Abstract
Spinal cord injuries (SCI) cause serious neurological impairment and psychological, economic, and social consequences for patients and their families. Clinically, more than 50% of SCI affect the cervical spine1. As a consequence of the primary injury, a cascade of secondary mechanisms including inflammation, apoptosis, and demyelination occur finally leading to tissue scarring and development of intramedullary cavities2,3. Both represent physical and chemical barriers to cell transplantation, integration, and regeneration. Therefore, shaping the inhibitory environment and bridging cavities to create a supportive milieu for cell transplantation and regeneration is a promising therapeutic target4. Here, a contusion/compression model of cervical SCI using an aneurysm clip is described. This model is more clinically relevant than other experimental models, since complete transection or ruptures of the cord are rare. Also in comparison to the weight drop model, which in particular damage the dorsum columns, circumferential compression of the spinal cord appears advantageous. Clip closing force and duration can be adjusted to achieve different injury severity. A ring spring facilitates precise calibration and constancy of clip force. Under physiological conditions, synthetic self-assembling peptides (SAP) self-assemble into nanofibers and thus, are appealing for application in SCI5. They can be injected directly into the lesion minimizing damage to the cord. SAPs are biocompatible structures erecting scaffolds to bridge intramedullary cavities and thus, equip the damaged cord for regenerative treatments. K2(QL)6K2 (QL6) is a novel SAP introduced by Dong et al.6 In comparison to other peptides, QL6 self-assembles into β-sheets at neutral pH6.14 days after SCI, after the acute stage, SAPs are injected into the center of the lesion and neural precursor cells (NPC) are injected into adjacent dorsal columns. In order to support cell survival, transplantation is combined with continuous subdural administration of growth factors by osmotic micro pumps for 7 days.
Keywords: Medicine, Issue 96, Spinal cord injury (SCI), cervical trauma, aneurysm clip SCI model, intraspinal injection, stem cells, self-assembling peptides, neural precursor cells, growth factors
Introduction
More than 50% of spinal cord injuries are related to the cervical spine. In the clinical setting two major pathophysiological mechanisms are described: the initial contusion of the spinal cord and subsequently, the ongoing compression caused by bone fractures, hemorrhages or tissue swelling.
The aneurysm clip contusion/compression model mimics both pathophysiological mechanisms: snapping the clip produces a contusion and the duration of clipping represents the compression component, conceding that the compression in clinical settings caused by bone fractures, hemorrhages or tissue swelling last significant longer. The used aneurysm clip is modified by a ring spring guaranteeing exact and reproducible clipping force. Especially in comparison to the hemi-transection or the contusion model, this aneurysm clip model mimics best clinical settings. While patients with thoracic injuries suffer from paraplegia, most patients with cervical injuries are tetraplegic and completely dependent. The anatomical structure of the cervical cord, however, shows significant differences compared to the thoracic or lumbar spine, and thus, is addressed in particular in this protocol.
The development of intramedullary cavities and tissue scarring are obstacles for recovery and regeneration. To overcome these barriers the use of scaffold material is a promising approach. Self-assembling peptides can be injected directly into the epicenter of the lesion. There they assemble into nano-fiber scaffolds bridging the cavity and improve the inhibitory environment by reducing inflammation and tissue scaring. While rigid materials cause considerable damage of the spinal cord during implantation, the fluid peptides can be injected safely and without severe additional damage.
Improving the inhibitory environment with self-assembling peptides before stem cell transplantation, hence, support cell integration, differentiation and finally, functional recovery, after cervical spinal cord injury.
Protocol
NOTE: The following experimental protocol was approved by the animal care committee of the University Health Network (Toronto, Canada) and is in accordance with the policies established in the guide to the care and use of experimental animals prepared by the Canadian council of animal care.
1. Cervical Aneurysm Clip Contusion/Compression Model
Before surgery autoclave instruments and keep sterile conditions during the entire surgcial procedure by putting the instruments in a 70% alcohol bath.
Anaesthetize Wistar rats (250-270 g) with a combination of oxygen (O2), nitrous oxide (N2O) (1:1), and 1.8-2.2% Isoflurane and support spontaneous breathing via a gas anesthesia mask. For induction of anesthesia start with 5% Isoflurane for 1 minute and reduce afterwards. Before starting surgery, control depth of anesthesia by giving a painful stimulus (e.g. at the paws). Apply fatty ointments in the eyes to avoid dryness and to prevent subsequent infections.
Put the rats onto a heating cushion (37 °C) and fix the head into a stereotactic frame.
Shave the surgical region around the cervical spine and disinfect with povidone iodine and 70% alcohol.
Make a midline incision above the spine from the cervical vertebra (C2) reaching to the prominent processus of the thoracic vertebral body 2 (T2).
Cut through the outer layer of the vertebral muscles directly on the midline (to avoid bleeding) in a cranio-caudal direction and further dissect the deeper muscle layers bluntly until you reach the processus spinosus and the laminae. Insert retractors.
Orientate the prominent processus spinosus of the vertebra body T2 to observe the targeted levels for laminectomy. After identification and micro-surgical preparation of the selected laminae, cut through the ligamenti flavae to loosen the laminae and the processus spinosus. Finally, cut through the laminae with a bone clipper lateral to the spinal cord and remove those gently, avoiding any compression of the spinal cord itself. NOTE: Most common levels are C5/6, C6/7, or C7/T1. The perioperative mortality rate increases the more rostral the level of injury. Bleeding from the paravertebral venous sinus is common and can be addressed by careful compression with sponge.
Before inserting the clip to traumatize the cord, identify emerging nerve roots to spare them from clipping (especially at level C5/6).
In order to ensure smooth clip induction, loosen the ventral dura from the dorsal side of the vertebral bodies with a hook and prepare a corridor for the clip.
Finally, insert the open clip and let it snap shut (rapid closure) to achieve a contusion injury. Clip closing force and duration of clip closure determine the intensity of the trauma and the extent of compression. Commonly used are clip forces of between 15-35 g and a clipping duration of e.g. 1 min. (Figure 1).
After removal of the clip, adapt muscles in 2 layers and close the wound.
Stop anesthesia and let the animal wake up under your continuous observation until it regains sufficient consciousness for sternal recumbency. Finally, put the rat in a single cage and follow post-operative treatment guidelines.
- Since animals struggle with the severity of this type of injury, you must pay special attention to post-operative treatments:
- Administer painkillers (Buprenorphine and Meloxicam for 3 days and 5 days, respectively and according to the clinical symptoms).
- Give additional saline solution subcutaneously for 3 days (2 times a day, 5-10 milliliter (ml)).
- Provide antibiotics in the drinking water 2 days prior and until 7 days post surgery (e.g. Moxifloxacine)
- Squeeze the urinary bladder 2-3 times a day until recovery of bladder function is constantly apparent.
- Observe neurological deficits and physiological condition of the operated animals at least once a day.
2. Injecting SAPs and NPCs (14 days after injury)
Induce anesthesia as described in 1.1. to 1.3, fix the head of the rat in a stereotactic frame, remove the stitches or wound clips, and disinfect the wound and the surgical area with povidone iodine and 70% alcohol.
Carefully dissect the paravertebral muscles, insert retractors, remove scar tissue microscopically from the dura, and re-expose the lesion site.
Prepare SAPs in a concentration of 1% (w/v) to perform an extracellular matrix gel. QL6 SAPs have a physiologically compatible pH and do not need to be buffered prior to injection. For visualization of SAPs in the spinal cord, use a fluorescent derivate of QL6 (QL6-FITC).
Inject SAPs (5 microliter (μl) into the center of the lesion, distributed in 2 portions, each 2.5 μl bilateral of the midline. Use a Hamilton syringe connected to the stereotactic frame with a micro glass capillary (100 micrometer (μm) outer diameter (OD)). Open the dura carefully with the tip of a sharp needle, and insert the glass capillary stereotactically 2 millimeter (mm) into the traumatized spinal cord.
After injecting 1/3 of the volume, remove the needle to 1.5 mm depth, and after a further 1/3 to 1 mm. After injection of the entire volume, and before removal of the syringe, wait 5 minutes to stabilize gel formation.
To generate NPCs, use adult DsRed mice (or YFP positive mice, green) and isolate and cultivate them from the paraventrical zone4,18,21.
Assess viability of NPCs by Trypan Blue staining indicating the presence of ~90% live cells in the cell suspension. Dilute the cells in growth medium (50 x 103 live cells/μl) and then use them for cell transplantation.
Make four 2 μl (8 μl total volume, containing 4 x 105 NPCs) intraspinal injections bilaterally at 2 mm rostral and caudal of the injury site. After opening of the dura, insert the Hamilton micro glass capillary 1.5 mm below the dorsal surface of the spinal cord and inject 2 μl of the cell suspension. Choose an injection rate at about 0.5 μl /min (min).
At the end of each injection and before removal of the capillary out of the cord, wait for at least 1 min allowing tissue stretching to accommodate the new cell volume. (Figure 2)
3. Implantation of Subdural Pumps for Growth Factor Application
In order to enrich the cerebral spinal fluid (CSF) with growth factors supporting cell survival, use micro-osmotic pumps diluting growth factors sub-durally throughout 7-14 days, with a dilution rate of 0.5 μl/hr, a catheter diameter of 0.04 cm OD, and a reservoir volume of 100 μl.
Choose preferred growth factors (e.g., brain derived growth factor (BDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF)), fill the pumps 6-10 hr prior implantation, and equilibrate pumps in a water bath at 37 °C.
Immediately after NPC injections, prepare a subcutaneous recess to place the pump. Preferred locations are the lateral flanks of thoraco- abdominal region avoiding major local discomfort caused by the pump itself.
To gain the highest concentration of growth factors, ensure that the open tip of the catheter ends close to the lesion site. Therefore, perform a skip-laminectomy of the adjacent upper or lower level. For example, if the SCI is at C7/T1, perform a small laminectomy of C5.
Put the pump into the subcutaneous recess, shorten the catheter to the necessary length, and secure it with several sutures (6.0) on the paravertebral muscles avoiding any movement-associated dislocation.
After opening the dura (e.g., at C5) with the sharp tip of a needle, introduce the catheter into the subdural space and let it glide in a caudal direction without any resistance and without injuring the cord. The skipped lamina of e.g. C6 serves as additional point of fixation and stabilization of the catheter.
Make sure that the catheter runs smoothly and does not fold.
Close muscles by layer, and skin, with sutures or clips. (Figure 3)
Stop anesthesia and let the animal wake up under your continuous observation until it regains sufficient consciousness for sternal recumbency. Finally, put them back in a single cage and follow post-operative treatment guidelines.
- Provide special postoperative treatment, even though, animals might have recovered partly at this time-point:
- Administer painkillers (Buprenorthine and Meloxicam for 3 days and 5 days, respectively and according to the clinical symptoms).
- Provide antibiotics in water bottles 2 days prior until 7 days post-surgery (e.g. Moxifloxacine).
- Continue squeezing the urinary bladder, if still necessary.
- Give additional subcutaneous fluids, if the rats appear dehydrated.
- Keep observing the neurological function and physiological condition of the operated animals at least once a day.
- Administer immunosuppression treatment 2 days prior NPC injection and until 7 days post transplanation (minocycline) and until sacrifice (sandimmune), respectively.
4. Tissue Assessment
Sacrifice animals at the end of the observation time (e.g. 4 weeks after SCI) in deep anesthesia (5% Isoflurane for 2-3 min) and perfuse them transcardially with 50 ml cold (4 °C) saline followed by 150 ml cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS).
Remove the spinal cord and put it in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) for 24 hr.
Put the spinal cords in 25% sucrose solution for cryoprotection for 48 hr.
Provide longitudinal cryosections with a thickness of <30 μm.
For immunohistochemical staining of all cell-nuclei providing a background use DAPI (1:1,000). DsRed positive NPCs appear red, QL-6 FITC appears green, and both thus need not to be stained specially. (Figure 4).
For scanning electron microscopy (SEM) let samples soak in glutaraldehyde at 4 °C for 2 hr, dehydrate them slowly in 10% increment steps of ethanol for 5 min and place them in a pressurized liquid CO2 siphon for 1 hr. Coat scaffolds with gold using a sputter coater. Take images with a Hitachi S-3400N scanning electron microscope. (Figure 5)
Representative Results
When performing the above-described procedure, you will get a SAP scaffold bridging the cavity and offering an improvement of the inhibitory environment, less tissue scaring and an increase in NPC survival. Figure 4 shows a longitudinal section of a rat spinal cord obtained at the injury site 6 weeks after SCI and 4 weeks after QL6 SAP injection and NPC transplantation. QL6 peptides were successfully injected into the cord, aggregated in the epicenter and diffused rostro-caudally in the penumbra. Electron microscope imaging further shows, in Figure 5, the assembly of 1% (w/v) QL6 peptides to a nanofiber scaffold within 2 hr diluted in PBS solutions.
This matrix provides an improvement of the inhibitory environment contributing to increased cell survival and cell differentiation, less tissue scarring and finally leads to a better chance for functional recovery.
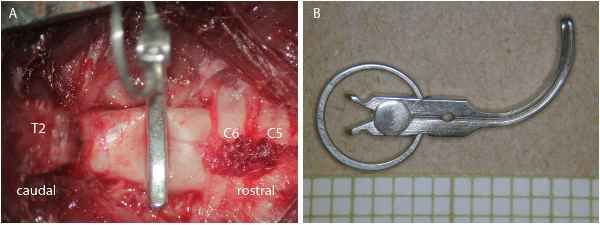 Figure 1: Clip contusion/compression aneurysm model.(A) Photograph through the surgical microscope after laminectomy of C7/T1 and clip contusion/compression of the spinal cord of a rat. (B) Picture of the clip with the ring spring guaranteeing precise closing force.
Figure 1: Clip contusion/compression aneurysm model.(A) Photograph through the surgical microscope after laminectomy of C7/T1 and clip contusion/compression of the spinal cord of a rat. (B) Picture of the clip with the ring spring guaranteeing precise closing force.
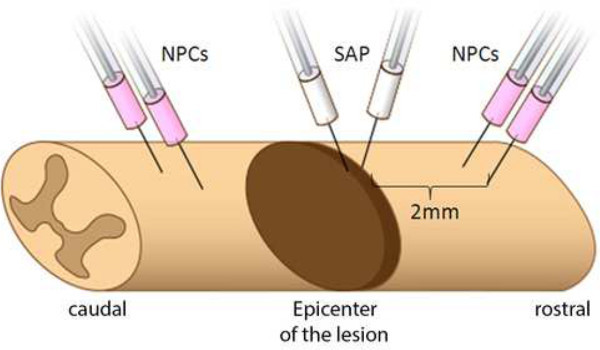 Figure 2: Injection points of SAPs and stem cells. Graphical illustration of the injection points: 2 stereotactically performed SAP injections in the epicenter of the lesion, followed by 4 injections of NPCs in the adjacent dorsal columns with a distance of 2 mm caudal and rostral from the epicenter.
Figure 2: Injection points of SAPs and stem cells. Graphical illustration of the injection points: 2 stereotactically performed SAP injections in the epicenter of the lesion, followed by 4 injections of NPCs in the adjacent dorsal columns with a distance of 2 mm caudal and rostral from the epicenter.
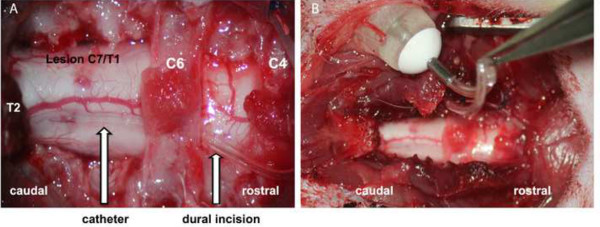 Figure 3: Subdural catheter for growth factor administration. Photograph through the surgical microscope: (A) Implanted subdural catheter in order to administer growth factors: the catheter is fixed by several 6.0 sutures at the paraspinal muscles; small opening of the dura at C5; subdural positioning of the catheter with the open catheter end close to the lesion site at C7/T1. (B) Catheter connected to a micro pump placed in a subdural recess on the lateral flank.
Figure 3: Subdural catheter for growth factor administration. Photograph through the surgical microscope: (A) Implanted subdural catheter in order to administer growth factors: the catheter is fixed by several 6.0 sutures at the paraspinal muscles; small opening of the dura at C5; subdural positioning of the catheter with the open catheter end close to the lesion site at C7/T1. (B) Catheter connected to a micro pump placed in a subdural recess on the lateral flank.
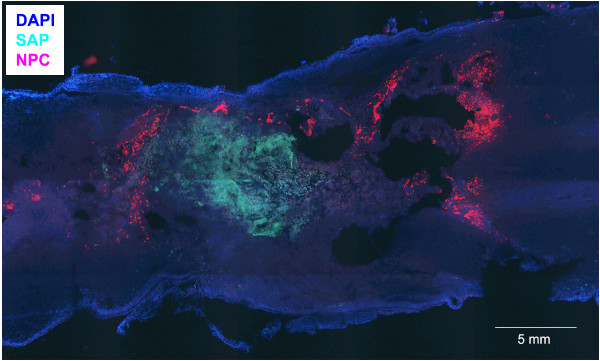 Figure 4: Successful delivery of SAPs and NPCs into the cervical spinal cord. Fluorescent staining (DAPI background, blue) of a longitudinal section of a traumatized spinal cord of a rat. Labeled SAPs (QL6-FITC, green) have aggregated in the epicenter of the lesion. Injected NPCs (DsRed positive, red) have diffused in rostral and caudal directions. Scale bar refers to 5 mm.
Figure 4: Successful delivery of SAPs and NPCs into the cervical spinal cord. Fluorescent staining (DAPI background, blue) of a longitudinal section of a traumatized spinal cord of a rat. Labeled SAPs (QL6-FITC, green) have aggregated in the epicenter of the lesion. Injected NPCs (DsRed positive, red) have diffused in rostral and caudal directions. Scale bar refers to 5 mm.
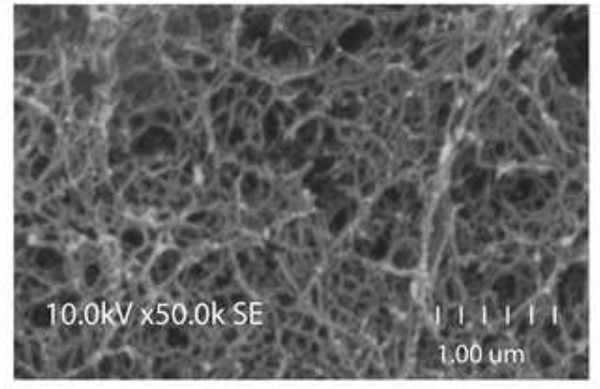 Figure 5: Nanofiber scaffold formation. Scanning electron microscope (SEM) image showing nanofiber scaffold formation of assembled SAPs. Scale bar refers to 1 μm).
Figure 5: Nanofiber scaffold formation. Scanning electron microscope (SEM) image showing nanofiber scaffold formation of assembled SAPs. Scale bar refers to 1 μm).
Discussion
This protocol has been developed to enable the reader to perform a cervical injury model in rats and to use a combined treatment approach with SAPs and NPCs promoting better recovery after cervical SCI.
Particularly in comparison to other cervical trauma models, such as the (hemi)-transection model or the weight drop and concussion models, the clip contusion/compression model represents both major pathophysiological trauma mechanisms – contusion and compression- and therefore mimics best clinical conditions. While it is well established for the use in rat and mouse thoracic spine7-11, it has recently been adapted for use in the cervical spine. This was an important step since cervical spinal cord injuries are the most frequently seen in the clinic and cervical anatomy varies significantly from the thoracic spine. Furthermore, cervical SCI patients suffering from tetraparesis are keen to regain at least partial motor function of their upper extremities.
Challenging aspects of an experimental cervical SCI, however, are a high mortality rate that increases in a rostral direction from C7 to C5 and can reach up to 20-30% in the middle and upper cervical spine. Furthermore, emerging nerve roots (especially at C5/6) are sensitive to manipulation, and in the case they are not preserved, damage and irritation might cause chewing of the frontal paws resulting in the necessity of an early sacrifice of the animals. In general, animals with cervical spinal cord injury need a lot of post-operative attention and care and show a longer course of recovery. On the other hand, in addition to careful surgical preparation, these issues can partly be addressed by adapting the severity of the injury by selecting an appropriate clip closing force (e.g. 15 to 35 g) and an adequate clipping time (e.g. 1 min). Modifying these parameters lead to different severities of injury (mild, moderate, or severe SCI). Furthermore, there might be a risk of surgeon dependency or an unequal distribution of the damage. To address these issues the use of a clip applicator is an option where the clip always is released and therefore closed with the same speed and hence velocity. Before snapping the clip it is mandatory to ensure that the clip has its correct position and encloses the entire spinal cord equally. Special surgical challenge lies in the re-exposure of the lesion site removing the scar tissue from the dura. It is recommended that this procedure is executed micro-surgically, using sharp preparation, and avoiding any pressure or pulling of the fixed cord.
Self-assembling peptides have been identified having potentials to bridge the cavity and, furthermore, to improve the inhibitory environment at the lesion site finally even leading to axonal regeneration and sprouting5,12,13,14. QL-6 nanofibers assemble to scaffolds bridging the intramedullary cavity and, thus, can provide a matrix for axonal sprouting and regeneration and improve the inhibitory environment before cell transplantation. The advantages of these peptides lay in their low viscosity (fluid) and the neutral pH. Especially in comparison to high-viscous hydrogels or tissue scaffolds, SAPs can easily injected into the injured spinal cord and additional injuries derived from the injection itself are limited.
Although QL6 nanofibers themselves might improve cell survival4,15, nevertheless, the use of growth factors seems to be advantageous16,17,18,19,20,21. They can be administered either by hydrogels releasing growth factors22, or by application via osmotic pumps (as described above). Osmotic pumps connected to subdural catheters offer the advantage of a continuous and controlled release and thus, enrichment of the cervical spinal fluid (CSF). Placing the subdural catheter, however, is challenging especially with respect to vast scarring that might have occurred after some weeks post injury.
In order to provide good tissue conditions for NPC survival and integration, several studies have chosen injection points in the adjacent white matter, 2 mm rostral or caudal to the lesion site and not directly in the epicenter of the lesion4,15,18. There, NPCs have a better chance for survival, integration, and differentiation into astrocytes, oligodendrocytes or neurons, and can migrate towards or into the lesion resulting in axonal sprouting and improved axonal connectivity.
After mastering these techniques this proposed protocol might to be adopted by the individual users according their necessities and interests encompassing modifications of the level and severity of the injury, the use of different cell types or growth factors, or the addition of other neuro-protective substances.
In summary, combined treatment with SAPs and NPCs might offer a novel and promising approach overcoming the most challenging obstacles in the treatment of SCI: cavities and tissue scarring.
Disclosures
Covidien supplied the QL6 SAP for this study.
Acknowledgments
We would like to acknowledge the funding support for this work from the Canadian Institutes of Health Research (CIHR), the Krembil Family Foundation, the Halbert Chair in Neural Repair and Regeneration, Phillip and Peggy DeZwirek, and Gordon Yao for the contribution to Figure 2. Klaus Zweckberger was funded by a grant from the “Deutsche Forschungsgesellschaft” (DFG).
References
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH, Linden RD. The relationships among the severity of spinal cord injury, motor and somatosensory evoked potentials and spinal cord blood flow) Electroencephalogr Clin Neurophysiol. 1989;74:241–259. doi: 10.1016/0168-5597(89)90055-5. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Wilcox JT, Nishimura Y, Zweckberger K, Suzuki H, Wang J, Liu Y, Karadimas SK, Fehlings MG. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD. Self-assembly of multidomain peptides: balancing molecular frustration controls conformation and nanostructure. J Am Chem Soc. 2007;129:12468–12472. doi: 10.1021/ja072536r. [DOI] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- Poon PC, Gupta D, Shoichet MS, Tator CH. Clip compression model is useful for thoracic spinal cord injuries: histologic and functional correlates. Spine (Phila Pa 1976) 2007;32:2853–2859. doi: 10.1097/BRS.0b013e31815b7e6b. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J Neurotrauma. 2002;19:191–203. doi: 10.1089/08977150252806956. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma. 2002;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- Cigognini D, Satta A, Colleoni B, Silva D, Donegà M, Antonini S, Gelain F. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffolds. PLoS One. 2011;6(5):e19782. doi: 10.1371/journal.pone.0019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Wu T, Wang L, Liu Y, Li M, Long Z, Chen H, Li Y, Wang Z. Cellular prostheses fabricated with motor neurons seeded in self-assembling peptides promotes partial functional recovery afters spinal cord injury in rats. Tissue eng Part A. 2012;18(9-10) doi: 10.1089/ten.TEA.2011.0151. [DOI] [PubMed] [Google Scholar]
- Gelain F, Cigognini D, Caprini A, Silva D, Colleoni B, Donegà M, Antonini S, Cohen BE, Vescovi A. New bioactive motifs and their use in functionalized self-assembling peptides for NPC differentiation and neural tissue engineering. Nanoscale. 2012;4(9):2946–2957. doi: 10.1039/c2nr30220a. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ye H, Satkunendrarajah K, Yao GS, Bayon Y, Fehlings MG. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075–8088. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Rosner J, Avalos P, Axosta F, Liu J, Drazin D. The potential for cell therapy combined with growth factors in spinal cord injury. Stem Cell Int. 2012. p. 826754. [DOI] [PMC free article] [PubMed]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynsky MH. Long distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1265–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Schut D, Wang J, Fehlings MG. Chondrioitinase and grwoth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PLoS One. 2012;7(5):e37589. doi: 10.1371/journal.pone.0037589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad BI, Carmody MA, Steinmetz MP. Potential role of growth factors in the management of spinal cord injury. World Neurosurg. 2013. pp. 1875–8750. [DOI] [PubMed]
- Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002;19(2):223–238. doi: 10.1089/08977150252806974. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Cindi MM, Fehlings MG. Delayed trasplantation of adult neural presursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–459. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]


