Abstract
Zebrafish (Danio rerio) embryos have proven to be a powerful model for studying a variety of developmental and disease processes. External development and optical transparency make these embryos especially amenable to microscopy, and numerous transgenic lines that label specific cell types with fluorescent proteins are available, making the zebrafish embryo an ideal system for visualizing the interaction of vascular, hematopoietic, and other cell types during injury and repair in vivo. Forward and reverse genetics in zebrafish are well developed, and pharmacological manipulation is possible. We describe a mechanical vascular injury model using micromanipulation techniques that exploits several of these features to study responses to vascular injury including hemostasis and blood vessel repair. Using a combination of video and timelapse microscopy, we demonstrate that this method of vascular injury results in measurable and reproducible responses during hemostasis and wound repair. This method provides a system for studying vascular injury and repair in detail in a whole animal model.
Keywords: Developmental Biology, Issue 96, Zebrafish, hemostasis, vascular injury, wound healing, inflammation, microscopy
Introduction
Zebrafish have been used extensively to study a variety of topics in vascular biology, including vascular development, angiogenesis, and hematopoietic development and pathology1-3. Embryos develop a functional circulation as well as leukocytes and other components of the innate immune system by 1 day post fertilization (dpf) 1,4,5. The conservation of the inflammatory and leukocyte response to injury has made the zebrafish embryo an informative model for such diverse inflammatory processes as tuberculous infection, enterocolitis, and tissue regeneration6-9. Zebrafish embryos have been used to study injury-related inflammation particularly in the context of epithelial wounding and the neutrophil response10,11. Injury to the embryo results in a highly conserved cellular response from cells at the injury site and the innate immune cells recruited to respond to the injury and regulate its resolution11,12. Other injury models have used focused laser pulses to spatially localize injury to specific cell types including neurons, muscle cells, and cardiomyocytes13-15.
Zebrafish embryos have been used as a model to study hemostasis and thrombosis in conditions of pharmacological and genetic manipulation, using both mechanical and laser-induced thrombus formation16-19. Components of the coagulation cascade appear to be well-conserved and transgenics have allowed for detailed studies of thrombocyte and fibrin deposition at the site of coagulation17,20,21. The procedure presented in this paper complements these methods by providing a system for studying mechanical vessel injury resulting in vessel breach, thrombus formation and resolution, and vessel repair.
Protocol
NOTE: Procedures using zebrafish were approved by UCSF's Institutional Animal Care and Use Committee.
1. Preparation of Tools
Insert minutia pin into a pin holder and clamp the pin.
Using fine tip forceps, carefully bend the tip of the pin to create a slight hook.
For manipulation and stabilization of the embryo during injury, bend the end of a 28 guage ½ inch needle mounted on an insulin syringe using needle-nose pliers.
2. Preparation of Zebrafish Embryos for Injury
Set up zebrafish breeding pairs and collect eggs in egg water (60ug/mL aquarium salts) as shown previously22.
Add 0.003% N-Phenylthiourea (PTU) to the egg water when the embryos are approximately 8 hr post fertilization (hpf) to prevent melanization.
Dechorionate two day post fertilization (dpf) embryos prior to the experiment using fine tip forceps. NOTE: Embryos can be injured anytime after circulation begins. The data presented here is for 2 dpf embryos, but the technique has been successfully applied in embryos up to 5 dpf.
Anesthetize the embryos with 0.02% buffered 3-aminobenzoic acid (Tricaine) approximately 10 min prior to manipulations.
3. Mechanical Vessel Injury of Embryos
Transfer anesthetized embryos to a depression slide on a dissecting stereomicroscope using a transfer pipet.
Using the short flat side of the syringe needle to manipulate the embryo with the dominant hand, position the embryo on its side with the ventral surface facing away from the needle.
Position the minutia pin with the tip pointed directly against the ventral surface of the fish posterior to the urogenital opening. Position the minutia pin at a slight angle such that the curved tip is able to pierce through the periderm directly into the caudal vein (Figure 1).
Using the syringe needle to manipulate the embryo, pierce the caudal vein with the minutia pin by tapping the embryo into the pin to slightly hook the pin into the vein.
Using the syringe needle, pull the embryo away from the minutia pin to create a small tear in the vessel. NOTE: A successful injury will result in immediate bleeding from the vein.
4. Analysis of Hemostasis
Choose only embryos with visibly circulating blood cells for this procedure.
Prepare to begin the timer as soon as the minutia pin is pulled from the vessel.
Start the timer as soon as blood loss can be visualized from the wound. When blood loss from the wound ceases, stop the timer and record total time as Bleeding Time. If coagulation is inhibited, record the time to when there are no longer visibly circulating blood cells.
5. Analysis of Wound Healing
Transfer post-injury animals onto glass-bottom imaging dishes for microscopy.
Remove the majority of the egg water.
Cover the embryos in 0.3-1.2% low melting agarose dissolved in egg water, heated to between 42 and 45 ºC and supplemented with 0.02% Tricaine.
Position embryos on their sides using forceps.
After the agarose cools, fill the dish with 0.02% Tricaine in egg water.
Acquire images using brightfield, epifluorescence, or confocal microscopy.
Remove embryo from the agarose using forceps and transfer back to egg water.
Representative Results
Mechanical vessel injury was performed on 2 dpf embryos (Figure 2A-C). Injury results in a rapid and reliable coagulation response as measured by time to cessation of bleeding (Figure 2D). To determine whether or not differences in the coagulation response could be measured, the anticoagulant hirudin was administered to the embryos by injection into the Duct of Cuvier immediately prior to wounding (5-10 nl of 1 unit per µl hirudin dissolved in water)(for demonstration of injections into the Duct of Cuvier, see previous JoVE article23)24. The administration of hirudin prior to injury resulted in significantly increased bleeding times versus vehicle control (Figure 2D).
Evidence of vessel damage and coagulation can be seen immediately post-injury using transgenic lines for endothelial (kdrl:egfp) and red blood cell (gata1a:dsred) markers25,26. Images were acquired sequentially every 5 min for a 12 hr period using epifluorescence. Representative still images are shown throughout different stages of wound repair (Figure 3). Using a combination of differential interference contrast (DIC) and fluorescence microscopy, it is possible to measure distinct parameters of wound repair. In order to determine whether or not wound repair followed a reproducible pattern across experiments, the time to reestablished blood flow was measured in 4 groups of fish. Vessel injury resulted in a reliable stereotypical response of 253 ± 16 min to the reestablishment of blood flow through the wounded vessel (n = 4-5 fish per experiment, average ± SEM).
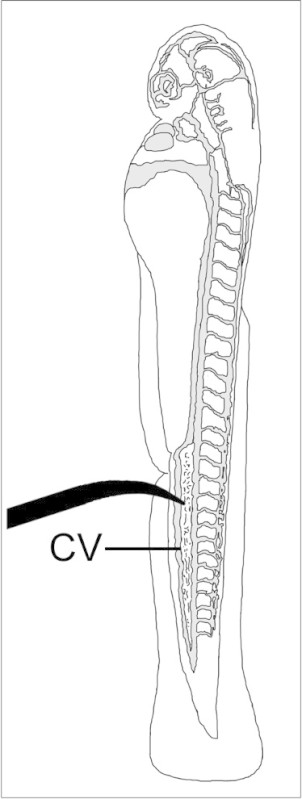 Figure 1:Diagram of 2 dpf embryo showing placement of minutia pin for performing mechanical injury of the caudal vein (CV). Vascular compartment is shaded in grey.
Figure 1:Diagram of 2 dpf embryo showing placement of minutia pin for performing mechanical injury of the caudal vein (CV). Vascular compartment is shaded in grey.
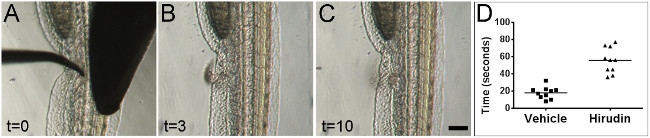 Figure 2:Bleeding times can be visually measured after mechanical injury. Stills from real-time video of zebrafish vessel injury on 2 dpf embryos. Images are shown at the time of injury (A), during active blood loss from the wound (B), and after cessation of blood loss (C). All times indicated are in seconds. Embryos are oriented laterally with anterior at top and ventral surface facing to the left. Scale bar 100 µm. Administration of the anticoagulant hirudin led to significantly increased bleeding times versus vehicle control (D) (p <0.0001, Student’s t-test).Please click here to view a larger version of this figure.
Figure 2:Bleeding times can be visually measured after mechanical injury. Stills from real-time video of zebrafish vessel injury on 2 dpf embryos. Images are shown at the time of injury (A), during active blood loss from the wound (B), and after cessation of blood loss (C). All times indicated are in seconds. Embryos are oriented laterally with anterior at top and ventral surface facing to the left. Scale bar 100 µm. Administration of the anticoagulant hirudin led to significantly increased bleeding times versus vehicle control (D) (p <0.0001, Student’s t-test).Please click here to view a larger version of this figure.
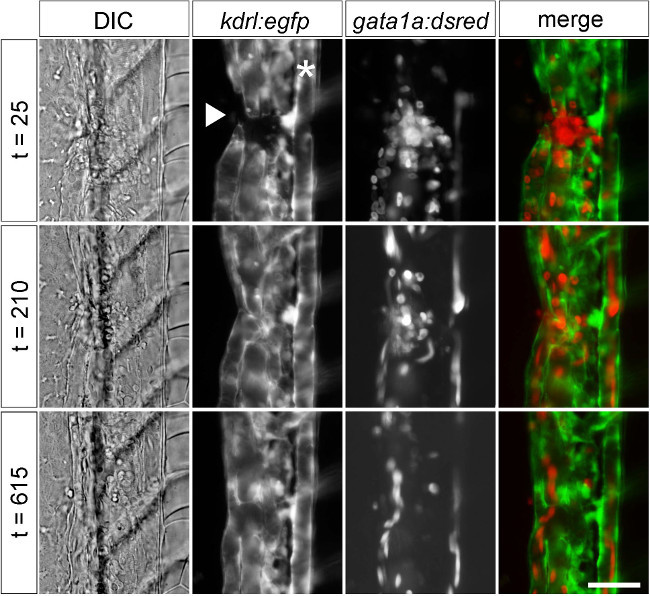 Figure 3:Visualizing mechanical injury and repair using transgenic markers. Stills from timelapse DIC and fluorescence microscopy after vessel injury using markers for vascular endothelium (kdrl:egfp) and red blood cells (gata1a:dsred) in 2 dpf embryos. Images showed a gap in vessels and local red blood cell accumulation (t = 25), partial repair with re-established blood flow (t = 210), and apparently complete restoration of normal vessel structure (t = 615). Time is indicated in minutes. Embryos are oriented laterally with anterior at top and ventral surface facing to the left. * indicates the position of the dorsal aorta. The injury (arrowhead) disrupted the caudal vein and part of the caudal plexus. Scale bar 25 µm.
Figure 3:Visualizing mechanical injury and repair using transgenic markers. Stills from timelapse DIC and fluorescence microscopy after vessel injury using markers for vascular endothelium (kdrl:egfp) and red blood cells (gata1a:dsred) in 2 dpf embryos. Images showed a gap in vessels and local red blood cell accumulation (t = 25), partial repair with re-established blood flow (t = 210), and apparently complete restoration of normal vessel structure (t = 615). Time is indicated in minutes. Embryos are oriented laterally with anterior at top and ventral surface facing to the left. * indicates the position of the dorsal aorta. The injury (arrowhead) disrupted the caudal vein and part of the caudal plexus. Scale bar 25 µm.
Discussion
Zebrafish have been used successfully as a model for different types of wounds including laser injury13-15, laser-induced thrombosis16, and epithelial wounding10. We report a method of mechanical wounding that is simple to execute and produces a controlled injury in an in vivo model that is highly amenable to real-time microscopy. Injury results in a rapid and measurable hemostatic response and a reproducible wound repair program that can be monitored using video and timelapse microscopy.
Their simple and stereotyped vascular anatomy, which permits reproducible injury at a defined and microscopically accessible site, and the presence of most vascular and hematopoietic cell types make zebrafish embryos particularly useful for studying responses to injury. However, zebrafish embryos do not have functional lymphocytes during the first weeks of development5,6, making this system most appropriate for studying the contribution of innate immunity in inflammation and repair. At present, a wide variety of transgenic zebrafish exist with markers for cells and proteins that participate in thrombus formation, coagulation, inflammation, and wound repair, including lines that label thrombocytes, fibrinogen, erythrocytes, leukocytes and vascular endothelium17,21,25-31. These and other tools should make it possible to follow processes involved in hemostasis and repair in significant detail.
Mechanical injury complements laser injury for the study of hemostasis in zebrafish. While laser-induced injury has been used for years to trigger thrombus formation in zebrafish embryos and mouse models, the mechanisms by which laser injury triggers coagulation and thrombocyte/platelet activation are not fully known16,32. Mechanical injury provides a physiologically relevant method for inducing coagulation by vascular breach and, presumably, tissue-factor-dependent initiation of coagulation cascade. The finding that hirudin treatment significantly increased bleeding times after injury suggests that this model is thrombin-dependent. Mechanical injury additionally complements laser injury by providing sufficient disruption of a blood vessel to provide an opportunity to follow vessel repair. Previous studies have successfully used mechanical injury by scalpel incision and needle puncture to show differences in bleeding times in conditions of pharmacological and genetic manipulation19,33. The minutia pin injury used in the current model may complement other injury models by providing a more reproducible injury due to the small and defined size of the wound it produces and by providing an opportunity to better study vessel recanalization and repair.
Epithelial wounding in the zebrafish has proven to be a powerful model for studying inflammation and wound repair10. The ability to introduce a vascular injury provides an opportunity to assess repair of more complex wounds in settings where fibrin provides a provisional matrix, thrombi and debris are cleared, and vessels regenerate. As these processes participate in normal tissue repair and in acute and chronic inflammation and vascular pathology, this method should help to model aspects of human disease in a system where cellular behaviors can be monitored in real time in a whole animal model.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The authors would like to thank Drs. Stephen Wilson and Lisa Wilsbacher for helpful discussions. This work was supported in part by NIH HL054737.
References
- Gore AV, Monzo K, Cha YR, Pan W, Weinstein B. Vascular development in the zebrafish. Cold Spring Harb Perspect Med. 2012;2(5):a006684. doi: 10.1101/cshperspect.a006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman S, et al. Assaying hematopoiesis using zebrafish. Blood Cells Mol Dis. 2013;51(4):271–276. doi: 10.1016/j.bcmd.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Lieschke GJ. Zebrafish as a model for vertebrate hematopoiesis. Curr Opin Pharmacol. 2010;10(5):563–570. doi: 10.1016/j.coph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Liu J, Stainier DY. Zebrafish in the study of early cardiac development. Circ Res. 2012;110(6):870–874. doi: 10.1161/CIRCRESAHA.111.246504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, et al. The zebrafish as a model organism to study development of the immune system. Adv Immunol. 2003;81:253–330. [PubMed] [Google Scholar]
- Lieschke GJ, Trede NS. Fish immunology. Curr Biol. 2009;19(16):R678–R682. doi: 10.1016/j.cub.2009.06.068. [DOI] [PubMed] [Google Scholar]
- Lesley R, Ramakrishnan L. Insights into early mycobacterial pathogenesis from the zebrafish. Curr Opin Microbiol. 2008;11(3):277–283. doi: 10.1016/j.mib.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, et al. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev Dyn. 2011;240(1):288–298. doi: 10.1002/dvdy.22519. [DOI] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29(11):611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBert DC, Huttenlocher A. Inflammation and wound repair. Semin Immunol. 2014. [DOI] [PMC free article] [PubMed]
- Henry KM, Loynes CA, Whyte MK, Renshaw SA. Zebrafish as a model for the study of neutrophil biology. J Leukoc Biol. 2013;94(4):633–642. doi: 10.1189/jlb.1112594. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M. In vivo nerve-macrophage interactions following peripheral nerve injury. J Neurosci. 2012;32(11):3898–3909. doi: 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten C, Abdelilah-Seyfried S. Laser-inflicted injury of zebrafish embryonic skeletal muscle. J Vis Exp. 2013. p. e4351. [DOI] [PMC free article] [PubMed]
- Matrone G, et al. Laser-targeted ablation of the zebrafish embryonic ventricle: a novel model of cardiac injury and repair. Int J Cardiol. 2013;168(4):3913–3919. doi: 10.1016/j.ijcard.2013.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P, Carrillo M, Radhakrishnan UP, Rajpurohit SK, Kim S. Laser-induced thrombosis in zebrafish. Methods Cell Biol. 2011;101:197–203. doi: 10.1016/B978-0-12-387036-0.00009-8. [DOI] [PubMed] [Google Scholar]
- Vo AH, Swaroop A, Liu Y, Norris ZG, Shavit JA. Loss of fibrinogen in zebrafish results in symptoms consistent with human hypofibrinogenemia. PLoS One. 2013;8(9):e74682. doi: 10.1371/journal.pone.0074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Targeted mutagenesis of zebrafish antithrombin III triggers disseminated intravascular coagulation and thrombosis, revealing insight into function. Blood. 2014. [DOI] [PMC free article] [PubMed]
- Jagadeeswaran P, Liu YC. A hemophilia model in zebrafish: analysis of hemostasis. Blood Cells Mol Dis. 1997;23(1):52–57. doi: 10.1006/bcmd.1997.0118. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P, Gregory M, Day K, Cykowski M, Thattaliyath B. Zebrafish: a genetic model for hemostasis and thrombosis. J Thromb Haemost. 2005;3(1):46–53. doi: 10.1111/j.1538-7836.2004.00999.x. [DOI] [PubMed] [Google Scholar]
- Lin HF, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106(12):3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JN, Sweeney MF, Mably JD. Microinjection of zebrafish embryos to analyze gene function. J Vis Exp. 2009. [DOI] [PMC free article] [PubMed]
- Benard EL, et al. Infection of zebrafish embryos with intracellular bacterial pathogens. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Jagadeeswaran P, Sheehan JP. Analysis of blood coagulation in the zebrafish. Blood Cells Mol Dis. 1999;25(3-4):3–4. doi: 10.1006/bcmd.1999.0249. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132(23):5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Traver D, et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4(12):1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248(2):307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Renshaw SA, et al. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108(13):3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:48. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117(4):e49–e56. doi: 10.1182/blood-2010-10-314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C, et al. Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb Haemost. 2011;105(5):811–819. doi: 10.1160/TH10-08-0525. [DOI] [PubMed] [Google Scholar]
- Bellido-Martin L, Chen V, Jasuja R, Furie B, Furie BC. Imaging fibrin formation and platelet and endothelial cell activation in vivo. Thromb Haemost. 2011;105(5):776–782. doi: 10.1160/TH10-12-0771. [DOI] [PubMed] [Google Scholar]
- Bielczyk-Maczynska E, et al. A loss of function screen of identified genome-wide association study Loci reveals new genes controlling hematopoiesis. PLoS Genet. 2014;10(7):e1004450. doi: 10.1371/journal.pgen.1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]


