1016/j.jacc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Dake MD, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4(5):495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- Bosiers M, et al. Results of the Protege EverFlex 200-mm-long nitinol stent (ev3) in TASC C and D femoropopliteal lesions. J Vasc Surg. 2011;54(4):1042–1050. doi: 10.1016/j.jvs.2011.03.272. [DOI] [PubMed] [Google Scholar]
- Duda SH, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13(6):701–710. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17(10):2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- Sata M, et al. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32(11):2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- Roque M, et al. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20(2):335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73(5):792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- Edelman ER, Adams DH, Karnovsky MJ. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman MB, Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouchet L, et al. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103(3):423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- Filipe C, et al. Estradiol accelerates endothelial healing through the retrograde commitment of uninjured endothelium. Am J Physiol Heart Circ Physiol. 2008;294(6):H2822–H2830. doi: 10.1152/ajpheart.00129.2008. [DOI] [PubMed] [Google Scholar]
- Feuls R, et al. Microvascular denudation of the femoral artery of the mouse as a model for restenosis. Rofo. 2003;175(7):952–957. doi: 10.1055/s-2003-40429. [DOI] [PubMed] [Google Scholar]
- Holt AW, Tulis DA. Experimental Rat and Mouse Carotid Artery Surgery: Injury & Remodeling Studies. ISRN Minim Invasive Surg. 2013;2013 doi: 10.1155/2013/167407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrati MD, et al. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3(5):545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- Sullivan TR, et al. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96(5):2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Zhao X, Fang Y, Huang L. Carotid artery wire injury mouse model with a nonmicrosurgical procedure. Vascular. 2010;18(4):221–226. doi: 10.2310/6670.2010.00031. [DOI] [PubMed] [Google Scholar]
- Nam D, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297(4):H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam D, et al. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Kuhlmann MT, et al. Implantation of a carotid cuff for triggering shear-stress induced atherosclerosis in mice. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Carmeliet P, et al. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol. 1997;150(2):761–776. [PMC free article] [PubMed] [Google Scholar]


