Abstract
The number of acceptable donor lungs available for lung transplantation is severely limited due to poor quality. Ex-Vivo Lung Perfusion (EVLP) has allowed lung transplantation in humans to become more readily available by enabling the ability to assess organs and expand the donor pool. As this technology expands and improves, the ability to potentially evaluate and improve the quality of substandard lungs prior to transplant is a critical need. In order to more rigorously evaluate these approaches, a reproducible animal model needs to be established that would allow for testing of improved techniques and management of the donated lungs as well as to the lung-transplant recipient. In addition, an EVLP animal model of associated pathologies, e.g., ventilation induced lung injury (VILI), would provide a novel method to evaluate treatments for these pathologies. Here, we describe the development of a rat EVLP lung program and refinements to this method that allow for a reproducible model for future expansion. We also describe the application of this EVLP system to model VILI in rat lungs. The goal is to provide the research community with key information and “pearls of wisdom”/techniques that arose from trial and error and are critical to establishing an EVLP system that is robust and reproducible.
Keywords: Medicine, Issue 96, EVLP, VILI, tidal volume, PEEP, lung transplant, positive pressure ventilation
Introduction
Clinical Relevance
There is currently a paucity of suitable lungs available for transplantation with only 19% of lungs being able to be utilized nationally leading to protracted wait-list time or patients dying awaiting transplant1. The shortage can be due to older donors, trauma, infection, multi-system organ failure and sometimes injured donor lungs upon harvest2. In addition, the lung is a frail organ outside of the thoracic cavity and standard transport and preservation techniques can lead to deterioration and non-viable lungs. Therefore, maintaining and improving lung viability ex-vivo has recently become a major focus in lung transplant medicine.
Ex-vivo Lung Perfusion (EVLP)
Ex-vivo lung perfusion (EVLP) has evolved to continuously perfuse organs being evaluated for transplantation and enables a period of assessment that allows for the potential of lung resuscitation or reconditioning. EVLP can prolong total out of body organ ischemic time and allow the donated organs to travel further distances3. Typically, the lungs are ventilated at 50% of total lung capacity or 20 cmH2O of peak airway pressure with a fraction of inspired oxygen (FiO2) of 30% to 50%4. Preservation solution is perfused at 40-60 ml/kg (approximately 40% of the predicted cardiac output of 100 ml/kg) in humans and large animals5,6, but is perfused at around 20% of the cardiac output for rats7. The inclusion of STEEN solution has allowed human lungs to travel in RT environments without development of pulmonary edema9. This pioneering work has been refined by the University of Toronto Lung Transplant Program10-13 and is being evaluated for improved assessment of marginal donor lungs for transplantation14,15. However, the optimal ventilation and perfusion conditions needed to regenerate marginal and/or sub-standard lungs for transplantation is not known and is currently an active area of research.
Isolated lung perfusion systems have been used in small animals to cause lung injury, re-create respiratory diseases, and perfuse the lungs with different solutions to prevent ischemic damage. Investigators have created a small-animal model of lung transplantation by using the isolated lung-perfusion system to mimic the EVLP protocols that could be used in humans and larger animals16-18. However, this experimental model has many challenges with regards to the various techniques and parameters used to mimic human physiology. In particular, there are many subtleties in maintaining lung viability during EVLP. These subtleties can arise due to differences in harvesting technique, positive pressure ventilation settings, perfusate composition and flow conditions and cannulation of the lung. Therefore, the goal here is to provide the research community with a number of troubleshooting and implementation tips that we have found lead to a robust method for implementing EVLP in a rodent model.
Protocol
NOTE: All procedures were performed according to the guide-lines of the Institutional Animal Care and the National Research Council’s Guide for the Humane Care and Use of Laboratory Animals (IACUC) and has undergone approval by The Ohio State University IACUC committee.
1. Initial Setup
Set up the EVLP circuit and have warm (37 °C) perfusate circulating through the system prior to incorporating the ex-planted lung (Figure 1).
Set the warm water bath, used to jacket the perfusate reservoir, heat exchanger, and artificial thorax, to 37 °C and circulating (Figure 1).
Run a de-oxygenation solution (e.g., 6% O2, 8% CO2, 84% N2) counter currently through the perfusate in the gas filter to ensure the perfusate has ~6% dissolved oxygen for the experiment. NOTE: This de-oxygenated perfusate enables the assessment of the lung function by measuring the oxygen introduced into the perfusate, post-organ.
Open the data acquisition program and connect the pulmonary artery pressure transducer, tracheal differential pressure transducer, respiratory flow differential pressure transducer, lung weight transducer, and pump speed transducer to the EVLP circuit and the data-acquisition / analog-to-digital converter box (Figure 2).
Set up the operating table and operating tools at the EVLP circuit (Figure 3).
Set up a small container of liquid nitrogen near the EVLP circuit if samples will be obtained. NOTE: The author’s system has been modified to collect pre-organ and post-organ perfusate without interrupting the pressure-flow dynamics that may potentially injure the lung.
2. Preparation of Anesthetics and Heparin, Anesthetization of Rat
Put on the following personal protective equipment (PPE) before handling rats and rat tissue: surgical mask, surgical gloves, and disposable gown.
Weigh the rat and record the weight.
Prepare 1,200 U/kg heparin.
Prepare both 60 mg/kg ketamine and 5 mg/kg Xylazine in the same syringe, preparing the ketamine first.
Intraperitoneally inject the mixture of ketamine and Xylazine into the rat and allow 5 min for the rat to become unconscious.
Confirm proper anesthetization by checking toe pinch reflex. If the rat does not withdraw its toe, it is not feeling pain.
Move rat to operating table, secure in supine position, and spray with alcohol for sterilization.
3. Extraction and Initial Ventilation of the Rat Lungs
Prepare 4-20 cm long silk sutures (3-0 or 4-0 should suffice).
Begin recording data using the data acquisition program.
Check for appropriate depth of anesthesia, using surgical scissors enter peritoneal cavity by a midline laparotomy and inject heparin into the inferior Vena cava.
Carry the incision cranially past the manubrium into the neck until the trachea is exposed. Do not rupture the thoracic cavity (Figure 4A).
Dissect posterior to the trachea in the midline and slide a silk suture posterior to the trachea (Figure 4B).
Raise the anterior section of the trachea and make a transverse incision between the cartilaginous rings, high on the trachea. Do not cut through the posterior membranous portion of the trachea at this point (Figure 4C).
Cannulate the trachea with the trachea cannula and secure with the silk suture (Figure 4D). Ensure that the suture ligature is secured in the notching to mitigate migration of the cannula.
Connect the trachea cannula to the ventilation circuit.
Turn on the mechanical ventilator to start mechanically ventilating the lungs. NOTE: Initial settings were chosen to be a tidal volume of 4 ml/kg and positive end-expiratory pressure (PEEP) of 2 cmH2O. These settings are the initial settings and depending on the experimental conditions may be adjusted once the organ is in the ex-vivo perfusion system.
Enter the thoracic cavity through the sternum/xyphoid and continue cranially towards the suprasternal notch. Take care to avoid touching the lungs. NOTE: As the rat lung is fragile, any inadvertent manipulation can lead to trauma and pulmonary edema (Figure 5A).
Using 2 retractors, retract the thoracic cavity to properly expose the anatomy (Figure 5A). Once again, take care to avoid touching the lungs.
Remove the thymus with slight elevation and blunt dissection.
Shift the abdominal contents to a side to expose either the inferior vena cava (IVC) or the mesenteric vein (MV).
Incise either the IVC or the MV to exsanguinate the rat, providing euthanasia.
Place a silk suture posterior to the pulmonary artery and aorta in preparation for securing the pulmonary artery cannula (Figure 5B).
Make a 2-3 mm incision on the anterior surface of the right ventricular outflow tract and place the cannula in the incision and into the main pulmonary artery and secure with the silk suture (Figure 5C).
Transect the apex of the heart to allow access to the left ventricle and flush any clots within the pulmonary vasculature by flowing ~15 ml of a low K+ electrolyte solution through the pulmonary artery and out through the apex of the heart into the chest cavity (Figure 5D).
Connect the pulmonary artery (PA) cannula to the EVLP circuit. Ensure the inflow line coming from the circuit to the PA cannula is primed with perfusate to avoid any air entering the heart and lungs.
Turn on the main peristaltic pump and set it to a low (~2 ml/min) speed to allow perfusate to run through the pulmonary artery and out the left ventricle into the chest cavity. **CRITICAL STEP** Ensure the PA pressure does not spike as this is a sign of either a blockage or poor cannulation (Figure 6).
Turn off the peristaltic pump.
Position a silk suture behind the heart, around the ventricles (Figure 7).
Begin the process of cannulating the left atrium by inserting a small pair of surgical forceps into the apex, through the mitral valve, and into the left atrium. NOTE: This will dilate the mitral valve and facilitate the cannulation. Aggressive dilation, or too deep dilation, can inadvertently lacerate the left atrium rendering the procurement ineffective.
Remove the forceps from the heart.
Insert the left atrium cannula into the apex through the mitral valve and into the left atrium.
Secure the left atrium cannula with the silk suture behind the heart (Figure 8). NOTE: This suture can be “pre-tied” to facilitate cannulation.
Connect the pulmonary artery cannula to the ex-vivo lung perfusion circuit (Figure 9A). Do not connect the left atrium cannula to the EVLP circuit until the heart-lung block has been completely removed from the body.
Clamp the esophagus with a hemostat and cut below the clamp (between the clamp and diaphragm) so that the esophagus can be used to raise the cardiopulmonary structures cephalad.
Bluntly dissect the surrounding tissue and cut the descending aorta and auxiliary vessels to free the heart-lung block as it is being raised via the esophagus (Figure 9B).
Transect the trachea cephalad to the tracheal cannula to completely free the heart-lung block.
Remove the heart-lung block and place in the designated location on the EVLP circuit (Figure 9C).
Connect the left atrium cannula to the outflow line and start the main peristaltic pump (Figure 9D).
4. Ex Vivo Perfusion of the Lungs
Quickly remove the ventilation line from the top of the EVLP apparatus and attach the housing with the pressure sensors, then insert the ventilation line on top of the housing on the top of the EVLP apparatus. NOTE: This will allow the ventilation data to be recorded and pressure monitored.
Ensure the bubble trap is filled with an adequate amount of perfusate so that no air bubbles (i.e., air emboli) are introduced to the lungs.
Slowly change ventilation and perfusion settings to desired experimental levels during the initial 15 min. Additionally, during this initial ramp-up phase, increase the perfusion flow rate to the desired rate and/or pressure. NOTE: Programming the ventilator to produce intermittent sigh breaths, which facilitate movement of fluid out of the lung space and therefore delay the onset of edema, is recommended. These can be produced by ventilators equipped with the sigh function.
Define “Time 0” as the time when ventilation parameters are at a tidal volume of 4 ml/kg, PEEP at 2 cm H2O, and perfusion parameters are at their expected levels and remaining constant.
If necessary, take perfusate samples from the sample port, flash freeze in liquid nitrogen, and note the time of the samples.
When the experiment is complete, isolate any necessary anatomical pieces for collection and either flash freeze in liquid nitrogen or place in fixing solution for further studies.
Representative Results
The real-time mechanical data collected through the data acquisition program can easily be analyzed to test any number of hypotheses. For example, Figure 10A shows the average lung weight through 60 min from 10 rat experiments where animals were ventilated with a low tidal volume/low PEEP of 4 ml/kg and 2 cmH2O. Although there is a very minor increase in lung weight throughout the experiment, this increase is not statistically significant (ANOVA, p = 0.92). Figure 10B shows the average pulmonary arterial pressure (PAP) through 60 min from 12 rat experiments. The lower PAP at the 0 min time point is a result of lower flow and ventilation settings used at the beginning of all experiments and the PAP remains constant after this time point with no statistically significant changes after t=10 min (ANOVA on ranks, p = 0.89). Figure 10C shows the pulmonary vascular resistance (PVR) through 60 min from 12 rat experiments and although there is a small decrease in PVR after t=20 min, there was no statistically significant difference in PVR during this experiment (ANOVA on ranks, p= 0.65). In comparison to the PVR data shown here, Noda et al. has shown the PVR to increase slightly over time for 4 hr. However, those authors report data in PVR starting at 1 hr instead of the start of the experiment and no standard deviation values are provided7. Noda et al. also does not show pulmonary edema data for the 4 hr experiments so no comparison can be made with the data presented here in Figure 10A. Major differences in Noda et al. procedure compared to what is shown in this paper include: a 1 hr cold preservation in LPS solution before EVLP, rats were initially ventilated with a gas mixture including isoflurane to render them unconscious, the perfusate solution was supplemented with 50 mg of methylprednisolone and 50 mg of cephalosporin, total flow was defined as 20% of the calculated cardiac output, perfusate samples were taken only after the lung had been ventilated on 100% O2 for 5 min prior and the experiment was run for 4 hr.
Samples taken during the experiment from the perfusate can also be analyzed for many purposes. As an example, in Figure 11 we demonstrate how high tidal volume/high PEEP ventilation can induce a pro-inflammatory response in 60 min. For these experiments, the perfusate from 4 rats ventilated under injurious conditions, i.e., high tidal volume of 10 ml/kg and high PEEP of 8 cmH2O, were analyzed for pro- and anti- inflammatory cytokines IL1β, TNFα and IL4 using standard ELISA techniques. As shown in Figure 11, compared to cytokine levels before ventilation (0 min), 60 min of injurious ventilation resulted in a statistically significant increase in IL-1β and TNFα (pro-inflammatory cytokines) and no change in IL-4 (an anti-inflammatory cytokine) concentration. Therefore, this EVLP system is able to generate lung injury profiles commonly observed during mechanical ventilation.
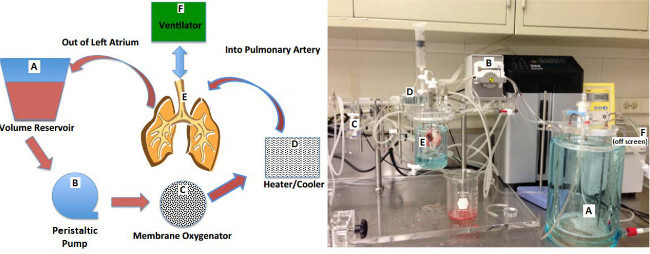 Figure 1. Diagram and photograph of small animal ex-vivo lung perfusion (EVLP) circuit. Letters in the diagram match up with letters in the photograph. Please click here to view a larger version of this figure.
Figure 1. Diagram and photograph of small animal ex-vivo lung perfusion (EVLP) circuit. Letters in the diagram match up with letters in the photograph. Please click here to view a larger version of this figure.
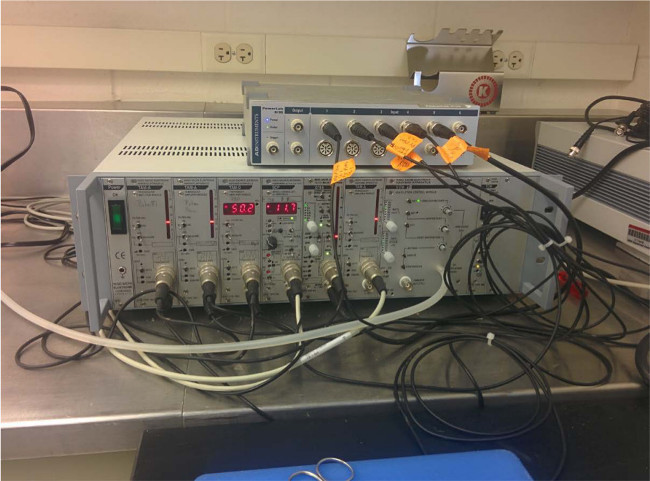 Figure 2.
All transducers are securely connected to the control boxes.
Please click here to view a larger version of this figure.
Figure 2.
All transducers are securely connected to the control boxes.
Please click here to view a larger version of this figure.
 Figure 3.
The rat operating table is securely set up adjacent to the ex vivo lung perfusion (EVLP) circuit.
Please click here to view a larger version of this figure.
Figure 3.
The rat operating table is securely set up adjacent to the ex vivo lung perfusion (EVLP) circuit.
Please click here to view a larger version of this figure.
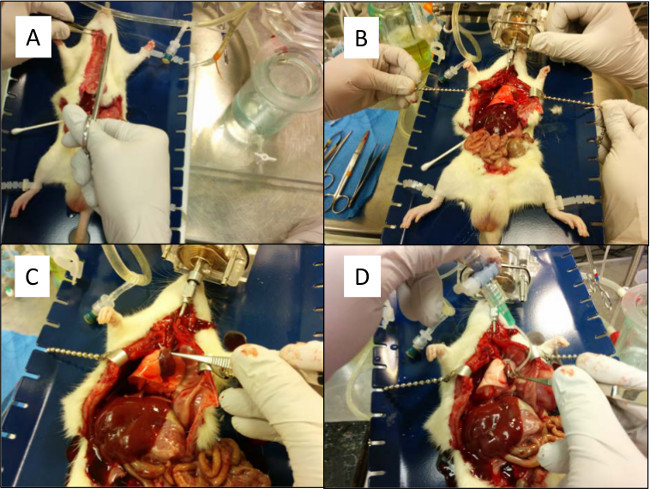 Figure 4. (A) An incision is made cranially to expose the trachea. The chest cavity is not exposed. (B) A silk suture is placed behind the trachea. (C) The trachea is partially cut to prepare for cannulation. (D) The trachea cannula is placed into position and secured with a silk suture. Please click here to view a larger version of this figure.
Figure 4. (A) An incision is made cranially to expose the trachea. The chest cavity is not exposed. (B) A silk suture is placed behind the trachea. (C) The trachea is partially cut to prepare for cannulation. (D) The trachea cannula is placed into position and secured with a silk suture. Please click here to view a larger version of this figure.
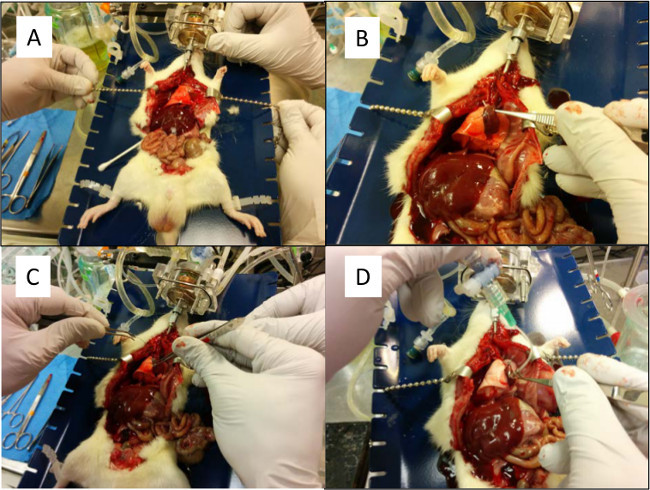 Figure 5. (A) The chest cavity is pulled back to allow access to the heart and lung. (B) Preparation for placing suture behind the pulmonary artery. (C) The pulmonary artery is cannulated and tied with the previously placed silk suture.(D) A low K+ electrolyte solution is flushed through the pulmonary artery and out the left atrium to remove any blood clots. Please click here to view a larger version of this figure.
Figure 5. (A) The chest cavity is pulled back to allow access to the heart and lung. (B) Preparation for placing suture behind the pulmonary artery. (C) The pulmonary artery is cannulated and tied with the previously placed silk suture.(D) A low K+ electrolyte solution is flushed through the pulmonary artery and out the left atrium to remove any blood clots. Please click here to view a larger version of this figure.
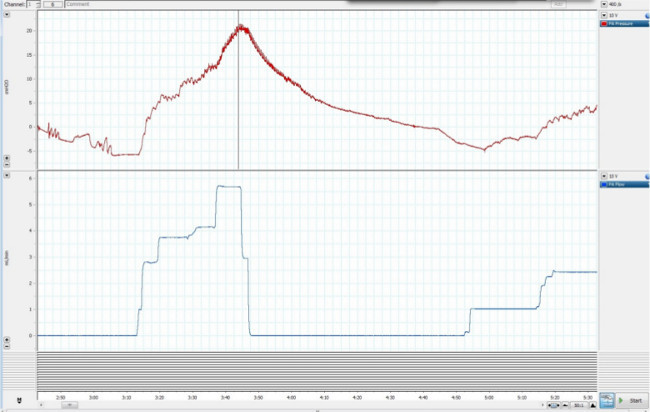 Figure 6.Increasing the pulmonary arterial flow when flushing the lung may cause the pulmonary arterial pressure to increase dramatically. If cannulation was performed correctly and there is no major blockage, the pressure should decrease. Please click here to view a larger version of this figure.
Figure 6.Increasing the pulmonary arterial flow when flushing the lung may cause the pulmonary arterial pressure to increase dramatically. If cannulation was performed correctly and there is no major blockage, the pressure should decrease. Please click here to view a larger version of this figure.
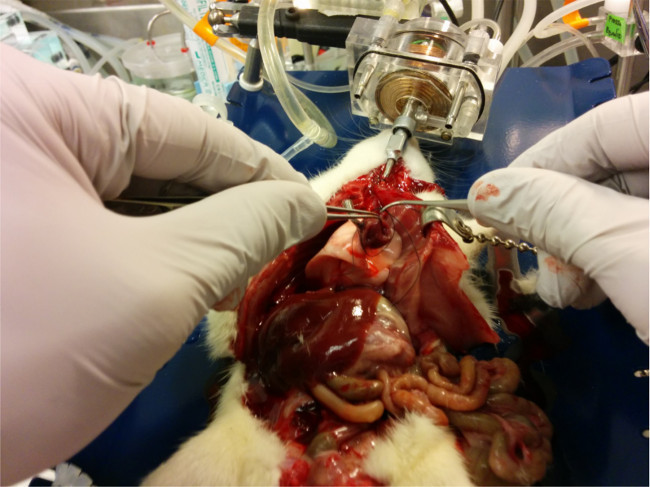 Figure 7.
Silk suture is placed around the entire heart in preparation for the left atrium cannulation.
Please click here to view a larger version of this figure.
Figure 7.
Silk suture is placed around the entire heart in preparation for the left atrium cannulation.
Please click here to view a larger version of this figure.
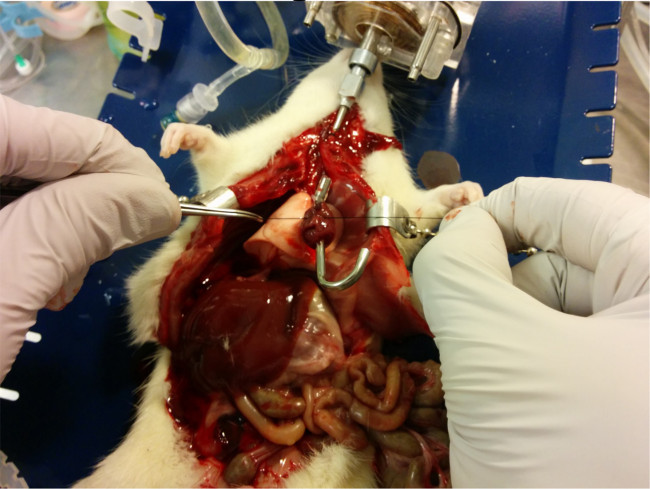 Figure 8.
The left atrium cannula is secured in place with a silk suture.
Please click here to view a larger version of this figure.
Figure 8.
The left atrium cannula is secured in place with a silk suture.
Please click here to view a larger version of this figure.
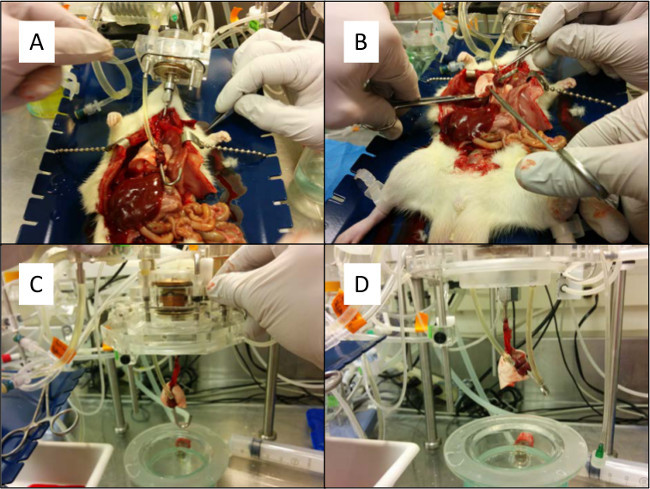 Figure 9. (A) The pulmonary artery cannula is connected to the ex-vivo lung perfusion circuit. (B) The esophagus is clamped and the connective tissue is bluntly dissected to remove the heart-lung bloc. (C) The heart-lung bloc is removed from the chest cavity and placed into the ex-vivo lung perfusion circuit. (D) The left atrium is connected to the ex-vivo lung perfusion circuit. Please click here to view a larger version of this figure.
Figure 9. (A) The pulmonary artery cannula is connected to the ex-vivo lung perfusion circuit. (B) The esophagus is clamped and the connective tissue is bluntly dissected to remove the heart-lung bloc. (C) The heart-lung bloc is removed from the chest cavity and placed into the ex-vivo lung perfusion circuit. (D) The left atrium is connected to the ex-vivo lung perfusion circuit. Please click here to view a larger version of this figure.
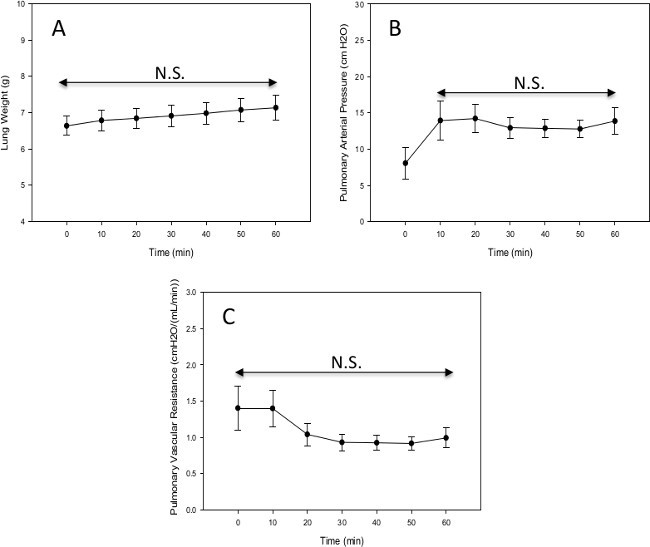 Figure 10. (A) Lung weight of male Sprague Dawley rats through 60 min of ex vivo lung perfusion (n=10). (B) Pulmonary arterial pressure of male Sprague Dawley rats through 60 min of ex vivo lung perfusion (n=12). (C) Pulmonary vascular resistance of male Sprague Dawley rats through 60 min of ex vivo lung perfusion (n=12), N.S. indicates no statistically significant difference. Please click here to view a larger version of this figure.
Figure 10. (A) Lung weight of male Sprague Dawley rats through 60 min of ex vivo lung perfusion (n=10). (B) Pulmonary arterial pressure of male Sprague Dawley rats through 60 min of ex vivo lung perfusion (n=12). (C) Pulmonary vascular resistance of male Sprague Dawley rats through 60 min of ex vivo lung perfusion (n=12), N.S. indicates no statistically significant difference. Please click here to view a larger version of this figure.
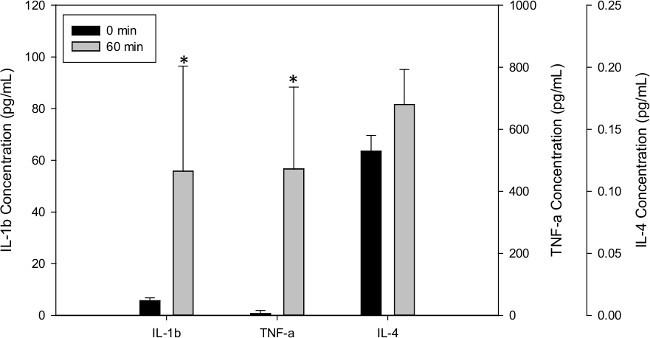 Figure 11. Effect of 1 hr ventilation at high tidal volumes (10 ml/kg) and high PEEP (8 cmH2O) on pro- and anti- inflammatory cytokine concentrations in the perfusate. n=4, * indicates statistically significant difference with respect to 0 hr sample (p < 0.05). Please click here to view a larger version of this figure.
Figure 11. Effect of 1 hr ventilation at high tidal volumes (10 ml/kg) and high PEEP (8 cmH2O) on pro- and anti- inflammatory cytokine concentrations in the perfusate. n=4, * indicates statistically significant difference with respect to 0 hr sample (p < 0.05). Please click here to view a larger version of this figure.
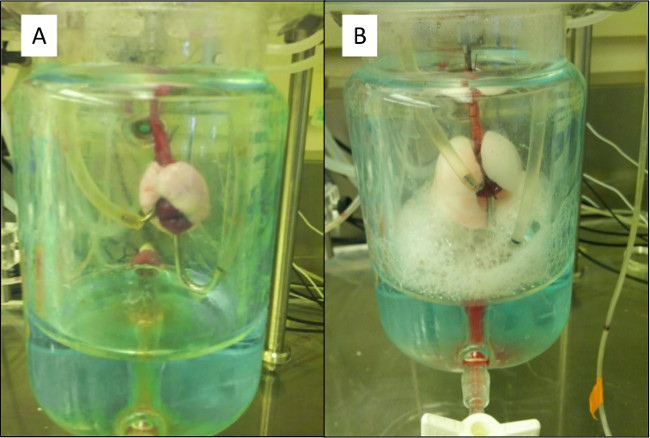 Figure 12. (A) A properly ventilating and perfusing lung connected to the EVLP circuit. (B) High positive end-expiratory pressure (PEEP) causes a tear at the trachea bifurcation causing bubbles to form at the injury and fill the artificial thorax. Please click here to view a larger version of this figure.
Figure 12. (A) A properly ventilating and perfusing lung connected to the EVLP circuit. (B) High positive end-expiratory pressure (PEEP) causes a tear at the trachea bifurcation causing bubbles to form at the injury and fill the artificial thorax. Please click here to view a larger version of this figure.
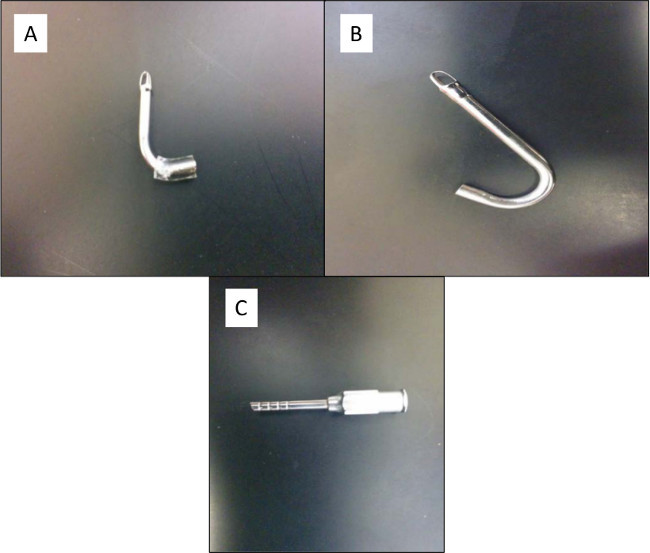 Figure 13. (A) Pulmonary artery cannula. This cannula is smaller than the left atrium cannula. (B) Left atrium cannula. This cannula is much larger than the pulmonary artery cannula. (C) Trachea cannula. This cannula has ribs to aid in securing the trachea with silk suture. The end that is inserted into the trachea is also slightly pointed to aid in inserting the cannula into the trachea. Please click here to view a larger version of this figure.
Figure 13. (A) Pulmonary artery cannula. This cannula is smaller than the left atrium cannula. (B) Left atrium cannula. This cannula is much larger than the pulmonary artery cannula. (C) Trachea cannula. This cannula has ribs to aid in securing the trachea with silk suture. The end that is inserted into the trachea is also slightly pointed to aid in inserting the cannula into the trachea. Please click here to view a larger version of this figure.
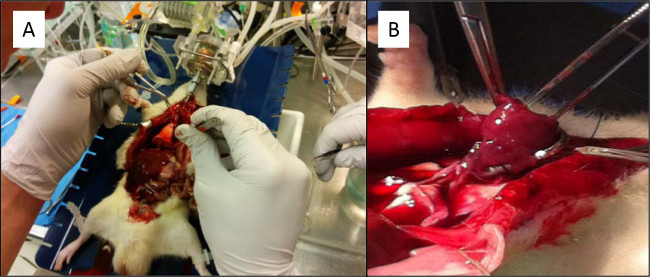 Figure 14. (A) The heart apex is held by a pair of forceps as the right ventricle is about to be incised in order to cannulate the pulmonary artery. (B) Dilation of the mitral valve annulus with a pair of small blunt-ended pick-ups makes it easier to visualize the tract into the left atrium. Please click here to view a larger version of this figure.
Figure 14. (A) The heart apex is held by a pair of forceps as the right ventricle is about to be incised in order to cannulate the pulmonary artery. (B) Dilation of the mitral valve annulus with a pair of small blunt-ended pick-ups makes it easier to visualize the tract into the left atrium. Please click here to view a larger version of this figure.
Discussion
SYSTEM MONITORING
What things look like when experiment is running well:
Once the cannulae have been placed in the circuit and the lungs are ventilating, there are multiple ways to ensure the system is working properly. There should be no leaks of perfusate throughout the line. The pulmonary vascular resistance (PVR) should remain relatively constant (assuming a constant flow). The oxygen exchange should increase once the ventilator is working properly and expanding the lungs to recruit more alveoli for gas exchange. Figure 12A shows properly ventilated and perfused lungs connected to the EVLP circuit inside the artificial thorax.
What things look like when experiment is not running well:
There are a few common issues that have had the highest rate of occurrence during the beginning stages of an EVLP experiment. The first and easiest to remedy is a leak in the line exiting from the lung. This is noticeable by a pool of perfusate pooling under part of the circuit and the level in the reservoir continually decreasing. Check and tighten any tube connectors around the spill area and inspect the tube itself for a leak. If this leak occurs before the lung, it may also introduce bubbles into the lung. This should be remedied as quickly as possible as air bubbles in the perfusate will result in tissue damage and cause a significant increase in the PVR. There may also be a leak coming from the lung or one of the cannulae. This may be caused by either slippage of a cannula or an obstruction in the exiting line causing a pressure buildup. Inspect the position for both cannulae to ensure neither has slipped or twisted. The PA pressure should also be monitored during this process because an instantaneous increase in PA pressure is an obvious sign that an obstruction of some kind has recently occurred. Figure 12B shows a ruptured lung that ruptured due to high pressures. A leak from the lung itself may also be caused by a tear in the tissue. This issue may or may not be reparable but repositioning and retightening the cannulae is the best option in this scenario.
Key Learning Points/Opportunities:
Trial and error development of the ex-vivo lung perfusion system has allowed us to identify several key issues that we outline here to facilitate efficient implementation of the EVLP system. First, with respect to procurement, it is important that standard anesthetic techniques are followed to properly anesthetize the animals (enough anesthetic, injection into the peritoneum) and adherence to all IACUC policies is required. The cannulae (shown in Figure 13 A, B, and C) should be repetitively flushed in order to remove any clot and/or debris within the pulmonary vasculature. With respect to animal selection, we suggest using Sprague Dawley or Lewis rats weighing 250–350 g. Special care should be taken when cannulating rats weighing close to 250 g since the vessels will be smaller and therefore much more difficult to cannulate without injuring the vasculature. If smaller rats, or a mouse model, is to be used, smaller cannula may need to be used.
Tracheal cannulation is not typically challenging as long as the suture is secured properly by first passing a silk suture posterior to the trachea after dissecting the surrounding fascia and prior to cannulation. Follow this with an anterior incision 1-2 tracheal rings above the suture to pass the cannula. Tie square knots in between the tracheal rings in order to secure it within a groove for better security (Figure 4C). Cannulation of the pulmonary artery (PA) is more challenging compared with the tracheal cannula. The following steps were used in this study for this procedure. First, grasp the heart apex with a pair of forceps. Pass another pair of forceps in the transverse sinus and thread a suture to secure the cannula in the proximal PA. Incise the right ventricle immediately before the right ventricular outflow tract (RVOT) (Figure 14A). After the incision into the RVOT, the cannula will be guided towards the pulmonary artery outflow tract. Having the suture in position behind the pulmonary artery/aorta before the right ventriculotomy increases efficiency (Figure 5C). The cannula should be secured into place with the suture to prevent dislodgement. A major complication can occur if the PA cannula is not in the correct anatomical orientation. The cannula may be inserted too far and only perfuse one branch or become mal-positioned with twisting of the heart-lung specimen upon removal from the chest cavity. This can easily be oriented back to the original position to preserve the proper angle of anatomical position. Finally, Left Atrial (LA) cannulation is the most challenging part of the procedure. The LA cannula needs to be placed within the left atrium. With the tissues being extremely friable, be mindful to not use significant force or twisting in order to prevent a tear within the pulmonary vein & left atrium which would then make the experiment unsalvageable. The PA cannula is best placed before the LA cannula. A left ventriculotomy with removal of the apex has been shown to disrupt the cordae tendinae and allow easier access through the mitral leaflets. Also, the ventriculotomy makes it easier to dilate and visualize the mitral valve and to feed the cannula through the mitral valve. Dilation of the mitral valve annulus with a pair of small blunt-ended pick-ups can be done in order to visualize the tract into the LA (Figure 14B). Suture should be placed behind the heart before cannulation. This can be done simply by lifting the heart up using a pair of small blunt-ended pick-ups and placing the suture underneath and across the heart. The LA is now ready to be cannulated. Feed the LA cannula through the pick-ups in order to properly visualize the placement of the cannula into the left atrium. Take special care not to dislodge the cannula back into the left ventricle. The suture should then be tightly secured along the myocardium of the left ventricle. Securing the suture to the left atrium could occlude the entire or part of the cannula.
During the procedure, it is crucial that no air remain in the inflow section of the apparatus. Any significant air can produce an air embolism increase the PVR (effectively an “air-lock”) which will result in a much lower perfusate flow for a given pressure. Various points can be used to remove air within the system. Air within the outflow section is expected and should not have any deleterious effect on the lungs. A porcine model for pulmonary hypertension has been shown to recreate the pathology from continuous small amounts of air over an 8-week period. The increased air decreases the amount of perfusion present while causing inflammation to the surrounding tissues 19.
The initiation of perfusion can occur once the cannulation is complete but before the tube coming from the LA is connected to the EVLP line. Perfusate should be run through to clear out any blood clots and this perfusate can empty into the chest wall without any issues. Switching the perfusate pump to manual mode and slowly increasing the flow rate to ~2 ml/min allows for close monitoring of the PA pressure. Pressures over 20-30 cmH2O can indicate an obstruction and watching for perfusate exiting the LA is also an indicator but this can be very hard to see. If the pressure does increase to over 20-30 cmH2O, stop the pump and recheck both cannulations. Once the pressure is constant around 10-20 cmH2O allow the perfusate to run through and into the chest cavity for 2 min. At this time the line from the LA can be connected to the EVLP circuit. The perfusate pump speed can be increased to 5-10 ml/min. As the fluid head progresses through the circuit, there will be an increase in the PA pressure due to the increase in height of the fluid head and therefore the static pressure. If the fluid cannot flow over the highest point in the line, it may be necessary to either apply a suction force on the opposite end of the line or attempt to lower the highest portion of the line. Once this issue is overcome, the perfusate should circulate without any issues.
A few issues should be monitored with respect to the ventilator. First, twisting of bronchi/trachea and heart-lung position may occur as the lungs become more edematous and the weight increases. It is important for the cannulae to remain in a relatively close anatomical position, therefore altering either or both cannulae may be necessary. Pressure or volume controlled ventilators as well as positive or negative ventilation can be used with this EVLP system. For the rat model, we have found using positive pressure, volume controlled ventilation works well at tidal volumes between 4-10 ml/kg and at positive end-expiratory pressures (PEEP) between 2-8 cmH2O. However, a PEEP of 8 cmH2O can cause a possible rupture at the bifurcation of the trachea. After each experiment (or set of experiments if performed back-to-back), the ventilation line leading to the trachea should be cleaned of any bronchoalveolar lavage (BAL) fluid that may have travelled up the trachea. This fluid will harden if left untouched and can completely block the ventilation line.
The perfusate composition is critical to a successful EVLP experiment. A 5% dextran mixture allows for lung perfusion that is close to physiologic conditions, maintains a stable oncotic pressure to drive fluid back into the vasculature to prevent edema and prevents thrombosis within the pulmonary vessels. It is important to note that some species of rats can be allergic to dextran which might cause pulmonary edema20. The contents of the perfusate was consistent across all experimental groups in this study, therefore the dextran content should not be a confounder. The oncotic pressure is a critical variable that has the potential to improve or produce tissue edema. Commercially available perfusion solutions that are optimized for cold static storage or normothermic perfusions have been used in this system to increase lung viability times. We note that some of these solutions contain albumin and one concern is the possibility of bovine albumin triggering an inflammatory response in the rodent lung. Although optimal perfusate composition is an ongoing subject of investigation, the perfusate needs to take into account the oncotic pressure, the osmotic pressure and buffering capacity. We recommend that the solution be based upon a modified Krebs–Henseleit solution or cell culture media. The oncotic pressure should be maintained by dextran or albumin, depending on the application. The perfusion pressure and flow rate affects the organ and supra-physiologic perfusion parameters can make the organ prone to mechanical trauma.
Visual Indicators during Experiment:
There are many visual cues as well as indications from real-time data that can be used to determine if an EVLP experiment is running well. The lung will remain the same size and will deflate to the same volume after every breath. There will also be no leaking from the lung itself. The PVR, lung weight, and compliance will remain relatively constant. Oxygen production will remain constant or increase slightly.
There are many visual indicators when the lung becomes compromised during an experiment. The lung becomes edematous and grows rapidly in size and weight. The color of the lung changes (from a tan-pink to white) and pockets of liquid can be identified in the tissue. If the trachea or lung ruptures from barotrauma or over distension, there will be bubbling from the point of injury (Figure 12B). Oxygen production will decrease and the PVR and compliance will dramatically increase as well.
The potential of using an EVLP model on small animals such as rodents opens the door for future studies improving the treatment of lung transplants. However, the small animal model requires a better understanding to truly mimic a lung transplantation. This model may be used in the future to improve medical treatments and define baseline parameters for future lung transplant studies.
Disclosures
None
Acknowledgments
The authors would like to acknowledge the assistance of Harvard Apparatus, especially Stephanie Pazniokas, MS (Physiology Systems & Regenerative Medicine) for their assistance in circuit assembly, modification and troubleshooting of the perfusion circuit and XVIVO Perfusion (Daniel Martinelli, CCP, CTP) for providing non-clinical use pulmonary plegia.
References
- United States Organ Transplantation, Organ Procurement and Transplantation Network & Scientific Registry for Transplant Recipients Annual Report 2011. 2011. Available from: http://srtr.transplant.hrsa.gov/annual_reports/2011.
- Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289–1298. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- Cardoso PF. New perspectives in lung transplantation: from conventional preservation to ex vivo lung perfusion and lung reconditioning. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2009;35:1057–1059. doi: 10.1590/s1806-37132009001100001. [DOI] [PubMed] [Google Scholar]
- DeCampos KN, Keshavjee S, Liu M, Slutsky AS. Optimal inflation volume for hypothermic preservation of rat lungs. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1998;17:599–607. [PubMed] [Google Scholar]
- Perrot M, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: donor-related risk factors and markers. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24:1460–1467. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Mulloy DP, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. The Journal of thoracic and cardiovascular surgery. 2012;144:1208–1215. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, et al. Successful prolonged ex vivo lung perfusion for graft preservation in rats. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2014;45:e54–e60. doi: 10.1093/ejcts/ezt598. [DOI] [PubMed] [Google Scholar]
- Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. American journal of respiratory and critical care medicine. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- Steen S, et al. Transplantation of lungs from a non-heart-beating donor. The Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- Perrot M, et al. Strategies to optimize the use of currently available lung donors. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2004;23:1127–1134. doi: 10.1016/j.healun.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Cypel M, et al. Technique for prolonged normothermic ex vivo lung perfusion. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27:1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Cypel M, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2262–2269. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Cypel M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. The New England journal of medicine. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- Cypel M, et al. Normothermic Human Ex Vivo Lung Perfusion (EVLP) for Improved Assessment of Extended Criteria Donor Lungs for Transplantation. The Journal of Heart and Lung Transplantation. 2009;28:S126–S126. [Google Scholar]
- Sanchez PG, et al. Normothermic Ex Vivo Lung Perfusion as an Assessment of. Marginal Donor Lungs - The NOVEL Lung Trial. J Heart Lung Transpl. 2013;32:S16–S17. [Google Scholar]
- Pego-Fernandes PM, et al. Experimental model of isolated lung perfusion in rats: first Brazilian experience using the IL-2 isolated perfused rat or guinea pig lung system. Transplantation proceedings. 2010;42:444–447. doi: 10.1016/j.transproceed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Pego-Fernandes PM, et al. Experimental model of isolated lung perfusion in rats: technique and application in lung preservation studies. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2010;36:490–493. doi: 10.1590/s1806-37132010000400015. [DOI] [PubMed] [Google Scholar]
- Niemeier RW. The isolated perfused lung. Environmental health perspectives. 1984;56:35–41. doi: 10.1289/ehp.845635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, et al. A pulmonary hypertension model induced by continuous pulmonary air embolization. The Journal of surgical research. 2011;170:e11–e16. doi: 10.1016/j.jss.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Harris JM. Differences in responses between rat strains and colonies. Food and cosmetics toxicology. 1965;3:199–202. doi: 10.1016/s0015-6264(65)80075-x. [DOI] [PubMed] [Google Scholar]


