Abstract
Eimeria species parasites, protozoa which cause the enteric disease coccidiosis, pose a serious threat to the production and welfare of chickens. In the absence of effective control clinical coccidiosis can be devastating. Resistance to the chemoprophylactics frequently used to control Eimeria is common and sub-clinical infection is widespread, influencing feed conversion ratios and susceptibility to other pathogens such as Clostridium perfringens. Despite the availability of polymerase chain reaction (PCR)-based tools, diagnosis of Eimeria infection still relies almost entirely on traditional approaches such as lesion scoring and oocyst morphology, but neither is straightforward. Limitations of the existing molecular tools include the requirement for specialist equipment and difficulties accessing DNA as template. In response a simple field DNA preparation protocol and a panel of species-specific loop-mediated isothermal amplification (LAMP) assays have been developed for the seven Eimeria recognised to infect the chicken. We now provide a detailed protocol describing the preparation of genomic DNA from intestinal tissue collected post-mortem, followed by setup and readout of the LAMP assays. Eimeria species-specific LAMP can be used to monitor parasite occurrence, assessing the efficacy of a farm’s anticoccidial strategy, and to diagnose sub-clinical infection or clinical disease with particular value when expert surveillance is unavailable.
Keywords: Infection, Issue 96, Loop-mediated isothermal amplification, LAMP, Coccidiosis, Eimeria, Chickens, Diagnostics, Field tools
Introduction
Global chicken production has increased ten-fold over the last 50 years with the developing world hosting nearly four times the expansion witnessed in the developed world (www.faostat.org). As the relevance of chicken production to world food security has grown so too has the profile of pathogens which can cause serious disease in chickens. One prime example are the Eimeria species, ubiquitous protozoan parasites which can cause the enteric disease coccidiosis1. Wherever chickens are reared one or more Eimeria species are likely to be common2-4. In the developed world Eimeria are primarily controlled by chemoprophylaxis, employing shuttle or rotation programmes to minimise the impact of resistance5. Live vaccines are also used in systems where bird value is sufficient to justify the cost (e.g., breeding stock, layers and some broilers5). As a result of these measures clinical coccidiosis is often well controlled, although sub-clinical infection is common5. In the developing world vaccination is rare and drug application frequently less well informed. As a consequence sub-clinical and clinical coccidiosis is more common and exerts a significant economic impact3.
Diagnosis of eimerian infection has traditionally relied on lesion scoring post-mortem, although even the authors of the most widely used scoring system commented that for some species “it seems doubtful whether such a procedure should be attempted in any but moderately severe infections”6. Supplementary evidence can be gathered through microscopic detection of the environmentally resistant oocyst lifecycle stage in faecal or litter samples, although overlapping morphology can confound all but the expert6,7. Molecular alternatives using polymerase chain reaction (PCR), random amplification of polymorphic DNA PCR (RAPD-PCR) and quantitative PCR technologies have been available for up to 20 years8-10, but to date they have failed to become popular. Relative expense and the requirement for specialist laboratory equipment or processing have limited their uptake, despite the often subjective and technically demanding nature of the older pathology- and microscopy-based approaches10,11. Such limitations can be exaggerated in many of the poorer regions of the world such as South East Asia, where the impact of coccidiosis on poverty may be proportionately greater12. In response there is a clear demand for new straightforward and sensitive, but cost effective, Eimeria species-specific diagnostic assays.
Loop-mediated isothermal amplification (LAMP) is an easy to prepare DNA polymerase-driven technique that is capable of amplifying large quantities of DNA. Most importantly, LAMP uses a Bst DNA polymerase instead of the Taq DNA polymerase commonly used in PCR, which facilitates DNA amplification at a single constant temperature without the requirement for thermal cycling13,14. LAMP can be amenable to application in even the most rudimentary laboratory or in the field. Characterised by relative resistance to many PCR inhibitors, high sensitivity and specificity, LAMP assays have been developed for a wide range of pathogens including infectious bursal disease virus, Clostridium perfringens and Cryptosporidium15-17. In response to demand for new cost-effective Eimeria species-specific diagnostics a panel of LAMP assays specific to each of the seven Eimeria species that infect chickens has been developed18. Applications for the new assays include monitoring parasite occurrence, of particular value given the association of species such as Eimeria maxima or Eimeria necatrix with poor economic performance3,4. Other applications include assessing the efficacy of a farm’s anticoccidial strategy, diagnosis of sub-clinical infection or clinical disease and evaluation of risk posed by Eimeria to a farm.
Protocol
1. Template Preparation
NOTE: Any genomic DNA template suspected to contain DNA derived from one of the seven Eimeria species which infect chickens can be used as template for LAMP-based Eimeria species identification. Intestinal tissue samples for field diagnostic analysis should be collected during routine post-mortem as described here.
Choose the section (or sections) of intestine to be tested. See Table 1 for a guide to sample site selection and the Eimeria species most likely to be present18 and Figure 1 for the species-specific range of distribution and location of the sampling sites. NOTE: This is of key importance since Eimeria are notably host site specific. Each species that infects chickens is defined by the intestinal region that it targets19. The decision may be influenced by previous experience of the farm, interest in one or more specific Eimeria species or other diagnostic indicators6.
Excise 5 cm or longer lengths of the intestinal section(s) selected to test for Eimeria using sterile scissors or a scalpel. Optionally, store the samples for subsequent analysis in a fixative such as for example in RNAlater18 or 95% ethanol. NOTE: If storing in ethanol the sample should be washed thoroughly in sterile tris-ethylenediaminetetraacetic acid (TE) buffer prior to use.
Cut the sample open longitudinally, remove most intestinal contents (if present) and scrape cells from the mucosal layer free using either the edge of a sterile glass microscope slide or an ethanol/flame sterilised scissor blade. Optionally for a pooled sample include cells from all four of the species-specific intestinal sites in a single tube.
Put the scraped material into a sterile 1.5 ml screw-top microcentrifuge tube containing 100 µl sterile TE buffer including 10% (w/v) Chelex 100 resin.
Shake each sample vigorously for 1 min. Ensure that the screw top is firmly closed and then incubate in a boiling water bath for 10 min.
After boiling allow each sample to cool at the ambient temperature for 1-2 min.
Centrifuge each sample using a microcentrifuge at top speed (e.g., ~10,000 x g) for 1 min.
Collect 2 µl of the resulting supernatant to be template in each LAMP assay to be undertaken. Optionally, pool more than one intestinal site in a single tube to provide a multi-site assay.
2. Eimeria LAMP Primer Preparation (Pre-assay)
- Prepare Eimeria LAMP primer stocks adequate for 100 assays:
- Reconstitute each lyophilised Eimeria LAMP primer by adding molecular grade water to a concentration of 100 µM (as specified by the manufacturer). If not specified, calculate the volume of molecular grade water required using the physical and molecular weights of each primer.
- Pipette 60 µl molecular grade water into a separate 0.5 ml flip-top microcentrifuge tube for each Eimeria species to be assayed.
- Add primers FIP, BIP, F3, B3, LF and LB specific to the target Eimeria species to the water using the volumes shown in Table 2, creating a series of seven species-specific primer mixes.
- Briefly vortex mix the primer solution, then pulse microfuge and freeze until required.
Prepare a LAMP reaction mastermix for each Eimeria species to be assayed. Multiply the volumes shown in Table 3 by the number of samples and add three to a positive control, negative control and pipetting spare. Pipette into a 0.5 or 1.5 ml flip-top microcentrifuge tube.
3. Eimeria LAMP Assay
Pipette 23 µl Eimeria species-specific Bst DNA polymerase/LAMP mastermix into a 0.5 ml microcentrifuge tube.
Add 2 µl DNA template (prepared in section 1), making a final reaction volume of 25 µl.
Add 2 µl Eimeria species-specific genomic DNA to one reaction (positive control). Add 2 µl molecular grade water to reaction (negative control). NOTE: If species-specific genomic DNA is not available a previous positive LAMP or standard PCR product may be used instead.
Incubate in a water bath or heat block at 62oC for 30 min. Optionally, de-activate the Bst DNA polymerase by heating to 80oC for 10 min if the reaction is not going to be read immediately.
4. LAMP Assay Read-out
At the conclusion of the incubation assess the colour of each reaction by eye under indoor light. Negative results appear pink to violet in colour, positive results appear sky blue20.
Optionally, confirm the LAMP assay result in a laboratory by mixing 5 µl LAMP reaction product with 1 µl DNA gel loading buffer for agarose gel electrophoresis using a 2% agarose gel in 1 x Tris/Borate/EDTA (TBE) buffer, pre-stained using a nucleic acid stain (5 µl per 50 ml agarose). Add 5 µl of a 1Kb molecular size DNA ladder to lane 1 of the gel to allow fragment size calculation.
Representative Results
Assay validation
During validation each Eimeria species-specific LAMP assay was tested using a panel of pure DNA samples representing all seven Eimeria species that infect the chicken, as well as chicken genomic DNA as a host control. Agarose gel electrophoresis was used to resolve each assay and demonstrated absolute species specificity with no host cross-reactivity18. Next, a ten-fold serial dilution series prepared using purified Eimeria tenella genomic DNA revealed an assay sensitivity limit of between one and ten genome copies18. No upper limit was determined with positive results achieved up to and including the highest concentration (100,000 genome copies)18.
Application with field samples
Samples collected for Eimeria testing are likely to be derived from chickens found dead, culled as a consequence of poor health or culled for sentinel health surveillance, indicating a likely sample size of between one and three when part of a routine. Testing three birds collected from a US broiler farm as part of a surveillance programme yielded three sets of intestinal samples. Targeted application of the species-specific LAMP assays, prioritising the preferred intestinal sites for each Eimeria species (Table 1), allowed visual identification of eimerian infection in all birds tested using hydroxynaphthol blue as an indicator (Figure 2A). The colour achieved with a negative LAMP reaction when using hydroxynaphthol blue can range from violet to pink, but is always distinct from the blue achieved by a positive result. Confirmation by agarose gel electrophoresis provided comparable results (Figure 2B). During field application the user may choose to apply the full screen against all seven species, or target only those species prioritised as important or known to be circulating on the farm or surrounding area.
The failure of PCR-based approaches to become established as diagnostics for the occurrence of Eimeria emphasises the requirement for simplicity in any new test. While LAMP offers simpler preparation and processing than PCR, the requirement to test multiple intestinal sites per bird remains discouraging. Production of a single pooled DNA sample per bird, which can then be tested with one or more LAMP assays, is likely to be more appealing. Processing one pooled sample per bird, representing material collected from each of the specific intestinal sites described in Table 1 and pooled prior to DNA preparation, for testing with all seven LAMP assays provided the same result as when each intestinal site was processed separately (Figure 2 compared with Figure 3).
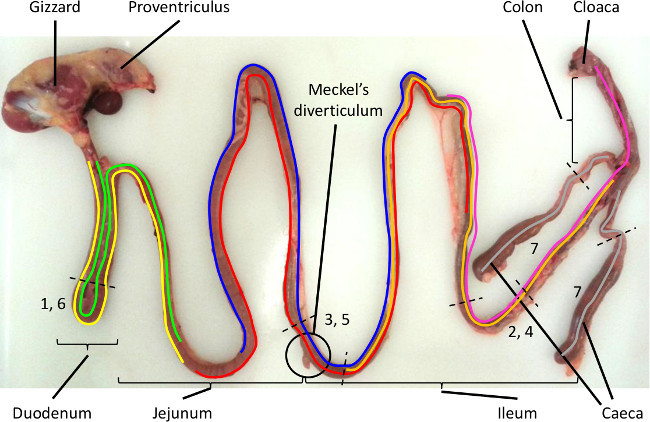 Figure 1. Intestinal sampling sites for LAMP detection of Eimeria species parasites that infect chickens. The intestinal regions targeted by each Eimeria species is highlighted by the coloured lines, with the preferred sites of sampling indicated by the number between the dotted black lines (E. acervulina: yellow/1, E. brunetti: pink/2, E. maxima: blue/3, E. mitis: orange/4, E. necatrix: red/5, E. praecox: green/6 and E. tenella: grey/7). Please click here to view a larger version of this figure.
Figure 1. Intestinal sampling sites for LAMP detection of Eimeria species parasites that infect chickens. The intestinal regions targeted by each Eimeria species is highlighted by the coloured lines, with the preferred sites of sampling indicated by the number between the dotted black lines (E. acervulina: yellow/1, E. brunetti: pink/2, E. maxima: blue/3, E. mitis: orange/4, E. necatrix: red/5, E. praecox: green/6 and E. tenella: grey/7). Please click here to view a larger version of this figure.
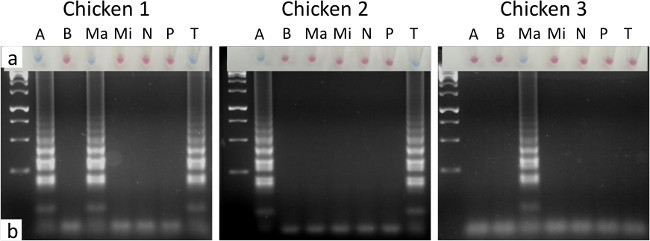 Figure 2. LAMP diagnosis of eimerian infection from three commercial broiler chickens. LAMP reactions resolved using (A) hydroxynaphthol blue, where a sky blue reaction was positive and a violet to pink reaction was negative, and (B) agarose gel electrophoresis. The intestinal sites sampled were as shown in Table 1 for each parasite species. A = E. acervulina, B = E. brunetti, Ma = E. maxima, Mi = E. mitis, N = E. necatrix, P = E. praecox and T = E. tenella. Lane 1 contained the GeneRuler 1Kb DNA ladder. Please click here to view a larger version of this figure.
Figure 2. LAMP diagnosis of eimerian infection from three commercial broiler chickens. LAMP reactions resolved using (A) hydroxynaphthol blue, where a sky blue reaction was positive and a violet to pink reaction was negative, and (B) agarose gel electrophoresis. The intestinal sites sampled were as shown in Table 1 for each parasite species. A = E. acervulina, B = E. brunetti, Ma = E. maxima, Mi = E. mitis, N = E. necatrix, P = E. praecox and T = E. tenella. Lane 1 contained the GeneRuler 1Kb DNA ladder. Please click here to view a larger version of this figure.
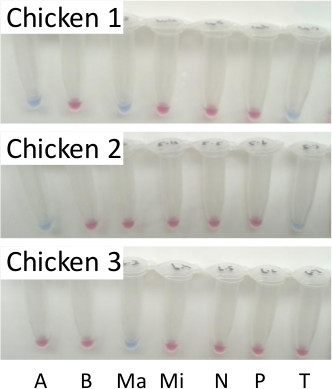 Figure 3. LAMP diagnosis of eimerian infection using pooled samples from three separate commercial broiler chickens. LAMP reactions resolved using hydroxynaphthol blue, where a sky blue reaction was positive and violet to pink reaction was negative. A = E. acervulina, B = E. brunetti, Ma = E. maxima, Mi = E. mitis, N = E. necatrix, P = E. praecox and T = E. tenella. Please click here to view a larger version of this figure.
Figure 3. LAMP diagnosis of eimerian infection using pooled samples from three separate commercial broiler chickens. LAMP reactions resolved using hydroxynaphthol blue, where a sky blue reaction was positive and violet to pink reaction was negative. A = E. acervulina, B = E. brunetti, Ma = E. maxima, Mi = E. mitis, N = E. necatrix, P = E. praecox and T = E. tenella. Please click here to view a larger version of this figure.
| Sample site | Eimeria species assay (most likely) |
| Duodenum (D) | E. acervulina, E. praecox |
| Jejunum/ileum* (J/I) | E. maxima, E. necatrix |
| Caeca (C) | E. necatrix, E. tenella |
| Terminal ileum (TI) | E. brunetti, E. mitis |
| Pooled sample (P) | E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, E. tenella |
Table 1. Intestinal region-specific selection of candidate Eimeria species assays. The choice of region to be sampled varies for each Eimeria species as illustrated in Figure 1. Pooled samples include material collected from all four specific sites which were then combined for DNA preparation.
| Primer* | Stock concentration (µM) | Volume (µl) |
| Water | - | 60 |
| Forward Inner Primer (FIP) | 100 | 40 |
| Backward Inner Primer (BIP) | 100 | 40 |
| Forward Outer Primer (F3) | 100 | 10 |
| Backward Outer Primer (B3) | 100 | 10 |
| Loop Forward (LF) | 100 | 20 |
| Loop Backward (LB) | 100 | 20 |
| Total | 200 |
Table 2. Preparation of a LAMP primer premix. The components and proportions required to prepare a primer premix for LAMP. Volumes shown are for 100 LAMP reactions. *Primer identities as shown in the Materials and Barkway et al (2011)18.
| Stock concn | Final reaction concn | Volume per reaction(µl) | |
| DDW | - | - | 10.1 |
| ThermoPol buffer | 10 x | 1 x | 2.5 |
| MgSO4 | 100 mM | 2 mM | 0.5 |
| Primer mix* | Table 2 | 2.5 | |
| dNTPs | 25 mM | 400 uM | 0.4 |
| Betaine | 5 M | 1 M | 5 |
| Hydroxynaphthol blue | 3 mM | 120 µM | 1 |
| Bst DNA polymerase | 8,000 U/ml | 8 U | 1 |
| Total | 23 |
Table 3. Preparation of a LAMP reaction mastermix. *Eimeria species-specific.
Discussion
The Eimeria species-specific LAMP assays described in this paper offer a new diagnostic tool kit in support of effective control of coccidia and the disease coccidiosis. The outcomes of eimerian infection can include severe economic loss as well as seriously compromised bird welfare and increased susceptibility to colonisation by zoonotic pathogens21. Opportunities to monitor flocks for the occurrence of some, or all Eimeria species can provide early warning of a breakdown in anticoccidial control efficacy. Key advantages of LAMP include robust target specificity, resulting from the requirement for six different DNA sequence targets, as well as high sensitivity, boosted by the inclusion of loop primers13, although the qualitative, not quantitative nature of LAMP may be considered a limitation. It is not currently possible to discriminate low level parasite escape from routine chemoprophylaxis or live vaccine replication from unchecked eimerian replication. Nonetheless, the technical ease of the protocol and definitive readout offers considerable improvement over the existing specialist and frequently subjective pathology- and morphology-led approaches6,7. Each assay may be completed at a cost of ~£0.75 per sample, independent of labour and equipment set up expenses. Thus, LAMP assays are also more cost effective than other molecular diagnostics such as PCR, since they require an isothermal incubation with no need for specialist equipment.
For many years access to Eimeria genomic DNA as template has limited the development and application of molecular field diagnostics. The oocyst is the most readily accessible phase of the eimerian lifecycle, but routine DNA extraction requires laboratory facilities22. Other, more labile intestinal lifecycle stages require purification prior to DNA preparation to prevent PCR inhibition and a consequent loss of sensitivity23,24. The ability to extract eimerian DNA of a quality suitable for LAMP using equipment no more specialised than a microcentrifuge and a water bath, supplemented by inhibitor adsorption using chelex resin, now promotes the wider use of molecular biology in eimerian diagnostics. Intriguingly, the reported detection of quantitative PCR-measurable Eimeria DNA in intestinal tissue 20 days after the initiation of parasite infection, 11 days after the last detectable oocyst output, raises the suggestion that LAMP may be used to detect resolved parasite exposure as well as ongoing infection, even after any visible lesions may have been resolved25.
The relatively low cost and low technical requirements of LAMP Eimeria diagnostics can promote their application in the developing world where other more established approaches may not be available or appropriate. For this to be applicable each assay must be capable of detecting all strains which may be circulating within each region. While understanding of the genetic diversity prevailing among Eimeria species is limited26, the use of target sequences previously validated for use in quantitative PCR with strains from Africa, Asia, Europe and South America provides some evidence of conservation, supporting the utility of these LAMP assays around the world10.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The work carried out in this study was supported in part by the Royal Veterinary College through the student research projects fund, as well as the Biotechnology and Biological Sciences Research Council and the Department for International Development (grant number BB/H009337/2). This manuscript has been assigned the reference PPB_00795 by the RVC.
References
- Chapman HD, et al. A selective review of advances in coccidiosis research. Adv Parasitol. 2013;83:93–171. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- Shirley MW, Smith AL, Tomley FM. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasitol. 2005;60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- Fornace KM, et al. Occurrence of Eimeria species parasites on small-scale commercial chicken farms in Africa and indication of economic profitability. PLoS ONE. 2013;8(12):e84254. doi: 10.1371/journal.pone.0084254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz RS, Jenkins MC, Klopp S, Miska KB. Genomic analysis of Eimeria spp. populations in relation to performance levels of broiler chicken farms in Arkansas and North Carolina. J Parasitol. 2009;95(4):871–880. doi: 10.1645/GE-1898.1. [DOI] [PubMed] [Google Scholar]
- Peek HW, Landman WJ. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q. 2011;31(3):143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970;28(1):30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Haug A, Gjevre AG, Skjerve E, Kaldhusdal M. A survey of the economic impact of subclinical Eimeria infections in broiler chickens in Norway. Avian Pathol. 2008;37(3):333–341. doi: 10.1080/03079450802050705. [DOI] [PubMed] [Google Scholar]
- Procunier J, Fernando M, Barta J. Species and strain differentiation of Eimeria spp. of the domestic fowl using DNA polymorphisms amplified by arbitrary primers. Parasitology Research. 1993;79(2):98–102. doi: 10.1007/BF00932253. [DOI] [PubMed] [Google Scholar]
- Schnitzler BE, Thebo PL, Mattsson JG, Tomley FM, Shirley MW. Development of a diagnostic PCR assay for the detection and discrimination of four pathogenic Eimeria species of the chicken. Avian Pathol. 1998;27(5):490–497. doi: 10.1080/03079459808419373. [DOI] [PubMed] [Google Scholar]
- Vrba V, Blake DP, Poplstein M. Quantitative real-time PCR assays for detection and quantification of all seven Eimeria species that infect the chicken. Vet Parasitol. 2010;174(3-4):183–190. doi: 10.1016/j.vetpar.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Morris GM, Gasser RB. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol Adv. 2006;24(6):590–603. doi: 10.1016/j.biotechadv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Perry B, Randolph T, McDermott J, Sones K, Thornton P. Investing in animal health research to alleviate poverty. Nairobi, Kenya: ILRI (International Livestock Research Institute); 2002. [Google Scholar]
- Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers) Mol Cell Probes. 2002;16(3):223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- Notomi T, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko I, et al. Detection of enterotoxigenic Clostridium perfringens in meat samples by using molecular methods. Appl Environ Microbiol. 2011;77(21):7526–7532. doi: 10.1128/AEM.06216-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanis P, et al. Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of cryptosporidium oocysts in fecal and water samples. Appl Environ Microbiol. 2007;73(17):5660–5662. doi: 10.1128/AEM.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C, et al. Rapid detection of Infectious bursal disease virus by reverse transcription loop-mediated isothermal amplification assay. J Vet Diagn Invest. 2009;21(6):841–843. doi: 10.1177/104063870902100612. [DOI] [PubMed] [Google Scholar]
- Barkway CP, Pocock RL, Vrba V, Blake DP. Loop-mediated isothermal amplification (LAMP) assays for the species-specific detection of Eimeria that infect chickens. BMC Vet Res. 2011;7(1):67. doi: 10.1186/1746-6148-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P, Joyner L, Millard B, Norton C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Veterinaria Latina. 1976;6(3):201–217. [PubMed] [Google Scholar]
- Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques. 2009;46(3):167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- Arakawa A, Baba E, Fukata T. Eimeria tenella infection enhances Salmonella typhimurium infection in chickens. Poult Sci. 1981;60(10):2203–2209. doi: 10.3382/ps.0602203. [DOI] [PubMed] [Google Scholar]
- Blake DP, Smith AL, Shirley MW. Amplified fragment length polymorphism analyses of Eimeria spp.: an improved process for genetic studies of recombinant parasites. Parasitol Res. 2003;90(6):473–475. doi: 10.1007/s00436-003-0890-x. [DOI] [PubMed] [Google Scholar]
- Lund M, Nordentoft S, Pedersen K, Madsen M. Detection of Campylobacter spp. in chicken fecal samples by real-time PCR. J Clin Microbiol. 2004;42(11):5125–5132. doi: 10.1128/JCM.42.11.5125-5132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj GD, et al. Real-time PCR-based quantification of Eimeria genomes: a method to outweigh underestimation of genome numbers due to PCR inhibition. Avian Pathol. 2013;42(4):304–308. doi: 10.1080/03079457.2013.790531. [DOI] [PubMed] [Google Scholar]
- Blake DP, Hesketh P, Archer A, Shirley MW, Smith AL. Eimeria maxima: the influence of host genotype on parasite reproduction as revealed by quantitative real-time PCR. Int J Parasitol. 2006;36(1):97–105. doi: 10.1016/j.ijpara.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Beck HP, et al. Molecular approaches to diversity of populations of apicomplexan parasites. Int J Parasitol. 2009;39(2):175–189. doi: 10.1016/j.ijpara.2008.10.001. [DOI] [PubMed] [Google Scholar]


